Abstract
Neutrophils are the most abundant cell type in the immune system and play an important role in the innate immune response. Using a diverse range of mouse models with either defective DC development or conditional DC depletion, we provide in vivo evidence indicating that conventional dendritic cells (cDC) play an important role in the regulation of neutrophil homeostasis. Flk2, Flt3L and Batf3 knockout mice, which have defects in DC development, have increased numbers of liver neutrophils in the steady state. Conversely, neutrophil frequency is reduced in DC-specific PTEN knockout mice, which have an expansion of CD8+ and CD103+ DCs. In chimeric CD11c-DTR mice, cDC depletion results in a systemic increase of neutrophils in peripheral organs in the absence of histological inflammation or an increase in pro-inflammatory cytokines. This effect is also present in splenectomized chimeric CD11c-DTR mice and is absent in chimeric mice with 50% normal bone marrow. In chimeric CD11c-DTR mice, DT treatment results in enhanced neutrophil trafficking from the bone marrow into circulation and increased neutrophil recruitment. Moreover, there is an increased expression of chemokines/cytokines involved in neutrophil homeostasis and reduced neutrophil apoptosis. These data underscore the role of the DC pool in regulating the neutrophil compartment in non-lymphoid organs.
Introduction
Neutrophils are the most abundant circulating leucocytes and are essential for innate immunity. They are recruited rapidly to sites of inflammation, where their primary role is to kill invading pathogens through phagocytosis by releasing preformed lytic enzymes, and by producing reactive oxygen species (1). Neutrophils exhibit rapid homeostatic turnover; it has been estimated that ~109 cells per kilogram of body weight leave the bone marrow per day (2). Neutrophil homeostasis is maintained through a delicate balance between granulopoiesis, bone marrow storage and release, intravascular margination, and migration into peripheral tissues. Neutrophils may also undergo apoptosis and clearance in peripheral tissues (3)(4). Tissue-resident macrophages, including Kupffer cells (5)(6), bone marrow stromal macrophages (7), and splenic marginal zone macrophages (8) are considered to play important roles in this process.
Conventional or classical dendritic cells (cDC) are professional antigen presenting cells of the immune system. FMS-related tyrosine kinase 3 (FLT3) and its ligand, FLT3L play a key role in the commitment and differentiation of the DC lineage (9). Tissue-resident DCs have been further characterized into Batf3-IRF8-Id2 dependent DC lineage (which includes lymphoid tissue CD8+DCs and non-lymphoid tissue CD103+CD11b-DCs) and the more heterogeneous Batf3-IRF8-Id2-independent DC lineage (10).
Besides macrophages, bone marrow-derived DCs have also been shown to potentially be involved in neutrophil homeostasis; however, this is mainly based on in vitro experiments (11). Herein, we provide in vivo evidence showing that cDCs play an important role in controlling peripheral neutrophil homeostasis at multiple levels by affecting bone marrow mobilization, recruitment and apoptosis of neutrophils.
Materials and Methods
Animals
Six week-old female Flt3LKO mice were purchased from Taconic. C57BL/6, CD11c-DTR, CD11c-Cre, Foxp3-DTR and Pten-flox mice were purchased from the Jackson Laboratory. Flk2KO mice were kindly provided by Ihor Lemischka from Ichan School of Medicine at Mount Sinai. Batf3KO mice were gifts from Kenneth Murphy from Washington University. DC-specific Pten knockout mice (DCΔPTEN) were generated by breeding CD11c-Cre with Pten-Flox mice. Cre-negative littermates (Pten-Flox) were used as control. All procedures were in accordance with Institutional Animal Care and Use Committee Protocols.
Bone Marrow Transplantation (BMT)
Recipient CD45.1 mice underwent lethal total body irradiation delivered in 2 doses of 660 rad each. 2×106 bone marrow (BM) cells from CD11c-DTR mice were injected retro-orbitally. All the mice were used 12 weeks after bone marrow transplantation.
In vivo cellular depletion
For cDC depletion, 25 ng/g of diphtheria toxin (DT; List Biological Laboratories Inc.) was administered intraperitoneally (IP) into the chimeric CD11c-DTR mice. Mice were sacrificed 12 hours later and bone marrow, blood, liver, spleen and lung were harvested for further analysis. For pDC depletion, mice were injected IP with 500 μg 120G8 every other day for 14 days. 120G8 and control antibody were purchased from Imgenex. Mice were sacrificed 24 hours after the last injection and livers were harvested. For Foxp3+CD4+T cell depletion, DT was injected IP at a dose of 50ng/g for 2 consecutive days into Foxp3-DTR mice. Mice were sacrificed 48h after the last dose.
Cell preparation
Bone marrow cells were flushed thoroughly from mouse femurs with 10 ml of PBS. Peripheral blood leukocytes were obtained through retro-orbital bleeding by heparinized capillary tubes. Single cell suspensions from spleens were prepared by mashing through 70 μM Cell Strainers (BD) without enzymatic digestion. Lungs were perfused via the right ventricle of the heart with PBS. The tissues were minced, digested in 0.4mg/ml collagenase IV (Sigma) for 45 minutes and filtered through 70 μM Cell Strainer (BD) to get the cells suspension. Red blood cells from the bone marrow, peripheral blood, spleen and lungs were removed by RBC lysis buffer (eBioscience). For liver leukocyte isolation, livers were perfused in situ via the portal vein with PBS. Perfused livers were dissected and digested with 0.1mg/ml collagenase IV (Sigma), except in cases where annexin V staining was to be performed. The resultant cell suspensions were layered onto a 2-step (40%/70%) discontinuous Percoll gradient (GE Healthcare) and centrifuged at 900g for 20 minutes at 25°C. Hepatic leukocyte populations collected at the interface were washed twice in wash medium and used for analysis.
