Abstract
Background
With growing evidence that anesthesia exposure in infancy affects cognitive development, it is important to understand how distinct anesthetic agents and combinations can alter long-term memory. Investigations of neuronal death suggest that combining anesthetic agents increases the extent of neuronal injury. However, it is unclear how the use of simultaneously combined anesthetics affects cognitive outcome relative to the use of a single agent.
Methods
Postnatal day (P)7 male rats were administered either sevoflurane as a single agent or the combined delivery of sevoflurane with nitrous oxide at 1 Minimum Alveolar Concentration for 4 h. Behavior was assessed in adulthood using the forced alternating T-maze, social recognition, and context-specific object recognition tasks.
Results
Animals exposed to either anesthetic were unimpaired in the forced alternating T-maze test and had intact social recognition. Subjects treated with the combined anesthetic displayed a deficit, however, in the object recognition task, while those treated with sevoflurane alone were unaffected.
Conclusion
A combined sevoflurane and nitrous oxide anesthetic led to a distinct behavioral outcome compared with sevoflurane alone, suggesting that the simultaneous use of multiple agents may uniquely influence early neural and cognitive development and potentially impacts associative memory.
Keywords: volatile anesthetics, sevoflurane +/− nitrous oxide, memory, neurotoxicity
Introduction
Every day, children around the world undergo general anesthesia for various procedures and operations. Epidemiologic studies have raised concerns that humans are susceptible to long-term effects on learning and memory following exposure to anesthesia at an early age1, 2. Animal models reveal that neonates exposed to anesthetics suffer extensive neuronal death and persistent memory deficits 3–12. With increasing evidence regarding the detrimental effects of neonatal anesthesia exposure, it is important to understand how anesthetic agents and combinations of agents might influence cognitive development.
Sevoflurane is a volatile anesthetic frequently used in children as a sole agent or in conjunction with nitrous oxide, a separate type of anesthetic. These anesthetics exert their effects via different mechanisms – sevoflurane is believed to be a Gamma-Aminobutyric Acid (GABAA) agonist 13, 14 while nitrous oxide acts as an N-methyl-D-aspartate (NMDA) receptor antagonist15–17. In the past, studies of neuronal death have indicated that the co-administration of a GABA agonist with an NMDA antagonist might result in greater neuronal death than either agent individually18. In addition, others have shown that the addition of nitrous oxide to another volatile anesthetic leads to increased neurotoxicity19, 20.
Although there are numerous studies of cell death5, 11, 18–22, there is a lack of behavioral experiments to accompany them. The important outcome of cognition and memory after anesthetic exposure is, therefore, understudied. As a result, it remains unclear whether anesthetics may induce different long-term effects on memory when used in combination rather than as individual agents.
Minimum Alveolar Concentration 23 is the minimum amount of inhaled anesthetic required to prevent movement in response to a painful stimulus and is a reliable measure of potency24, 25. Unlike in adult rodents, MAC in newborns is not a fixed concentration but decreases over time and involves continual adjustment of the concentration6, 26. By adjusting to 1 MAC, we are able to compare cognitive outcomes from anesthetics that are similar in potency8. In the present study, we investigated whether a combined anesthetic of sevoflurane and nitrous oxide would lead to a different behavioral outcome than sevoflurane alone. Following exposure to 1 MAC of either anesthetic as newborns, long-term memory was evaluated by testing subjects in the forced alternating T-maze, social recognition, and an object recognition task relying on associative learning.
Materials and Methods
Subjects
All experiments were conducted with approval from the Institutional Animal Care and Use Committee at University of California, San Francisco. Dams with postnatal-day 6 (P6) male Sprague-Dawley pups were purchased from Charles River Laboratories (Glilroy, CA). At P7, pups were randomized into three groups – control (n = 29), anesthesia with sevoflurane (n = 54), anesthesia with sevoflurane and nitrous oxide (n = 27). Following anesthesia, animals were cross fostered among the dams until weaning at P21. They were then kept in standard lab housing with 12-hour light-dark cycle and given ad libitum access to food and water prior to cognitive testing. During testing, animals were housed individually and food restricted as described for each experiment below.
Anesthesia
Anesthetic delivery was performed similarly to what we have reported before 8, 27. Briefly, treatment animals received either sevoflurane as a single agent or the simultaneously combined treatment of sevoflurane with nitrous oxide for a total of four hours. Each anesthetic regimen was adjusted to 1 Minimum Alveolar Concentration23. Sevoflurane was administered in air and oxygen (FiO2 25%), and MAC was determined by tail clamping every 15 min and anesthetic concentration was adjusted so that 50% of animals would respond to the stimulus 8, 27. In the combined treatment, nitrous oxide was held constant at 70% while sevoflurane concentration was adjusted to achieve 50% movement in response to tail clamping. Control rats were treated in an identical manner for four hours without being exposed to anesthetic. Animals were kept on a warming blanket in the chamber and temperatures were measured with an infrared laser thermometer and maintained with a goal of 35°C, the average skin temperature of non-anesthetized control pups in a huddle without the dam.
Forced Alternating T-maze
Apparatus
Testing was conducted in a T-maze apparatus made of wood with a detachable stem (length 55 cm, width 10 cm) and crosspiece (length 91 cm, width 10cm). Food wells (diameter 2.5 cm, depth 2 cm) at the ends of each arm were recessed into the maze track so they were not visible from the stem. The food wells contained a full size reward (Silly Circles, Safeway Kitchens) fixed between 2 cup-shaped filters so both baited and unbaited arms had the same smell. Clear acrylic was used for the maze walls with guillotine-style doors (width 10 cm, height 20 cm) at the maze arms and start area. Testing occurred in a 3 meter square area enclosed in black felt curtain with visual reference cues on each wall.
