Abstract
Dowling-Degos disease (DDD) is an autosomal dominant genodermatosis characterized by reticular pigmented anomaly mainly affecting flexures. Though KRT5 has been identified to be the causal gene of DDD, the heterogeneity of this disease was displayed: for example, POFUT1 and POGLUT1 were recently identified and confirmed to be additional pathogenic genes of DDD. To identify other DDD causative genes, we performed genome-wide linkage and exome sequencing analyses in a multiplex Chinese DDD family, in which the KRT5 mutation was absent. Only a novel 1-bp deletion (c.246+5delG) in POFUT1 was found. No other novel mutation or this deletion was detected in POFUT1 in a second DDD family and a sporadic DDD case by Sanger Sequencing. The result shows the genetic-heterogeneity and complexity of DDD and will contribute to the further understanding of DDD genotype/phenotype correlations and to the pathogenesis of this disease.
Introduction
Dowling-Degos disease (DDD [MIM 179850]) is an autosomal dominant genodermatosis characterized by reticular pigmented anomaly mainly affecting flexures, such as the neck, axilla and areas below the breasts and groin [1]. In 2006,Betz et al. performed a genomewide linkage analysis of two German families and identified loss-of-function mutations in the keratin 5 gene (KRT5) [2]. However, no KRT5 mutations were identified in more than 50% of DDD familial cases and sporadic cases [3], [15], suggesting the genetic heterogeneity of DDD.
Recently, next generation sequencing technologies, including whole genome and whole exome sequencing, have been successfully applied to human genetics research to identify pathogenic genes []–[6]. Equipped with this advanced technology, two genes, POFUT1 [7] and POGLUT1 [15], were identified and confirmed to be additional pathogenic genes of DDD during the past year.
Here, we investigated two DDD families and one sporadic DDD case which are absent from KRT5 mutations and performed genome-wide linkage and exome sequencing analyses in one DDD family. Only a novel mutation, c.246+5delG, in POFUT was identified to be potentially causal. This mutation was confirmed by Sanger sequencing to be present in all affected family members, absent in unaffected individuals that were sequenced except that the unaffected III8 in the family also carries it. III8, whose mother is a DDD case, is 12 years old and probably under the disease onset age. Sanger sequencing did not detect other novel mutations nor this deletion in POFUT1 in a second DDD family and a sporadic DDD case. The result shows the genetic heterogeneity and complexity of DDD.
Materials and Methods
Subjects
Our study recruited two unrelated DDD families and one sporadic DDD case of Chinese ethnicity, totaling 10 affected and 12 unaffected individuals (Figure 1). In addition, 100 unrelated healthy controls of Chinese ethnicity were also Sanger sequenced. All patients were carefully examined by at least two experienced dermatologists, and were diagnosed by clinical features (Figure 2) and histopathological findings (Figure S1). EDTA anticoagulated venous blood samples were collected from all participants. Genomic DNA was extracted from peripheral blood lymphocytes by standard procedures using FlexiGene DNA kits (Qiagen).
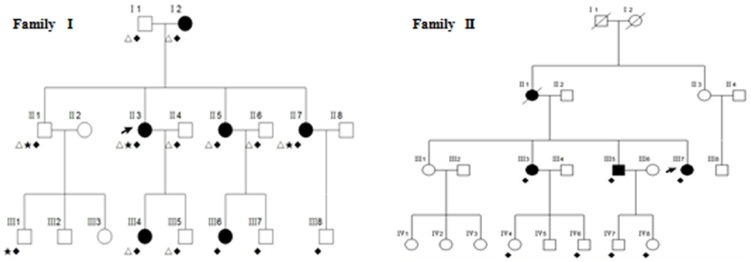
Figure 1. Family trees of Family I and Family II.
Shown are the pedigree of family I and family II with DDD. The arrow denotes the proband; “Δ” denotes the individuals used in the linkage analysis; “★” denotes the individuals used in exome sequencing analysis, “⧫” denotes the individuals that were Sanger sequenced for POFUT1.