Flow cytometry
Fluorochrome-conjugated or biotinylated mAb specific to mouse antigen were listed as below. PE-Ly6G (clone1A8) and APC-Cy7-CD45 (clone 30-F11) were purchased from BD Pharmingen. PerCP-Cy5.5-CD11b (clone M1/70), FITC-CD11b (clone M1/70), eFluor 450-CD19 (clone eBio1D3), eFluor 450-CD3 (clone 17A2), eFluor 450-NK1.1 (clone PK136), APC-MHCII (I-A/I-E clone M5/114.15.2), PE-Cy7-CD11c (clone N418), biotin-PDCA-1 (clone eBio927), FITC-PDCA-1 (clone eBio927), PE-CD103 (clone 2E7), PerCP-Cy5.5-CD3 (clone 145-2C11), and PE-Cy7-NK1.1 (clone PK136), PE-IFN-γ (clone XMG1.2), APC-IL-17 (clone eBio17B7), APC-CD34 (clone 4H11 (APG), PE-CD16/32(clone 93), FITC-Sca-1 (clone D7), PE-Cy7-C-kit (clone 2B8) were from eBioscience. Biotin-F480 (clone CI: A3-1) was purchased from Serotec. Secondary reagents PE-Cy7-streptavidin were purchased from BD Pharmingen. PE-streptavidin and APC-streptavidin were from eBioscience. Intracellular PE-Foxp3 (clone FJK-16s) staining was performed after fixation and permeabilization following the manufacturer’s protocol (eBioscience). For intracellular IL-17 staining, cells were stimulated for 4h with phorbol 12-myristate 13-acetate (PMA) (50 ng/ml), and ionomycin (1 μg/ml, both from Sigma-Aldrich) in the presence of monensin (2μM) (eBioscience) at 37°C. For detection of IL-17, cells were fixed and permeabilized according to manufacturer’s protocol (eBioscience). Multiparameter analyses of stained cell suspension were performed on an LSR II (BD) and analyzed with FlowJo software (TreeStar). Single DAPI-cells were evaluated for all analyses but intracellular staining and apoptosis. Plasmacytoid DCs (pDC) were gated as CD45+CD3e-CD19-NK1.1-PDCA-1+CD11cint. Conventional DCs (cDC) were gated as CD45+CD3e-CD19-NK1.1-PDCA-1-CD11chighMHCIIhigh. Neutrophils were defined as CD45+Ly6G+CD11b+. Th17 cells were gated as CD45+CD3+CD4+IL-17+. Apoptosis was evaluated by incubating the cells with APC-annexin V in binding buffer (eBioscience), as per manufacturer’s instructions.
Cell sorting and Hema3 staining
Neutrophils from the liver were sorted based on the expression of Ly6G and CD11b on FACSAria II (BD). The Hema 3 system (Fisher Scientific) was used to stain cytospin prepared slides through cytocentrifugation.
Histochemical neutrophil staining and quantification
Neutrophil specific esterase staining was performed on paraffin-embedded liver, spleen, bone marrow and lung sections using Naphthol AS-D chloroacetate esterase kit following the product instructions (Sigma-Aldrich). At least 20 pictures per section were acquired using an AxioImager M2 microscope (Zeiss). Neutrophils were manually counted in each 400× field.
Immunofluorescence staining
Goat-anti-mouse S100A9 (R&D, 100ng/ml) and rabbit- anti-mouse colIV (Abcam, 13.3μg/ml) immunofluorescence staining was performed on frozen tissue, followed with fluorescently conjugated secondary antibodies (chicken anti-goat AlexaFluor488 and donkey anti-rabbit AlexaFluor647, Invitrogen, 10μg/ml). Nuclei were visualized using DAPI. Images were acquired using a Leica SP5 DM confocal microscope. Neutrophils were manually counted in each 400× field.
Multiplex cytokine/chemokine assay
Mouse liver was homogenized in 0.15 M potassium chloride solution containing protease inhibitor cocktail (Roche). Cytokines/Chemokines expression in the liver was assessed by a magnetic bead-based array MILLIPLEX™ MAP (EMD Millipore) following the manufacture’s instruction. Multiplex plates were read using a Luminex 100 multiplex plate reader (Luminex, Austin, TX).
ELISA
Blood was collected by retro-orbital bleeding and plasma was isolated using BD Microtainer Plastic Capillary Blood Collectors. Plasma G-CSF level was detected with Mouse G-CSF Quantikine ELISA Kit (R&D) following the manufacture’s instruction.
Splenectomy
Mice were anesthetized with isoflurane (Isoflu; Dainippon Sumitomo Pharma). The spleen was exposed and removed after appropriate blood vessel ligation by clipping. Sham-operated animals underwent the laparotomy without a splenectomy.
Mix bone marrow chimera
Recipient CD45.1 mice underwent lethal total body irradiation delivered in 2 doses of 660 rad each. Bone marrow (BM) cells from CD11c-DTR mice (CD45.2) and WT mice (CD45.1) were mixed together (106 total, ratio 3:2) and injected via the retro-orbital vein. All the mice were used 12 weeks after bone marrow transplantation.