Habituation and Testing
Subjects from the control group (n = 29), sevoflurane group (n=41), and sevoflurane with nitrous oxide group (n = 26) began behavioral testing on P69. From P69–P85, subjects were food restricted and weighed daily to achieve 85%–90% normal bodyweight. At P69, animals were given two 5-minute trials of free exploration in the open T-maze with rewards placed in both food wells and along the floor throughout the maze. Animals not moving after 5 minutes were guided down the stem and given an additional minute for exploration. At P70–74, habituation continued without guided exploration or rewards along the track.
Subjects began forced-alternation testing in the T-maze at P76 between 0700 and 1900 h. Testing occurred over a period of 10 days with 6 trials per day. Each trial consisted of two runs – an “information” run and a “choice” run. During the information run, one of the two arms was closed so the rat would have only one option (left or right) in order to obtain the reward. In the subsequent choice run, both arms were open, and only the opposite arm as the previous run contained the reward. If the animal entered the same arm it had already visited, then it was negatively reinforced by being confined for 10 seconds within the arm lacking the reward. The direction of the choice and information runs were randomized for each trial using a computer so that every animal was given an equal number of left and right entries but the order was variable. Subjects were introduced into the maze facing away from the crosspiece during each run and given 3 minutes to commit to an arm. Commitment to an arm was established when a subject’s hind legs entered the arm. Any animal unable to commit to an arm within 3 minutes was returned to its cage without a reward. Trials in which the rat did not make a choice were not scored and only sessions during which an animal made a choice in at least 4 out of six trials were used in the final results. The maze was wiped clean between subjects using 70% ethanol and the same handlers were used throughout all behavioral experiments.
On days 1–8, the delay between the information and choice run was 5 seconds. During days 9 and 10, animals underwent delayed forced alternation testing with a 30 second delay between the information run and choice run (based on validation testing, animals had fewer correct choices but still performed the task at 30 seconds). After completing the information run, animals were placed in the closed start area and confined for 30 seconds before opening the door to begin the choice run.
Social Interaction
Upon completion of the T-maze test, rats were given unrestricted access to food and water. To assess social behavior, subjects were presented simultaneously with a female rat and novel object and assessed whether they spent more time investigating the social target. Six adult female Sprague Dawley rats were used as social targets and housed individually prior to testing. They were introduced within cages or “holders” and placed in the arena opposite the novel object. The male subject was given five minutes for exploration while interactions were recorded with a video camera (SONY HDR-CX190) mounted 2 m above the box.
Investigation of the female was defined as any direct contact with the nose or paws, as well as sniffing toward any part of the female including the tail if it extended outside of the holder. Not included was time spent sniffing toward the empty top portion of the holder or circling without pausing to sniff. Investigation of the object was defined as sniffing or placing the nose within 1 cm of and oriented toward the object. This excluded merely using the object as a support during rearing. Observers blind to group assignment were used to record the investigation times.
Social Recognition
Subjects were separately tested in social recognition using a two-trial discrimination model. Exposure to a single female was followed by a second exposure to the same (familiar) female and a novel female. Testing occurred during the light cycle, between 0900 and 1700 h, and each rat was tested in its home cage (20 cm × 23 cm × 46 cm). The same adult females were used as social targets, although testing occurred more than one week after the social interaction task so subjects would not recall the stimulus animals.
In the first exposure, the male subject was given five minutes to investigate a single female. After a 60-minute delay, the first female was presented simultaneously with a novel female. The male subject was then given three minutes to explore the two female rats. The test phase was recorded and investigation times were later scored by an observer blind to group assignment.
Object-Place-Context Recognition Task
Contexts
For object recognition, two testing arenas, hereafter referred to as “contexts,” were used that were distinct in texture and appearance. Each context had 61 cm square base and 30 cm high walls. Context 1 had a base covered with white PEVA shower liner and walls covered with brown cardboard. Context 2 had base and walls made of black plastic. Each context had different visual cues on three walls. All subjects were habituated to each context prior to testing.
Testing
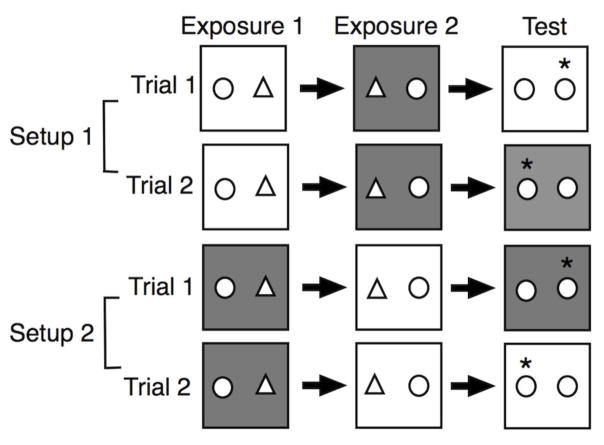
Subjects were tested in context-specific object recognition, which took place between 0700 and 1900 h using the arrangements shown in Figure 1. In the first exposure, the rat was placed in either context 1 or 2 with two different objects and observed remotely via video camera with a goal of 15 seconds of investigation per object. If the subject reached the goal of 15 seconds of exploration per object, then it was removed after two minutes (a minimum of two minutes ensured the subject was adequately exposed to the context). If the subject did not achieve 15 seconds of investigation per object, then it remained in the context for 5 minutes before it was removed. After a two-minute delay, the subject was placed in the opposite context with the same two objects, and the objects’ locations were reversed. Thus, each object was observed in both contexts and locations (left and right) after two exposures. Again, they were allowed to explore with a goal of 15 seconds per object for a minimum of 2 minutes and a maximum of 5 minutes.