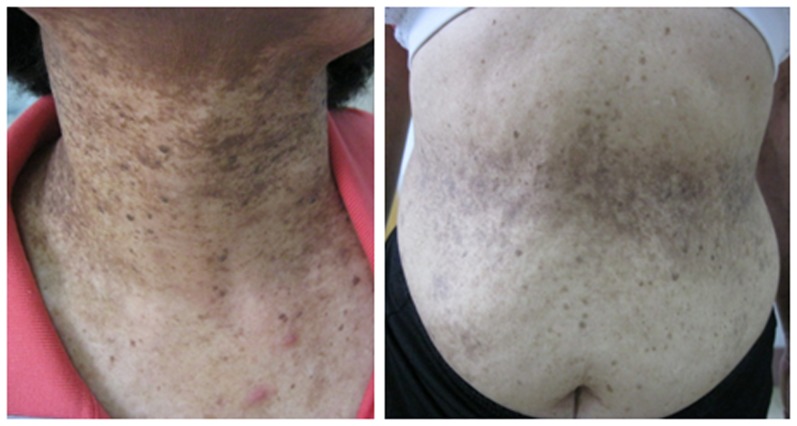
Figure 2. Clinical manifestations of DDD.
Reticular hyperpigmentation on the skin of neck and abdomen.
The study was approved by the institutional review board at Shandong Provincial Institute of Dermatology. Written informed consent was obtained from all participants, or their guardian.
Genome-wide linkage and exome sequencing analyses
Approximately 200 ng of genomic DNA was used for genotyping by Illumina Human 660W-Quad BeadChip for each of 10 individuals (Figure 1) in Family I. Multipoint parametric linkage analysis were performed in Merlin [8] by using the LD pruned autosomal SNPs (with LD<0.1 in population data) and assuming a dominant inheritance mode with a disease allele frequency of 0.001.
To pinpoint the causal mutations for DDD, exome capture was carried out using Agilent SureSelect Human All Exon Kit according to the manufacturer's protocols in two affected (II 3 and II 7) and two unaffected individuals (II 1 and III 1) in family I (Figure 1).
Each captured library was loaded on a HiSeq 2000 platform, and paired-end sequencing was performed with read lengths of 100 bp. Variants were called and filtered based on the “best practice variant detection with GATK (v3), that is “QD<2.0”, “MQ<40.0”, “FS>60.0”, “HaplotypeScore >13.0”, “MQRankSum <−12.5”, “ReadPosRankSum <−8.0” for single nucleotide variants (SNV), and “QD<2.0”, “ReadPosRankSum <−20.0”, “InbreedingCoeff <−0.8”, “FS>200.0” for indel.
Sanger sequencing
All seven coding exons including intron–exon boundaries as well as 5′ UTRs and 3′ UTRs of POFUT1 were amplified by polymerase chain reaction (PCR) using the published primers [7]. After amplification, products were purified and sequenced on ABI 3130xl Genetic Analyser. Mutations were identified by comparing with the reported DNA reference sequence (GenBank accession number: NG_032110). All the identified mutations were verified by the subsequent opposite-direction sequencing. Both the patients and control samples were analyzed using the same protocol.
Results
Linkage analysis
We firstly performed genome-wide linkage analyses in family I to determine the locus that co-segregates with the disease. Maximal LOD score of 1.5 was obtained on chromosome 8 and 20 (Figure S2). Haplotype co-segregation analyses by Haplopainter [9] mapped the linkage critical region to chr20: 17,722,478–36,009,145. There are 165 SNPs in chromosome 20 for linkage analysis after LD pruning.
Exome sequencing analysis
Sequence reads were mapped by BWA [18] to hg19 human reference genome. On average, each sample had a total of 102063181 uniquely mapped reads (Table S1). Each sample had a mean coverage of 115.85 and 91% of the 50 Mb target regions were covered at 10× or more (Figure S3 and Table S2). GATK Unified Genotyper with the recommended filtering criteria was invoked to call single nucleotide variants (SNV) and indels. For SNV, 18,606 coding variants were identified on average in each individual (Table S3). The percentage of novel SNVs (not in dbSNP132) was 6.2%; Ti/Tv ratios for novel SNVs and SNVs in dbSNP132 were 2.90 and 3.22 respectively. 2215 SNVs were shared by the two patients and absent in the two healthy controls; 81 of which were not in 1000 Genome Project (Phase I, Oct 2012), none of them lied in the linkage critical region (Table S4).