BrdU labeling
Mice were injected intraperitoneally with 1.5 mg bromodeoxyuridine (BrdU) (BDPharmingen). At various time points after injection, blood samples were obtained via retro orbital bleeding by heparinized capillary tubes and analyzed by flow cytometry using APC BrdU Flow Kit (BDPharmingen) following the manufacturer’s protocol.
Statistical analysis
Error bars represent mean+/−SD and were analyzed by Student’s t test, with Prism 4 software (GraphPad Software, Inc.). Values of P < 0.05 were considered significant. Statistical significance was expressed as follows: *, P < 0.05; **, P < 0.01; ***, P<0.001.
Results
Flt3LKO and Flk2KO mice have increased hepatic neutrophils
DC development critically depends on the cytokine FMS-like tyrosine kinase ligand (Flt3L), which signals through its receptor Flt3/Flk2 (9). Both Flk2KO and Flt3LKO mice have reduced pDC and cDC compared with wild type controls (9) (12). In the first set of experiments, we explored whether the neutrophil homeostasis in these strains of mice is altered in the steady-state. We noted an increase in hepatic neutrophils in Flk2KO mice both as a percentage of total CD45+ cells (neutrophil frequency) and by absolute number (Figure 1A–C) while in Flt3LKO mice only an increase in hepatic neutrophil frequency was observed (Figure 1A, D–E). Flt3L-dependent defects were associated with increased blood neutrophil frequencies in both mice strains (Figure 1A F–G).
Figure 1. Mice with developmental DC deficiency have increased neutrophil in the liver and blood.
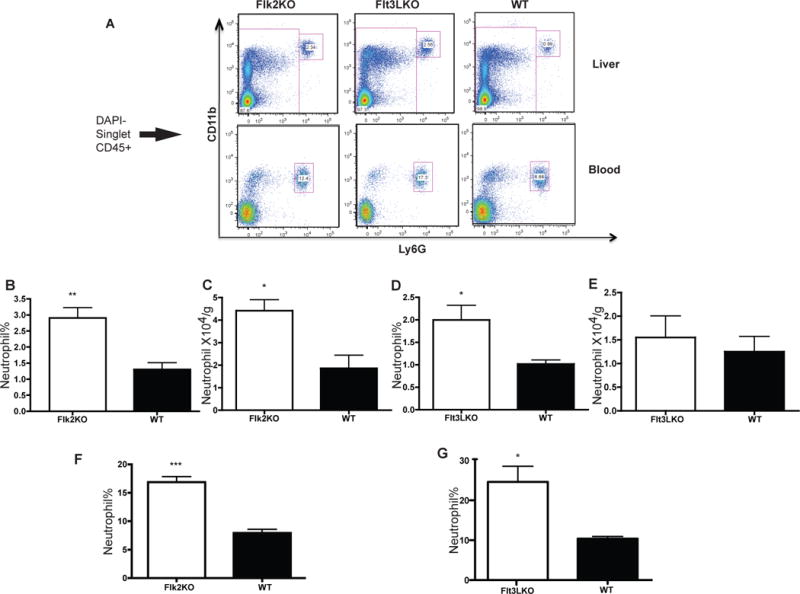
Representative FACS plots (A) of liver (top panel) and blood (bottom panel) neutrophils in WT, Flk2KO and Flt3LKO mice are displayed. Liver neutrophils are gated as CD45+CD11b+Ly6G+ (B–E) Increased neutrophils in the liver of Flk2KO and Flt3LKO as graphed by percentage of CD45+ cells (B, D) and absolute numbers (C, E). (F–G) Increased blood neutrophils frequencies of Flk2KO and Flt3LKO. Results are expressed as mean value +/− standard deviation (n=4–6 mice). The experiments were repeated three times with similar results.
Mice with abnormal CD103+DC development exhibit differences in peripheral neutrophil numbers
Consistent with these results, Batf3KO mice, well known to have developmental deficiencies of CD8+DC and CD103+DC (13) in lymphoid and non-lymphoid organs respectively, also have more neutrophils in their livers than WT controls (Figure 2A–C). It has been reported that PI3K-mTOR signaling downstream of Flt3L controls DC development, and DC-specific deletion of the PI3K-mTOR negative regulator PTEN causes a substantial expansion of DCs, especially CD8+ and CD103+ DCs (14) (data not shown). These mice with specific CD103 DC expansion had a decreased neutrophil frequency (Figure 2D–E). As expected due to the tumor suppressor function of PTEN, significant myeloproliferation is observed in these mice. No difference in the absolute neutrophil number was noted (data not shown). Similar to our model, in a myeloid-specific PTEN deletion model generated using Lysozyme-Cre, neutrophil numbers in peripheral blood was also unchanged from control mice (15).
Figure 2. Mice with altered CD103+DC development have changes in hepatic neutrophil.