Fig. 1.
Object-Place-Context Recognition Task. Two different objects are presented in separate contexts in the exposure phases, so that each object has been observed in each context and location (left and right). In the test phase, two identical objects are placed in either the original context (Trial 1) or the same context as the second exposure (Trial 2). One object appears in a location and context that was observed in a prior exposure, while the other “displaced” object (*) is in a location and context that has not been seen. Subjects were tested in two trials with half experiencing Setup1 and the other half Setup 2.
In the test phase, subjects either remained in the same context or were placed into the original context with two identical copies of one of the two objects. The location and context associated with one object were familiar, while the other “displaced” object appeared in a location and context in which it had not been observed. The paradigm assesses the subject’s ability to successfully associate an object with its unique location and context, relying on rodents’ propensity to explore objects that change locations and context. The subject was introduced into the final context for three minutes. The test phase was recorded and scored later by an observer blinded to group assignment to allow more accuracy in timing.
Each subject completed two trials with half the subjects in each group assigned to Setup 1 and the other half to Setup 2 (Fig. 2). The location (left versus right) of the displaced object in the test phase was counterbalanced within each group. Times for the two trials were combined for analysis and were included only if both objects were explored and the subject achieved a minimum of 10 seconds of exploration in the test phase for the two trials.
Fig. 2.
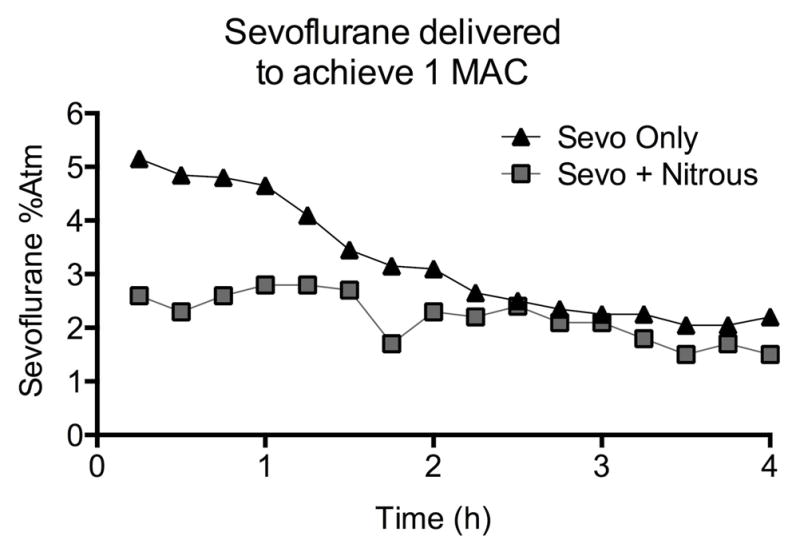
Anesthesia. Each anesthetic was administered for 4 hours and adjusted to achieve 1 Minimum Alveolar Concentration, or the concentration at which 50% of subjects respond to the same pain stimulus (tail-clamping), so the anesthetics were of comparable potency. Sevoflurane concentration was adjusted after tail-clamping every 15 min in each group, as shown. In the group that received sevoflurane plus nitrous oxide, nitrous oxide was held constant at 70%. The added concentration of sevoflurane required to achieve one MAC is shown.
Statistical Analysis
Data was analyzed using Prism 6 Software for Mac OSX (GraphPad, San Diego, CA). All comparisons were made using a two-tail P value, and a value less than 0.05 was considered statistically significant. Data were analyzed for Gaussian distribution using the D’agostino and Pearson test, and data following a normal distribution were analyzed using parametric tests. Paired data, such as a subject’s investigation times in behavioral tests, were assessed using the paired t-test and non-paired data used the unpaired t-test. One-way ANOVA was used for three or more groups with Tukey’s post-test for multiple comparisons. Data that were not normally distributed were analyzed using nonparametric tests. The Wilcoxon matched-pairs rank test was used to compare paired data and the Mann-Whitney test for non-paired data. The Kruskal-Wallis test was used for more than two groups with Dunn’s post-test. Two-way ANOVA was used to assess performance in the T-maze test and rat weights during food restriction in T-maze.
A “discrimination index” (DI) was calculated for recognition tasks to denote the relative time spent investigating the novel target relative to the familiar one. For example, in the social recognition task, the amount of time spent investigating the familiar target was subtracted from the time spent on the novel target, and this value was divided by the total time spent investigating the two (DI = (novel – familiar)/(total)) 28. A discrimination index of zero reflects equal amounts of time spent investigating two targets. DI significantly greater than zero using one sample t-test indicated that more time was spent investigating the novel target.
Excluded data: in the T-maze, four control and one sevo+nitrous animal were excluded for inability to complete the task. Additionally, sessions in which an animal failed to make a choice in 3 or more of 6 trials were not scored. This resulted in 16 of 200 (8.0%) exclusions in the control group, 13 of 328 (4.0%) in the sevoflurane group and 9 of 200 (4.5%) in the sevoflurane + nitrous group. In the object recognition task, 4 rats were excluded from the control group, 0 in the sevoflurane group, and 2 from the sevo-nitrous group due to inadequate investigation times.