5421 indels were identified on average in each individual. 170 were shared by the two patients and absent in the two healthy controls, 98 of which were not in 1000 Genome Phase I data (Oct 2012). Six out of these 98 indels lied in the linkage critical region, one of which is functional. This indel (c.246+5delG), 5 bp downstream of exon 2 in POFUT1, is a non-canonical donor splice site (within 3–8 bp of the exon-intron boundary).
Sanger sequencing
To verify the identified mutation, we screened POFUT1 coding sequences including intron-exon boundaries as well as 5′ UTRs and 3′ UTRs by Sanger sequencing most of DDD Family I, part of Family II members and one sporadic case (10 affected and 12 unaffected individuals together, Figure 1).
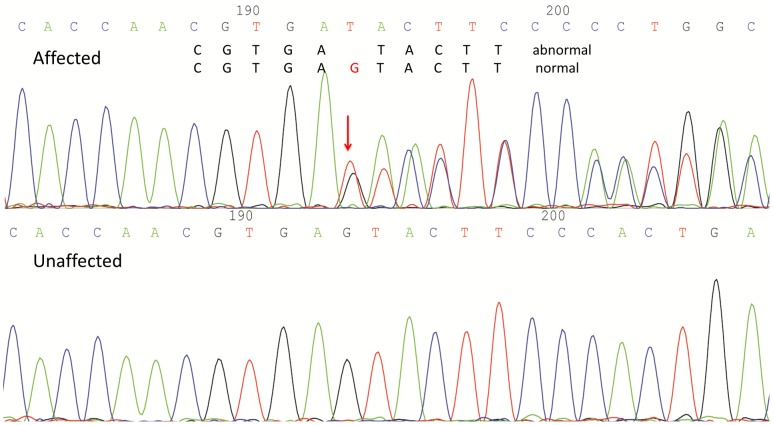
In Family I, the novel deletion (c.246+5delG) in intron 2 of POFUT1 was present in all affected and absent in all unaffected members that were sequenced (Figure 3), except that unaffected III8 carried it (Figure 1). However, III8, whose mother is a DDD case, is 12 years old and may well be under disease onset age (the average age at onset in Family I is about 20). This indel was absent in 100 unrelated healthy individuals of Chinese ethnicity that were Sanger sequenced. No other novel mutations or this mutation of POFUT1 were identified in Family II and the sporadic case.
Figure 3. The 1-bp deletion in POFUT1.
The Sanger sequencing traces around the position of 1-bp deletion c.246+5delG in one affected and one unaffected individuals of Family I.
Discussion
DDD is a relatively uncommon papillomatous pigmentary defect showing autosomal-dominant inheritance. It was first described by Dowling and Freudenthal in 1938 [10] and was termed by Degos and Ossipowski in 1954 [11].
After Betz et al. detected KRT5 as a DDD pathogenic gene in 2006 [2], Li et al identified POFUT1 as a new causal gene in 2013 [7]. This verified the genetic heterogeneity of DDD. POFUT1, located at 20q11, encodes two isoforms due to the alternative splicing. Researches have confirmed that this gene is an essential component of the canonical Notch signaling pathway [7], [12], which plays an important role in the regulation of melanocyte lineage development [13], [14].
Our linkage critical region (chr20: 17,722,478–36,009,145) includes gene POFUT1. Two variants were identified in POFUT1 in our exome sequencing data of Family I – one was a SNV (chr20:30795819) which did not co-segregate with the disease, the other was an indel (c.246+5delG) in intron 2 of POFUT1. This indel is different from the two mutations identified by Li et al [7] which lie in exon 4 of POFUT1. c.246+5delG, 5 bp downstream of exon 2, is a non-canonical donor splice site (within 3–8 bases of the exon-intron boundary). We invoked NetGene2 [16] and MaxEntScan [17] splice site prediction softwares to investigate the effect of the 1-bp deletion on the splicing. The scores for the reference sequence around exon 2-intron 2 boundary from NetGene2 and MaxExtScan were 0.86 (> threshold of 0.5) and 9.54 respectively, while the scores for the 1-bp deletion sequence were below the threshold and −2.88 respectively, suggesting that the deletion might prevent the splicing between exon 2 and intron 2. Conservation scores and multiple alignments around the deletion from UCSC genome browser (Fig. S4) showed that the position affected by the deletion was conserved across species.