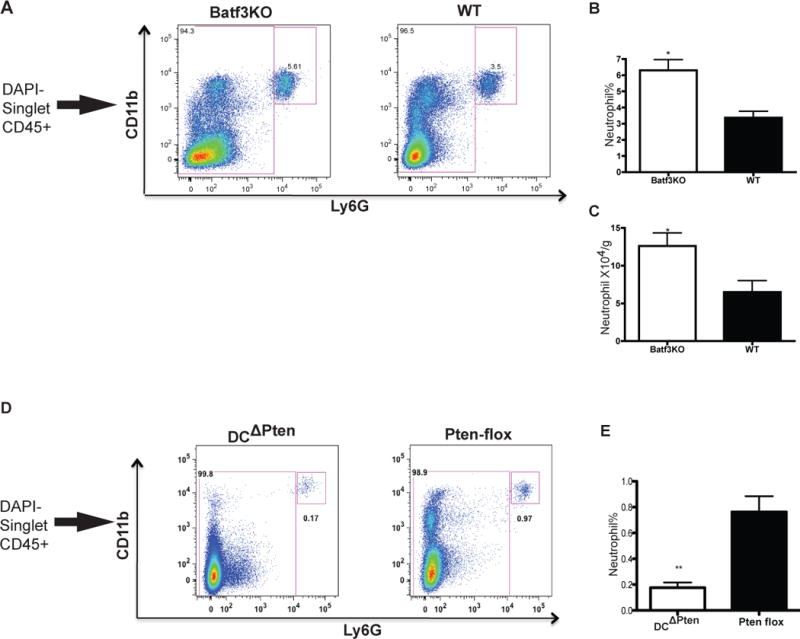
Batf3KO mice have increased neutrophil number in the liver as indicated by FACS (A) and graphed as percentage (B) and absolute number (C). DCΔPTEN mice have decreased neutrophil in the liver as indicated by FACS (D) and graphed as percentage (E) Results are expressed as mean value +/− standard deviation (n=3–5 mice). The experiments were repeated twice with similar results.
In addition to homeostatic conditions, in a chronic liver inflammation model induced using carbon tetrachloride (CCl4) administration, there was still an increase in liver neutrophils in both Flk2KO mice and Batf3KO mice compared to controls. However, the magnitude of neutrophilia was smaller than under steady state conditions (data not shown).
cDC depletion in CD11c-DTR chimeric mice results in neutrophilia and increased neutrophils in peripheral organs
Deficiency in the Flk2-Flt3L system affects both the pDC and cDC compartment (10). In addition, Flt3LKO mice also had impaired NK and B cell development (12). Depleting pDC with a depleting antibody 120G8 (Supplemental Figure 1A) did not result in increased neutrophils (Supplemental Figure 1B–D), and we therefore hypothesized that the increased neutrophil in Flk2KO mice is due to cDC deficiency. In order to test this, we used chimeric CD11c-DTR mice to deplete cDC. Injecting these mice with 25ng/g body weight of DT induces cDC depletion in the liver, spleen and lung without affecting pDCs (data not shown). We observed a systemic increase in neutrophils associated with cDC depletion as revealed by FACS analysis (Figure 3A–C) and neutrophil esterase specific staining (Figure 3D–E).
Figure 3.
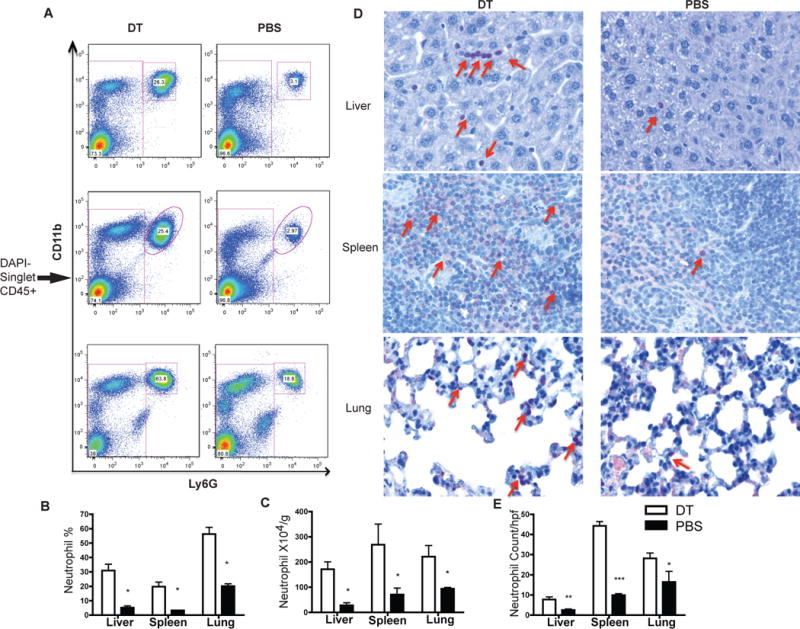
cDC ablation by DT injection into CD11c-DTR chimera leads to systemic neutrophil increase in liver, spleen and lung as shown by flow cytometry (A–C) and confirmed by Naphthol AS-D chloroacetate esterase staining (D) (400×) and counted per high power field (E)
Increased neutrophils in DT treated chimeric CD11c-DTR mice is not caused by nonspecific inflammation induced by DT
We next confirmed that the increase in neutrophil is not due to non-specific inflammation induced by dendritic cell necrosis/apoptosis. Firstly, we did not identify any differences in the expression of several inflammatory cytokines (IL-1α, IL-1β, TNF-α, IL-12 and IFN-γ) in the liver between the cDC-depleted group and control group (Figure 4A). In the plasma, these cytokines were either below the detection limit (IL-1β, TNF- TNF-α, IL-12 and IFN-γ) or did not differ (IL-1α) between the two (data not shown). We also performed intracellular cytokine staining for IFN-γ and there was no difference in the percentage of IFN-γ+ cells (data not shown). Secondly, the same dose (25ng/g) of DT injection into WT mice did not lead to a neutrophil increase, suggesting that increased neutrophil infiltration is not caused by contamination with LPS of DT or DT itself (Figure 4B–D). Thirdly, in another DTR mouse model, Foxp3-DTR mice, efficient CD4+Foxp3+ T cell depletion (50ng/g body weight for 2 consecutive days) (16) (Supplemental Figure 1E) did not result in increased neutrophils (Supplemental Figure 1F–H), suggesting that increased neutrophils in CD11c-DTR mice is due to specific cDC depletion rather than general cell death induced by the DTR system. Lastly, in order to prove a causal relationship between cDC depletion and neutrophil increase, we analyzed neutrophil numbers in mixed bone marrow chimeras (WT CD45.1+CD11cDTR CD45.2). DT injection in these chimeras leads to depletion of cDC only from CD45.2+ compartment originating from CD11c-DTR strain without affecting overall cDC number (Supplemental Figure 2A–B). However, in the presence of DC from the WT CD45.1 compartment, we did not observed increased neutrophil after DT depletion and depletion of all cells from CD45.2+DTR+ donor DC (Supplemental Figure 2C). Together, these data suggest that the increase in neutrophils is due to specific cDC depletion and not due to the DTR system, DT treatment or non-specific inflammation.