Results
Anesthesia and body growth
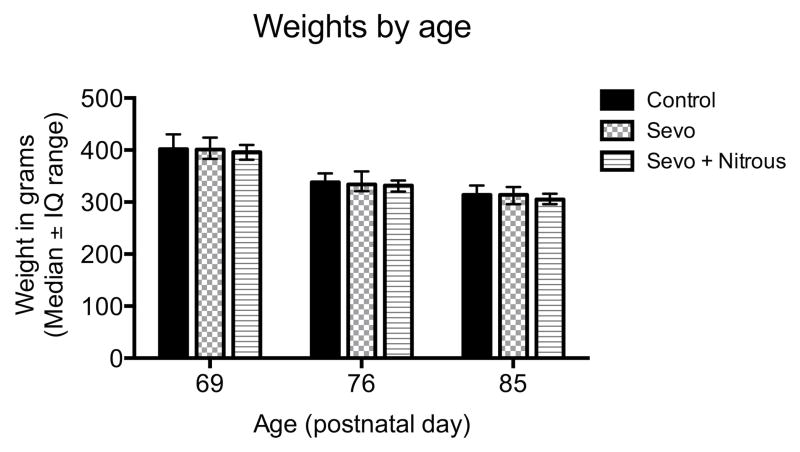
The concentration of sevoflurane required to achieve 1 MAC in both treatment groups decreased over time as expected6, 7, 26 (Fig. 2). Sevoflurane concentration required to achieve 1 MAC in the presence of 70% nitrous oxide was on average 72% of the value of sevoflurane alone in this study and in our previous experience 8 (Fig. 2). Forty-one of fifty-four pups anesthetized with sevoflurane survived, and twenty-six of twenty-seven animals from the combined treatment. There was a significant effect of food restriction on body weight (P < 0.001, two-way ANOVA, food restriction, Fig. 3) but no effects of anesthesia on body weight (P = 0.29, two-way ANOVA, anesthesia, Fig. 3).
Figure 3.
Weights by age. Animals were weighed and food-restricted throughout T-maze testing. There was a significant effect of food restriction on body weight (P < 0.001, two-way ANOVA, food restriction) but no effects of anesthesia on body weight (P = 0.29, two-way ANOVA, anesthesia).
Treatment subjects were unaffected in the forced alternating T-maze and social recognition tasks
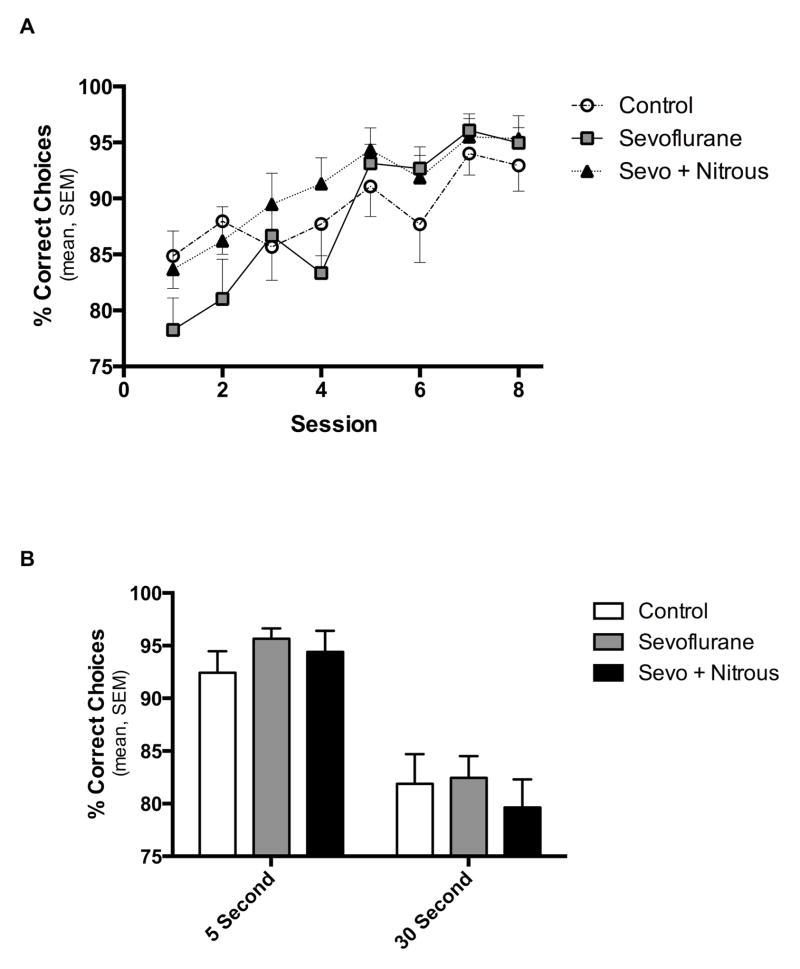
We found no difference among subjects in the rate of acquisition of the forced alternating T-maze over 8 days of testing with a 5 second delay between the information and choice runs (P = 0.12, two-way ANOVA, treatment, Fig. 4A). All groups improved over time (P < 0.0001, two-way ANOVA, session), but there was no effect of treatment (P=0.12) or interaction (P=0.67). Further post-hoc analysis of groups at each session found no difference between groups at any time-point (Tukey P>0.05 for all sessions). When we increased the delay between the information and choice runs to 30 seconds all groups also performed similarly (Fig. 4B), but the percent correct choices decreased for all groups with the longer delay as expected29 (two-way RM ANOVA, Session P < 0.0001, Treatment P = 0.595, matching P = 0.0025).
Fig. 4.
T-maze Results. Rats were placed in the maze for 6 consecutive trials daily. In each trial, rats were given an information run followed 5 seconds later by a choice run. A) Percent correct choices are recorded across sessions for each group. There was no difference between groups in the learning phase. B) The last two sessions with a 5 second delay and the subsequent two sessions with a 30 second delay are blocked together. There is no difference between treatment groups.