Recently, Basmanav et al [15] performed exome sequencing in five unrelated DDD individuals who were absent from mutations in KRT5 and identified POGLUT1 as another DDD causal gene. Only 2 SNPs in POGLUT1 were identified in our exome sequencing data of Family I – one was SNV chr3:119196334 which was present in one unaffected and two affected individuals, absent in the other unaffected individuals. The other was SNV chr3:119198873 which was present in one unaffected and one affected individuals, absent in the other unaffected and affected individuals. Furthermore, POGLUT1 is not in our linkage critical region, rendering the chance of POGLUT1 being causal for DDD in Family I slim.
We should have genotyped more samples in Family I to increase the statistical power of the linkage analysis. However, it may not change the identified causal gene and mutation as the linkage analysis of Family I in our study and that of the discovery family in Li et al [7] pointed to the similar linkage critical regions.
In conclusion, our result further verified the genetic heterogeneity and complexity of DDD and contributes to the additional understanding of DDD genotype/phenotype correlations.
Supporting Information
Histopathological findings for DDD. The lesion showed hyperkeratosis and filiform epithelial downgrowth of epidermal rete ridges. There were a large amount of melanins in the basal layer and some melanophages and melanins in the upper dermis. Left: HE 40×, right: HE 400×.
(TIF)
LOD score of parametric linkage analysis vs genetic map for 22 chromosomes.
(TIF)
Percentage of the target regions covered at 5×, 10×, 20×, etc for the four individuals used in exome sequencing analysis.
(TIF)
Conservation scores and multiple alignments from chr20: 30,797,995–30,798,005 (around deletion c.246+5delG) by UCSC genome browser.
(TIF)
Reads information for four exome sequenced individuals.
(PDF)
Percentage of the target regions covered at 5×, 10×, 20×, etc for the four individuals used in exome sequencing analysis.
(PDF)
Variant information for four exome sequenced individuals.
(PDF)
81 SNVs identified in exome sequencing analysis that were shared in two DDD cases, absent in two unaffected controls, and not present in 1000 Genome Project (Oct 2012).
(PDF)
Acknowledgments
We thank all the patients and their family members for their enthusiastic participation.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Natural Science Foundation of Shandong Province (ZR2011HQ003, ZR2011HQ037), to FZ; Project of Taishan scholar (2008-), to FZ; Project of clinical center for Dermatology and Venereology of Shandong Province (2010-), to FZ; and Project of Research Foundation of Shandong Provincial Institute of Dermatology and Venereology (2008-7), to FZ. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rebora A, Crovato F (1984) The spectrum of Dowling–Degos disease. Br J Dermatol 110: 627–630. [DOI] [PubMed] [Google Scholar]
- 2. Betz RC, Planko L, Eigelshoven S, Hanneken S, Pasternack SM, et al. (2006) Loss-of-function mutations in the keratin 5 gene lead to Dowling-Degos disease. Am J Hum Genet 78: 510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asahina A, Ishii N, Kai H, Yamamoto M, Fujita H (2007) Dowling-Degos disease with asymmetrical axillary distribution and no KRT 5 exon 1 mutation. Acta Derm Venereol 87 (6) 556–7. [DOI] [PubMed] [Google Scholar]
- 4. Chen WJ, Lin Y, Xiong ZQ, Wei W, Ni W, et al. (2011) Exome sequencing identifies truncating mutations in PRRT2 that cause paroxysmal kinesigenic dyskinesia. Nat Genet Nov 20;43 (12) 1252–5. [DOI] [PubMed] [Google Scholar]