Figure 4. Increased neutrophil in chimeric CD11c-DTR mice is not caused by non-specific inflammation related with cell apoptosis.
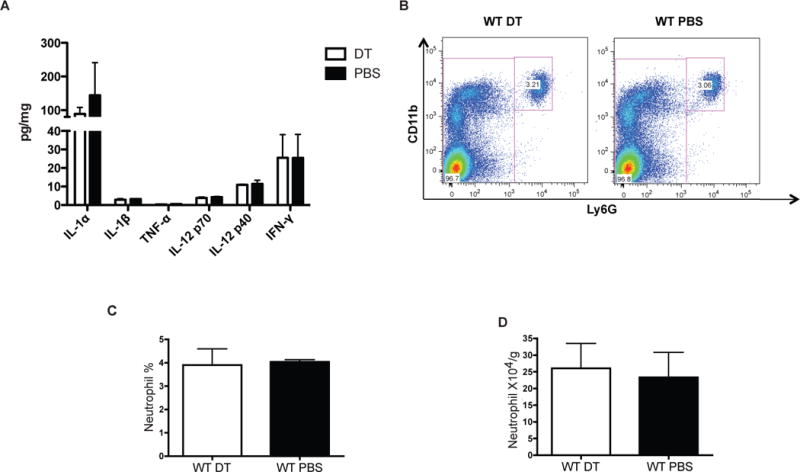
(A) No difference in pro-inflammatory cytokine expression in liver of cDC depleted mice versus control. (B–D) DT injection into wild type mice does not cause neutrophil increase as displayed by FACS plot (B) and graphed by frequency (C) and absolute number (D). Results are expressed as mean value +/− standard deviation (n=4 mice/group). The experiments were repeated three times with similar results.
Since CD11c-DTR chimeric mice enabled us to specifically deplete cDC, we utilized this model to further characterize the mechanisms underlying cDC depletion-induced neutrophilia.
Acute cDC depletion is associated with an increased mobilization of neutrophils from bone marrow
In order to elucidate the mechanism of increased neutrophils associated with DC depletion, we quantified first the neutrophil numbers in the bone marrow during cDC depletion. DT treatment led to a ~50% decrease in bone marrow neutrophils (Figure 5A–C) without any impact on the total CD45+ femoral cellularity (Figure 5D). Consistent with this finding, the frequency of cells expressing neutrophil-specific leukocyte esterase is decreased after DT administration (Figure 5E). As bone marrow neutrophil numbers decrease, a concomitant increase in neutrophil numbers is observed in the peripheral blood (Figure 5F). Neutrophil trafficking from the bone marrow is estimated by neutrophil distribution index (NDI), which is the percentage of neutrophils in the blood versus the total number of neutrophils in the bone marrow and blood (17). As indicated in Figure 5G, there is a ~3 fold increase in NDI after cDC depletion. In order to confirm the increased neutrophil mobilization, we injected CD11c-DTR chimeras with BrdU and administered one dose of DT to deplete cDC 12 hours later. The percentage of BrdU+ neutrophils in the blood was assessed from 24–144 hours after BrdU injection. BrdU+ neutrophils were observed in the periphery 24 hours earlier in cDC depleted mice than in controls, confirming the increased rate of release of neutrophils from the bone marrow into the periphery (Supplemental Figure 2D). Collectively, these data suggest that loss of cDC results in the mobilization of neutrophils from bone marrow into the periphery.
Figure 5. cDC depletion results in neutrophil release from bone marrow into periphery.