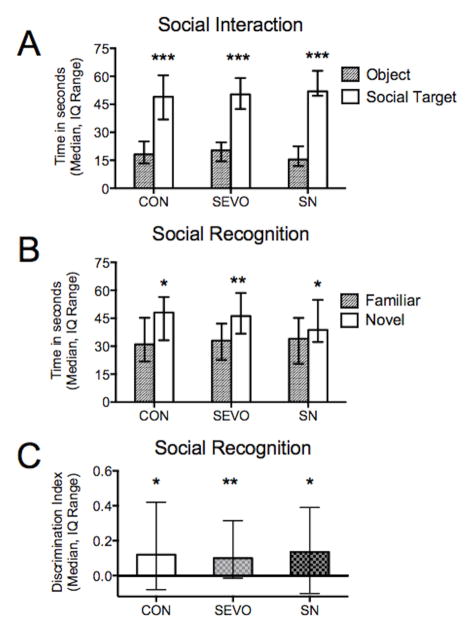
There was no evidence of impaired social behavior in either treatment group as evaluated by the relative time spent investigating a social target relative to an inanimate novel object. All subjects spent significantly more time investigating the social target (all P < 0.0001, social target vs object compared with Wilcoxon matched-pair rank test, Fig. 5A). There was also no effect of treatment on the ability to distinguish between familiar and novel animals. All groups demonstrated intact social memory and spent more time with the novel female (control P = 0.032; sevoflurane P = 0.0014; sevo-nitrous P = 0.015, ratio paired t test novel vs familiar for each group, Fig. 5B). The discrimination indexes for all three groups were significantly greater than zero (control P = 0.0350, sevoflurane P = 0.0012, sevo-nitrous P = 0.0155; one sample t test vs hypothetical value of 0, Fig. 5C), and there was no difference between indexes among groups (P = 0.9493, one-way ANOVA).
Fig. 5.
Social Interaction and Recognition. A) Subjects were simultaneously presented with an adult female social target and novel object in an open field. All subjects spent significantly more time investigating the social target. B) Subjects were then tested in social recognition using a two-trial discrimination model. The first exposure with a single female was followed by a test phase (shown here) in which the same (familiar) female and a novel female were presented after a delay. Each group spent more time investigating the novel female. C) The discrimination index (DI) indicates the proportion of time spent investigating the novel animal relative to the familiar animal. The three groups’ DIs were similar and all were significantly greater than zero. * P < 0.05, ** P < 0.01, *** P < 0.001, CON = control, SEVO = sevoflurane, SN = sevoflurane-nitrous oxide
Animals treated with the combined sevoflurane-nitrous oxide anesthetic were impaired in object recognition
Control and sevoflurane treatment animals demonstrated successful object-place-context recognition, while those treated with combined sevoflurane and nitrous oxide were impaired. Control and sevoflurane-treated subjects successfully identified the object presented in a familiar location and context and consequently spent more time with the “displaced” object in the novel configuration (control P = 0.0047, sevoflurane P = 0.0058; Wilcoxon matched-pairs rank test between familiar and displaced object, Fig. 6A). The subjects treated with combined sevoflurane and nitrous oxide spent the same amount of time with each of the two objects (sevo-nitrous P = 0.25, Wilcoxon matched-pairs rank test between familiar and displaced object, Fig. 6A), demonstrating an inability to associate an object with its specific location and context. In assessing the discrimination index (DI), only the sevo-nitrous group’s DI was not greater than zero (P = 0.3318; one sample t test vs hypothetical value of 0, Fig. 6B). The sevo-nitrous DI was also the lowest among the groups, although comparison did not reach statistical significance (P = 0.11, Kruskal-Wallis test).
Fig. 6.
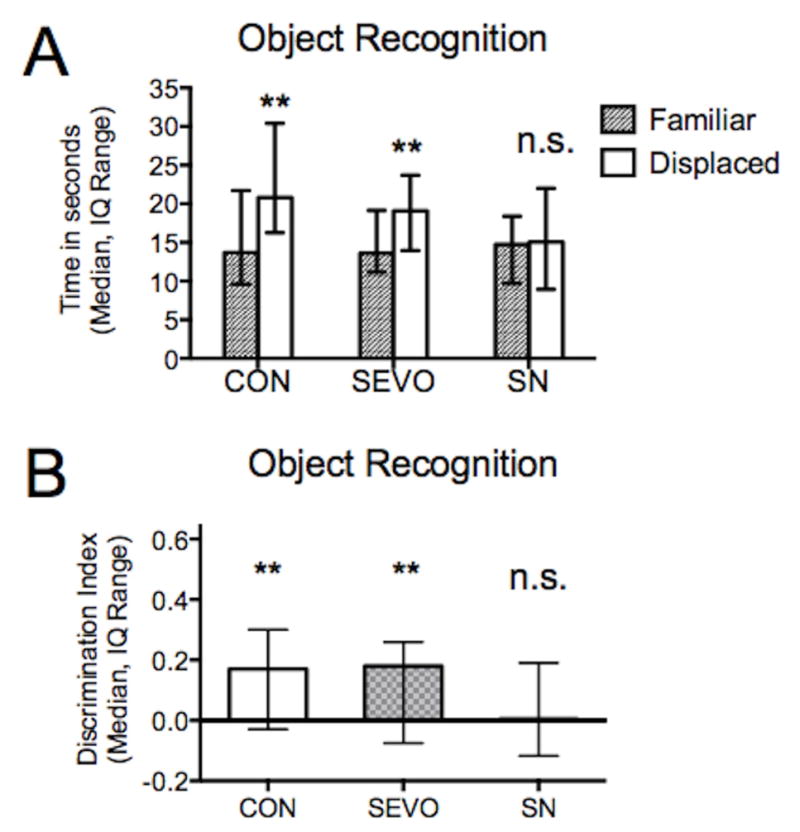
Object-Place-Context Task Results. In the task, subjects must be able to recognize an object, its location, and the context in which it appeared. The task relies on rodents’ propensity to explore objects that appear in new locations and contexts. Because the “displaced” object” is presented in an unfamiliar setup, the subject demonstrates a preference for that particular object. A) Both control and sevoflurane groups demonstrated successful object recognition and spent more time with the displaced object, while the sevo-nitrous group spent similar amounts of time with each object. B) In assessing discrimination index (DI), only the sevo-nitrous DI was not greater than zero and was also the lowest among the groups, although comparison did not reach statistical significance.