- 5. Yang J, Duan S, Zhong R, Yin J, Pu J, et al. (2013) Exome sequencing identified NRG3 as a novel susceptible gene of Hirschsprung's disease in a Chinese population. Mol Neurobiol 47 (3) 957–66. [DOI] [PubMed] [Google Scholar]
- 6. Liu H, Li Y, Hung KK, Wang N, Wang C, et al. (2014) Genome-wide linkage, exome sequencing and functional analyses identify ABCB6 as the pathogenic gene of dyschromatosis universalis hereditaria. . PLoS One Feb 3;9 (2) e87250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li M, Cheng R, Liang J, Yan H, Zhang H, et al. (2013) Mutations in POFUT1, Encoding Protein O-fucosyltransferase 1, Cause Generalized Dowling-Degos Disease. Am J Hum Genet Jun 6;92 (6) 1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abecasis GR, Cherny SS, Cookson WO, Cardon LR (2002) Merlin–rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet 30 (1) 97–101. [DOI] [PubMed] [Google Scholar]
- 9. Thiele H, Nürnberg P (2005) HaploPainter: a tool for drawing pedigrees with complex haplotypes. Bioinformatics 21 (8) 1730–1732. [DOI] [PubMed] [Google Scholar]
- 10. Dowling GB, Freudenthal W (1938) Acanthosis Nigricans. Proc R Soc Med 31: 1147–1150. [PMC free article] [PubMed] [Google Scholar]
- 11. Degos R, Ossipowski B (1954) Reticulated pigmentary dermatosis of the folds: relation to acanthosis nigricans. Ann Dermatol Syphiligr (Paris) 81: 147–151. [PubMed] [Google Scholar]
- 12. Yao D, Huang Y, Huang X, Wang W, Yan Q, et al. (2011) Protein O-fucosyltransferase 1 (Pofut1) regulates lymphoid and myeloid homeostasis through modulation of Notch receptor ligand interactions. Blood 117: 5652–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moriyama M, Osawa M, Mak SS, Ohtsuka T, Yamamoto N, et al. (2006) Notch signaling via Hes1 transcription factor maintains survival of melanoblasts and melanocyte stem cells. J Cell Biol 173: 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schouwey K, Delmas V, Larue L, Zimber-Strobl U, Strobl LJ, et al. (2007) Notch1 and Notch2 receptors influence progressive hair graying in a dose-dependent manner. Dev Dyn 236: 282–289. [DOI] [PubMed] [Google Scholar]
- 16. Brunak S, Engelbrecht J, Knudsen S (1991) Prediction of Human mRNA Donor and Acceptor Sites from the DNA Sequence,. Journal of Molecular Biology 220: 49–65. [DOI] [PubMed] [Google Scholar]
- 17. Eng L, Coutinho G, Nahas S, Yeo G, Tanouye R, et al. (2004) Nonclassical Splicing Mutations in the Coding and Noncoding Regions of the ATM Gene: Maximum Entropy Estimates of Splice Junction Strengths,. Hum Mut 23: 67–76. [DOI] [PubMed] [Google Scholar]
- 18. Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Histopathological findings for DDD. The lesion showed hyperkeratosis and filiform epithelial downgrowth of epidermal rete ridges. There were a large amount of melanins in the basal layer and some melanophages and melanins in the upper dermis. Left: HE 40×, right: HE 400×.
(TIF)
LOD score of parametric linkage analysis vs genetic map for 22 chromosomes.
(TIF)
Percentage of the target regions covered at 5×, 10×, 20×, etc for the four individuals used in exome sequencing analysis.
(TIF)
Conservation scores and multiple alignments from chr20: 30,797,995–30,798,005 (around deletion c.246+5delG) by UCSC genome browser.
(TIF)
Reads information for four exome sequenced individuals.
(PDF)
Percentage of the target regions covered at 5×, 10×, 20×, etc for the four individuals used in exome sequencing analysis.
(PDF)
Variant information for four exome sequenced individuals.
(PDF)
81 SNVs identified in exome sequencing analysis that were shared in two DDD cases, absent in two unaffected controls, and not present in 1000 Genome Project (Oct 2012).
(PDF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.