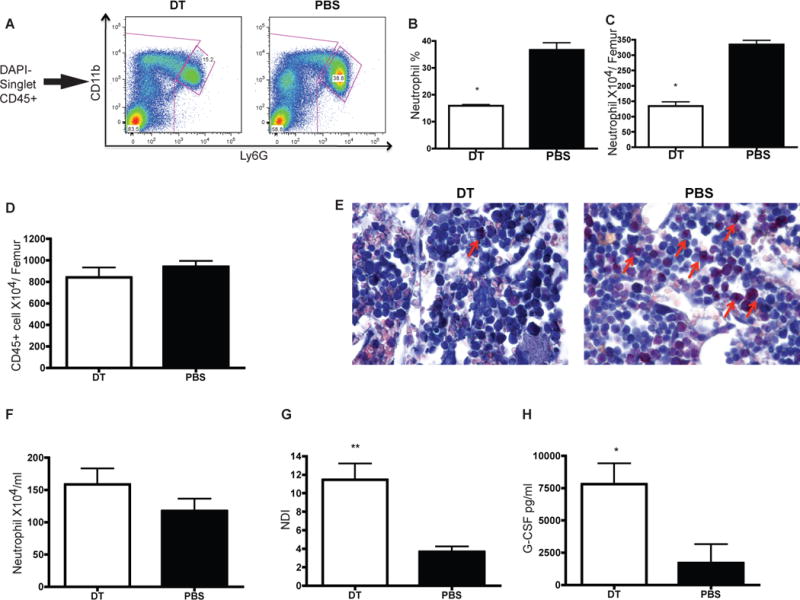
(A–D) DT administration leads to reduced neutrophil number in the bone marrow without affecting total hematopoietic cell number (D). Neutrophil reduction is shown by FACS (A) and graphed as percentage of total CD45+ cell (B) and absolute number (C). (E) Less neutrophil in bone marrow after cDC depletion is confirmed by Naphthol AS-D chloroacetate esterase staining (630×Oil). (F–G) Enhanced neutrophil mobilization into periphery as indicated by increased neutrophil number in blood (F) and increased NDI (G). (H) Increase plasma G-CSF is detected in cDC depleted mice by ELISA.
We did not observe the release of immature neutrophils based on Hema 3 staining of cytospin preparations. The majority of the sorted hepatic neutrophils from mice undergoing cDC depletion were mature segmented neutrophils (Supplemental Figure 3A). We did not detect histological features of extramedullary hematopoiesis in peripheral organs (liver and lung). In the bone marrow, cDC depletion led to an increase in common myeloid progenitor (CMP) and a decrease in granulocyte myeloid progenitor (GMP) (Supplemental Figure 3B–C). Furthermore, in Supplemental Figure 2D, cDC depletion resulted in an earlier appearance in BrdU+ neutrophils in the peripheral blood, while the area under curve did not differ between the two groups. Altogether, our data suggest that cDC depletion did not have a significant effect on neutrophil production.
We next sought to identify mediators responsible for increased neutrophil mobilization. G-CSF promotes neutrophil release from the BM into the circulation (17). Indeed, there was increased plasma G-CSF in cDC depleted mice, implicating this cytokine as a potential candidate to explain the increased mobilization (Figure 5H). Since IL-17 induces the expression of G-CSF (18), we compared the frequency of Th17 cells in the liver, spleen and lung in cDC depleted mice with control. However, after cDC depletion, there was no alteration in Th17 cell frequency in these organs, indicating that IL-17 is not likely to be responsible for up-regulation of G-CSF in our model (data not shown).
cDC depletion increases the expression of neutrophil-recruiting cytokines in the liver
To better understand the cause of increased neutrophils in the peripheral organs, we examined production of chemokines in the liver by a customized cytokine beads array focused on cytokines involved in neutrophils recruitment. KC, MCP-1, G-CSF and IP-10 were all shown to be increased in cDC depleted mice (Figure 6A). All these chemokines have been reported as involved in neutrophil mobilization and recruitment (19)(20)(21)(22)(23)(24). A similar increase was also noted in the plasma of cDC depleted mice. (Figure 6B). This indicates that increased neutrophil recruitment also contributes to cDC depletion-induced neutrophilia, in addition to increased mobilization. Supporting this increase recruitment, a calculated neutrophil recruitment index was higher in cDC depleted mice (Supplemental Figure 2E).
Figure 6. Increase neutrophil recruitment cytokines and reduced neutrophil apoptosis are present in cDC depleted mice liver.
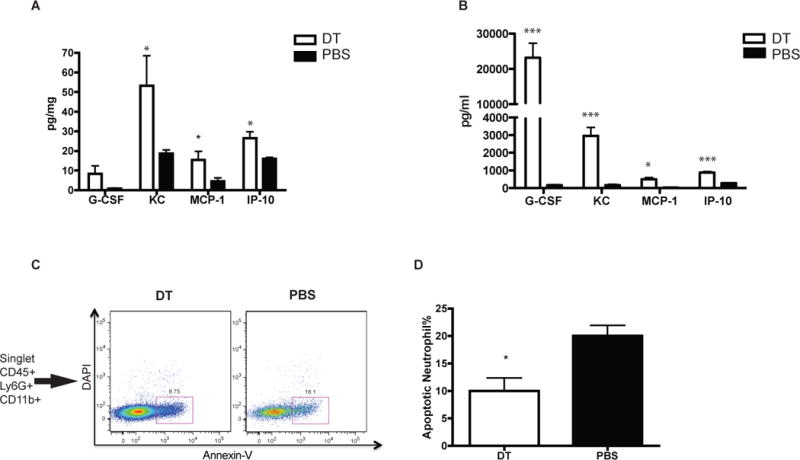
G-CSF, KC, MCP-1 and IP-10 are elevated in the liver (A) and plasma (B) of CD11c-DTR chimera receiving DT than control. (C–D) Apoptotic neutrophil (CD45+Ly6G+CD11b+DAPI-AnnexinV+) is reduced after cDC ablation in CD11c-chimera as indicated by FACS plots (C) and graphed as apoptotic cell percentage (D).