** P < 0.01, n.s. = not significant, CON = control, SEVO = sevoflurane, SN = sevoflurane-nitrous oxide
Discussion
Main Findings
The main finding of this study is that use of a combined anesthetic led to a distinct behavioral outcome compared with a single anesthetic. Exposure to the combined sevoflurane and nitrous oxide anesthetic on postnatal day 7 impaired long-term associative learning while sevoflurane treated animals were unaffected. Spatial working memory remained intact following early anesthetic exposure and there was no deficit in social behavior or recognition in anesthetized animals.
Single versus combined anesthetics
Distinct behavioral outcomes following anesthetic regimens of similar potency suggest that the two treatments influence cognitive development differently. One possible explanation is that, in the combined anesthetic, the composite action of agents functioning via different pathways may have a greater effect on brain development. Sevoflurane is believed to act predominantly as a GABAA agonist 13, 14 and nitrous oxide as an NMDA receptor antagonist15–17, and Frederikkson et al. demonstrated that co-administration of a GABA agonist (thiopental or propofol) with NMDA antagonist (ketamine) caused a significant increase in neuronal death compared with the agents delivered individually18. Their findings suggest a synergistic effect given that the cell death was more pronounced in the ketamine (25 mg) plus propofol (10 mg) group than in mice given a six-fold higher dose of propofol (60 mg).
Jevtovic-Todorovic et al. found that the addition of nitrous oxide to isoflurane and midazolam resulted in greater neurotoxicity than the double cocktail of midazolam and isoflurane3. Other studies of isoflurane neurotoxicity have shown greater cell death when isoflurane was combined with nitrous oxide19, 20, although the anesthetics used in these studies were not of equal potency and the depth of anesthesia likely increases with each additional agent. Our findings suggest that the effects of a combined anesthetic are not simply due to a greater anesthetic depth because the anesthetics were adjusted to the same MAC equivalent.
Anesthesia
We previously reported that MAC decreases over time in immature rodents 6–8, a finding confirmed by Kodama et al 26 and repeated in our present study. Combining sevoflurane with nitrous oxide decreased the dose of sevoflurane required to achieve one MAC as expected and sevoflurane decreased over time in both treatments. Sevoflurane concentration required to achieve 1 MAC in the combined sevoflurane and nitrous oxide anesthetic was on average 72% of the concentration for sevoflurane alone in this study and in our previous experience8. Based on these anesthetic curves, we postulate that the MAC value of 70% nitrous oxide in these animals is around 0.3 – 0.4 MAC.
Forced alternating T-maze & Object Recognition
The forced alternating T-maze can be solved using working memory, egocentric cues, and body turn rather than spatial cues30. We found no difference in performance of the T-maze alternation task in either anesthetized group, which is consistent with previous findings that working memory is often unaffected after neonatal anesthesia26, 31, 32. The behavior assessed in the object recognition task, however, is more complex and relies on different types of learning and memory. Recognition memory, for instance, is a type of episodic memory supported by the distinct processes of familiarity and recollection33. Familiarity is immediate and simply leads to recognition of an episode as old, while recollection involves recall of details associated with an event required for successful object recognition in a task like the object-place-context task34–36. Hippocampal and anterior thalamic circuits are important in recollection34, and cell death has consistently been documented in these regions3, 7, 37 which could potentially cause a deficit in recognition memory38, 39.
Studies by Eacott and Langston et al. also suggest an explanation of the behavioral deficit. Animals with hippocampal and fornix lesions were able to recognize a novel object but unable to complete the more difficult object-place-context task28, 40. In this task, the object, its location, and context are all familiar, although successfully performing the task relies on the ability to integrate these separate components. Studies have shown that novel object recognition is intact with thalamic lesions while tasks relying on associative memory are impaired41. The inability of the sevo-nitrous group to recognize an object and its context and location may be explained by problems with associative learning and recall of contextual details.
Social behavior
We did not identify any difference in social interaction or recognition among the groups that were tested, unlike Satomoto et al37 who found abnormal social behavior and recognition in mice after anesthesia. There are a number of differences between our studies that may account for the discrepancy in results, including subjects, treatment, and experimental model. In spite of the different testing paradigms, however, our results suggest that sevoflurane treated subjects are in fact able to develop social memory. Neither sevoflurane nor combined sevoflurane-nitrous oxide treated animals showed impairment in either their investigation of social targets or the subsequent ability to distinguish familiar and novel animals.
Conclusion
As evidence regarding anesthetic neurotoxicity continues to grow, it is important for investigations to address clinically relevant questions. For example, are certain anesthetic agents and treatments more toxic to the developing brain? Because anesthetics are often used in conjunction, it is meaningful to explore how unique combinations of agents influence development. Furthermore, since our understanding of anesthetic effects in humans is quite limited, we continue to rely on animal models to gain insight and potentially guide future studies of anesthetic effects in children.