Careful inspection of neutrophil staining by histochemistry localized the increased neutrophils with in the hepatic sinusoid. To further determine if margination was involved in increased neutrophils, we performed co-localization studies. Staining neutrophils with S100A9 (25) and basement membrane type collagen IV confirmed that many neutrophils resided in the sinusoidal space (Supplemental Figure 4A–B), indicating that some of the neutrophilia is due to margination.
Acute cDC depletion results in decreased hepatic neutrophil apoptosis
Given that cell death is an important element of neutrophil homeostasis in peripheral tissues, we measured neutrophils apoptosis by flow cytometry to determine whether cDC also affected neutrophil survival. As shown in Figure 6C–D, 12 hours after cDC depletion, the percentage of apoptotic neutrophils as defined by AnnexinV+DAPI-CD45+Ly6G+CD11b+ was reduced by ~50%, suggesting an enhanced survival of neutrophils after acute DT-induced cDC depletion.
Our mechanistic studies indicate that acute cDC depletion affects multiple steps involved in neutrophil homeostasis, including mobilization, recruitment, margination and survival.
Increased hepatic neutrophils after DT depletion in CD11c DTR chimeric mice is not due to depletion of splenic macrophages
One limitation of the CD11c-DTR mice is that DT injection not only depletes cDC but also depletes marginal zone and metallophilic macrophages from the spleen (26). Spleen marginal zone macrophages have been shown to play a critical role in maintaining the homeostasis of neutrophils through phagocytosis of senescent neutrophils (8). In order to rule out the role of spleen macrophages in our model, we analyzed neutrophils after cDC depletion in splenectomized CD11c-DTR chimeras. Three weeks after splenectomy, DT treatment induced cDC depletion and resulted in similarly increased neutrophils in splenectomized mice and sham-operated controls (Figure 7A–B). Similar to non-splenectomized mice, we observed decreased mature neutrophil numbers in the bone marrow (Figure 7A,C) and increased numbers in the peripheral blood (Figure 7A,D), corresponding to a significantly increased NDI (Figure 7E). Thus, we conclude that depletion of spleen macrophages is not responsible for increased neutrophil trafficking from the bone marrow into periphery after DT induced cDC depletion.
Figure 7. cDC depletion in splenectomized mice leads to neutrophil mobilization similar to sham operated mice.
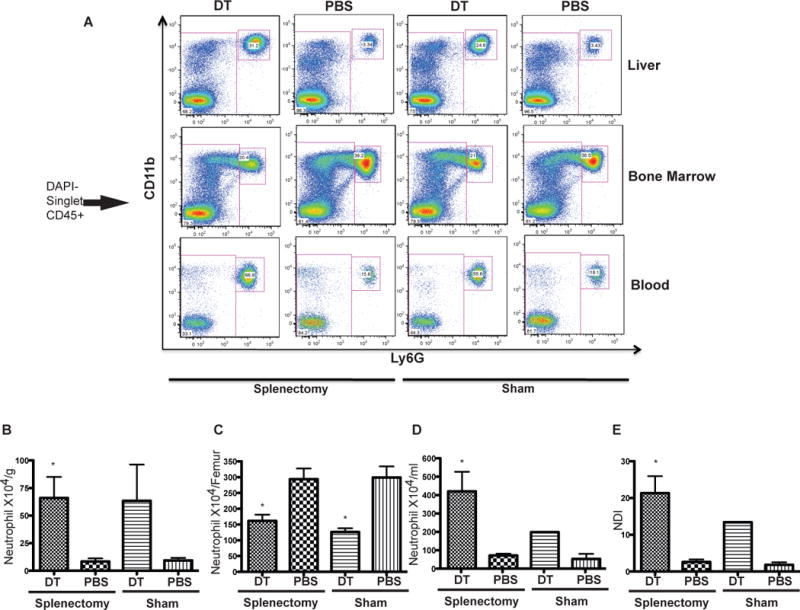
DT injection leads to liver neutrophil increase in splenectomized mice as indicated by FACS (A, top panel). A similar decrease of neutrophil in bone marrow (A, middle panel) and increase in peripheral blood (A, bottom panel) were also observed. Neutrophil absolute numbers are graphed in B–D.
(E): cDC depletion in splenectomized mice also results in enhanced neutrophil trafficking, as graphed by increased NDI.
Discussion
In this study, we have shown using a variety of murine models that cDCs are involved in neutrophil migration from the bone marrow and their recruitment and local survival in peripheral organs. Our results provide in vivo evidence for an important role for dendritic cells in neutrophil homeostasis.
Our analysis shows increased neutrophil counts in mice with DC developmental deficiencies (Flk2KO, Flt3LKO and Batf3KO) and decreased frequencies in a model with DC expansion (loss of Pten in the CD11c+ compartment). Abnormal neutrophil homeostasis has also been reported in other transgenic murine models that exhibit abnormal DC development. Firstly, two strains of CD11c-DTA mice generated by independent labs have a constitutive absence of cDC and both exhibit neutrophilia(27)(28). Secondly, dampened DC survival induced by DC-specific knockout of transforming growth factor beta-activated kinase 1 (TAK1) causes a myeloid proliferative disorder characterized by expansion of neutrophils and inflammatory monocytes (29). Thirdly, mice that are deficient in IRF8, a critical transcriptional factor involved in the development of DCs, especially CD8+DC (30) also have a significant systemic increase of neutrophils (31). Most recently, in zDC-DTR mice, another transgenic mouse strain which carries DTR in the 3′ UTR of the zDC (a zinc finger transcription factor specifically expressed by cDC and committed cDC precursors), an increase in neutrophils was reported following cDC depletion (32). Furthermore, mutations that impair IRF8 transcriptional activity in human patients lead to selective depletion of CD11c+CD1c+ circulating dendritic cells and are associated with a very high neutrophil count in the blood (33). Together, these data strongly point to a potential link between DC and neutrophil homeostasis.