Epidemiologic studies in children exploring learning and memory outcomes after anesthesia look at factors such as sex, age at exposure, number and duration of exposures1, 42–44. Effects of different anesthetic agents and regimens are areas requiring further study. Given a possible difference in outcomes after combining anesthetics, a relevant study may be to compare outcomes after use of single or combined anesthetics in humans - whether retrospectively or prospectively.
The use of anesthetics in children may not be avoidable, but we can modify clinical practice to administer anesthesia more safely. If certain combinations prove to be more detrimental to neurocognitive development, then they should be avoided. Also, if particular agents demonstrate minimal or no long-term effects, then they would be preferable for clinical use in children. These are all important implications to consider as anesthetic neurotoxicity research progresses.
Limitations
Our findings in rodents do not necessarily translate to humans with a different developmental time frame and longer life span. However it brings an important issue to our attention – whether combined agents affect the developing brain and eventual cognitive outcome differently than the use of a single agent.
Highlights.
Different volatile anesthetics act via unique mechanisms
In clinical practice, anesthetics are often used in combination
Combined anesthetics may affect long-term memory differently than single agents
Acknowledgments
Funding: Grant award GM086511 to JWS from National institutes of Health, Bethesda, MD. UCSF Department of Anesthesia and Perioperative Care Hamilton Award to JWS. Medical Student Research Program to AA from University of Arizona College of Medicine, Tucson.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flick RP, Katusic SK, Colligan RC, et al. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:e1053–61. doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilder RT, Flick RP, Sprung J, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nikizad H, Yon JH, Carter LB, et al. Early exposure to general anesthesia causes significant neuronal deletion in the developing rat brain. Ann N Y Acad Sci. 2007;1122:69–82. doi: 10.1196/annals.1403.005. [DOI] [PubMed] [Google Scholar]
- 5.Istaphanous GK, Howard J, Nan X, et al. Comparison of the neuroapoptotic properties of equipotent anesthetic concentrations of desflurane, isoflurane, or sevoflurane in neonatal mice. Anesthesiology. 2011;114:578–87. doi: 10.1097/ALN.0b013e3182084a70. [DOI] [PubMed] [Google Scholar]
- 6.Stratmann G, May LD, Sall JW, et al. Effect of hypercarbia and isoflurane on brain cell death and neurocognitive dysfunction in 7-day-old rats. Anesthesiology. 2009;110:849–61. doi: 10.1097/ALN.0b013e31819c7140. [DOI] [PubMed] [Google Scholar]
- 7.Shih J, May LD, Gonzalez HE, et al. Delayed environmental enrichment reverses sevoflurane-induced memory impairment in rats. Anesthesiology. 2012;116:586–602. doi: 10.1097/ALN.0b013e318247564d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramage TM, Chang FL, Shih J, et al. Distinct long-term neurocognitive outcomes after equipotent sevoflurane or isoflurane anaesthesia in immature rats. Br J Anaesth. 2013 doi: 10.1093/bja/aet103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boscolo A, Starr JA, Sanchez V, et al. The abolishment of anesthesia-induced cognitive impairment by timely protection of mitochondria in the developing rat brain: the importance of free oxygen radicals and mitochondrial integrity. Neurobiol Dis. 2012;45:1031–41. doi: 10.1016/j.nbd.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jevtovic-Todorovic V, Boscolo A, Sanchez V, et al. Anesthesia-induced developmental neurodegeneration: the role of neuronal organelles. Front Neurol. 2012;3:141. doi: 10.3389/fneur.2012.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizzi S, Ori C, Jevtovic-Todorovic V. Timing versus duration: determinants of anesthesia-induced developmental apoptosis in the young mammalian brain. Ann N Y Acad Sci. 2010;1199:43–51. doi: 10.1111/j.1749-6632.2009.05173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentry KR, Steele LM, Sedensky MM, et al. Early developmental exposure to volatile anesthetics causes behavioral defects in Caenorhabditis elegans. Anesth Analg. 2013;116:185–9. doi: 10.1213/ANE.0b013e31826d37c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–14. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- 14.Ishizeki J, Nishikawa K, Kubo K, et al. Amnestic concentrations of sevoflurane inhibit synaptic plasticity of hippocampal CA1 neurons through gamma-aminobutyric acid-mediated mechanisms. Anesthesiology. 2008;108:447–56. doi: 10.1097/ALN.0b013e318164cfba. [DOI] [PubMed] [Google Scholar]
- 15.Sanders RD, Weimann J, Maze M. Biologic effects of nitrous oxide: a mechanistic and toxicologic review. Anesthesiology. 2008;109:707–22. doi: 10.1097/ALN.0b013e3181870a17. [DOI] [PubMed] [Google Scholar]
- 16.Jevtovic-Todorovic V, Todorovic SM, Mennerick S, et al. Nitrous oxide (laughing gas) is an NMDA antagonist, neuroprotectant and neurotoxin. Nat Med. 1998;4:460–3. doi: 10.1038/nm0498-460. [DOI] [PubMed] [Google Scholar]
- 17.Nagele P, Metz LB, Crowder CM. Nitrous oxide (N(2)O) requires the N-methyl-D-aspartate receptor for its action in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2004;101:8791–6. doi: 10.1073/pnas.0402825101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fredriksson A, Ponten E, Gordh T, et al. Neonatal exposure to a combination of N-methyl-D-aspartate and gamma-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology. 2007;107:427–36. doi: 10.1097/01.anes.0000278892.62305.9c. [DOI] [PubMed] [Google Scholar]
- 19.Ma D, Williamson P, Januszewski A, et al. Xenon mitigates isoflurane-induced neuronal apoptosis in the developing rodent brain. Anesthesiology. 2007;106:746–53. doi: 10.1097/01.anes.0000264762.48920.80. [DOI] [PubMed] [Google Scholar]
- 20.Zhen Y, Dong Y, Wu X, et al. Nitrous oxide plus isoflurane induces apoptosis and increases beta-amyloid protein levels. Anesthesiology. 2009;111:741–52. doi: 10.1097/ALN.0b013e3181b27fd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Creeley CE, Dikranian KT, Dissen GA, et al. Isoflurane-induced apoptosis of neurons and oligodendrocytes in the fetal rhesus macaque brain. Anesthesiology. 2014;120:626–38. doi: 10.1097/ALN.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng M, Hofacer RD, Jiang C, et al. Brain regional vulnerability to anaesthesia-induced neuroapoptosis shifts with age at exposure and extends into adulthood for some regions. Br J Anaesth. 2014 doi: 10.1093/bja/aet469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macbeth AH, Edds JS, Young WS., 3rd Housing conditions and stimulus females: a robust social discrimination task for studying male rodent social recognition. Nat Protoc. 2009;4:1574–81. doi: 10.1038/nprot.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eger EI, 2nd, Saidman LJ, Brandstater B. Minimum alveolar anesthetic concentration: a standard of anesthetic potency. Anesthesiology. 1965;26:756–63. doi: 10.1097/00000542-196511000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Merkel G, Eger EI., 2nd A comparative study of halothane and halopropane anesthesia including method for determining equipotency. Anesthesiology. 1963;24:346–57. doi: 10.1097/00000542-196305000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Kodama M, Satoh Y, Otsubo Y, et al. Neonatal desflurane exposure induces more robust neuroapoptosis than do isoflurane and sevoflurane and impairs working memory. Anesthesiology. 2011;115:979–91. doi: 10.1097/ALN.0b013e318234228b. [DOI] [PubMed] [Google Scholar]
- 27.Stratmann G, Sall JW, May LD, et al. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–48. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 28.Langston RF, Wood ER. Associative recognition and the hippocampus: differential effects of hippocampal lesions on object-place, object-context and object-place-context memory. Hippocampus. 2010;20:1139–53. doi: 10.1002/hipo.20714. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-Santed F, de Bruin JP, Heinsbroek RP, et al. Spatial delayed alternation of rats in a T-maze: effects of neurotoxic lesions of the medial prefrontal cortex and of T-maze rotations. Behav Brain Res. 1997;84:73–9. doi: 10.1016/s0166-4328(97)83327-x. [DOI] [PubMed] [Google Scholar]
- 30.Aggleton JP, Hunt PR, Nagle S, et al. The effects of selective lesions within the anterior thalamic nuclei on spatial memory in the rat. Behav Brain Res. 1996;81:189–98. doi: 10.1016/s0166-4328(96)89080-2. [DOI] [PubMed] [Google Scholar]
- 31.Loepke AW, Istaphanous GK, McAuliffe JJ, 3rd, et al. The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg. 2009;108:90–104. doi: 10.1213/ane.0b013e31818cdb29. [DOI] [PubMed] [Google Scholar]
- 32.Callaway JK, Jones NC, Royse AG, et al. Sevoflurane anesthesia does not impair acquisition learning or memory in the morris water maze in young adult and aged rats. Anesthesiology. 2012;117:1091–101. doi: 10.1097/ALN.0b013e31826cb228. [DOI] [PubMed] [Google Scholar]
- 33.Yonelinas AP. Components of episodic memory: the contribution of recollection and familiarity. Philos Trans R Soc Lond B Biol Sci. 2001;356:1363–74. doi: 10.1098/rstb.2001.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowles B, Crupi C, Mirsattari SM, et al. Impaired familiarity with preserved recollection after anterior temporal-lobe resection that spares the hippocampus. Proc Natl Acad Sci U S A. 2007;104:16382–7. doi: 10.1073/pnas.0705273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yonelinas AP, Otten LJ, Shaw KN, et al. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci. 2005;25:3002–8. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manns JR, Hopkins RO, Reed JM, et al. Recognition memory and the human hippocampus. Neuron. 2003;37:171–80. doi: 10.1016/s0896-6273(02)01147-9. [DOI] [PubMed] [Google Scholar]
- 37.Satomoto M, Satoh Y, Terui K, et al. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–37. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 38.Aggleton JP, Dumont JR, Warburton EC. Unraveling the contributions of the diencephalon to recognition memory: a review. Learn Mem. 2011;18:384–400. doi: 10.1101/lm.1884611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aggleton JP, O’Mara SM, Vann SD, et al. Hippocampal-anterior thalamic pathways for memory: uncovering a network of direct and indirect actions. Eur J Neurosci. 2010;31:2292–307. doi: 10.1111/j.1460-9568.2010.07251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eacott MJ, Norman G. Integrated memory for object, place, and context in rats: a possible model of episodic-like memory? J Neurosci. 2004;24:1948–53. doi: 10.1523/JNEUROSCI.2975-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cross L, Brown MW, Aggleton JP, et al. The medial dorsal thalamic nucleus and the medial prefrontal cortex of the rat function together to support associative recognition and recency but not item recognition. Learn Mem. 2012;20:41–50. doi: 10.1101/lm.028266.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ing C, DiMaggio C, Whitehouse A, et al. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 2012;130:e476–85. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- 43.DiMaggio C, Sun LS, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. 2011;113:1143–51. doi: 10.1213/ANE.0b013e3182147f42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sprung J, Flick RP, Katusic SK, et al. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012;87:120–9. doi: 10.1016/j.mayocp.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]