The cDC effect on neutrophils homeostasis appears to be class specific as we did not observe increased neutrophils in mice that received a pDC depleting antibody. This data is consistent with previously published data showing that successful pDC depletion obtained with a BDCA-2 DTR system results in similar numbers of neutrophils as controls (34). Thus, neutrophil homeostasis is likely regulated by cDC rather than pDC, despite the fact that both DC subsets are Flt3L dependent. It should be noted that an increase of neutrophils after DC depletion in the CD11c-DTR model has been previously mentioned (35) but a causal link between DCs and neutrophils in the steady state and a mechanistic characterization of this phenomenon have been missing.
In our methodology, we explored a number of other mechanisms that have frequently been claimed to increase neutrophils induced by DT. In CD11c-DTR bone marrow chimeric mice, we first performed a series of experiments to rule out that DT induced cell death is responsible for neutrophilia. We did not observe any differences in the levels of several inflammatory cytokines (IL-1α, IL-1β, TNF-α, IL-12 and IFN-γ) assessed using a cytokine bead array technology. Moreover, in mixed bone marrow chimeras (50% wild type and 50% CD11cDTR bone marrow) DC depletion of the DTR+ DC fraction does not induce neutrophilia, in spite of DT treatment and the associated cell death. Furthermore, by performing a splenectomy of CD11c-DTR chimeric mice, we confirmed for the first time that splenic macrophages, which have also been found to be depleted with cDC in this model system, were not involved in the development of neutrophilia. Together, we have provided a causal relationship between cDC depletion and neutrophilia; a relationship that has previously been attributed mainly to the macrophage population.
Next we explored the mechanism underpinning the increase in neutrophils associated with DC depletion. DT induced cDC depletion results in decreased neutrophil numbers in the bone marrow and a concordant increase in the periphery. A significant increase in neutrophil distribution index (NDI) and an earlier appearance of BrdU-labeled neutrophils in the circulation indicate that neutrophilia results from increased neutrophil mobilization from the bone marrow. Consistent with our data, another group has also recently reported that dendritic-cell depletion causes neutrophil release from the bone marrow using different strains of CD11c-DTR mice (36). Considering the well-characterized role of G-CSF in neutrophil release (17) and an up-regulation of this cytokine in cDC depleted mice, we consider G-CSF as a plausible candidate to trigger neutrophil release after acute DC depletion induced by DT administration. The cellular sources of G-CSF in cDC depleted mice are still unknown. We hypothesize that in the liver the loss of cDC is sensed by endothelial cells which serve as source of G-CSF (37), although the detailed mechanism is still unclear.
Recently, in 2 infection models caused by uropathogenic Escherichia coli (UPEC) and Yersinia enterocolitica (Ye), DC depletion using the CD11c-DTR system resulted in a neutrophil increase and was associated with improved bacterial clearance (36)(38). Besides CD11c-DTR models, studies using DC developmental deficiency models must be re-interpreted. For example, in the Salmonella typhimurium oral infection model, salmonella dissemination to mesenteric lymph nodes (MLN) was strongly reduced in Flt3LKO mice compared with wild type control 2 days after infection. The phenotype was previously interpreted to result from impaired dissemination of bacteria to the MLN in the absence of lamina propria CD103+CD11b+ DC in Flt3LKO mice. Considering the increased neutrophil in Flt3LKO mice, we cannot rule out the possibility that increased neutrophil may also contribute to reduced bacteria dissemination in this system (39). Further, experiments exploring immune responses in these models should take into account the effect of increased neutrophils on generation on adaptive immunity.
In summary, cDC are well known to orchestrate immune response by linking innate and adoptive immunity. Our studies add to the enlarging repertoire of DC function by providing compelling evidence for their involvement in liver neutrophil homeostasis.
Supplementary Material
Acknowledgments
Costica Aloman was supported by grant 1K08DK088954. Ana-Cristina Dragomir was supported by The Levine Family Foundation. Scott Friedman is supported by NIH Grants DK56621 and AA020709.
References
- 1.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Dancey JT, Deubelbeiss KA, Harker LA, Finch CA. Neutrophil kinetics in man. J Clin Invest. 1976;58:705–715. doi: 10.1172/JCI108517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. J Immunol Baltim Md 1950. 2008;181:5183–5188. doi: 10.4049/jimmunol.181.8.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi J, Fujieda H, Kokubo Y, Wake K. Apoptosis of neutrophils and their elimination by Kupffer cells in rat liver. Hepatol Baltim Md. 1996;24:1256–1263. doi: 10.1053/jhep.1996.v24.pm0008903407. [DOI] [PubMed] [Google Scholar]
- 6.Shi J, Kokubo Y, Wake K. Expression of P-selectin on hepatic endothelia and platelets promoting neutrophil removal by liver macrophages. Blood. 1998;92:520–528. [PubMed] [Google Scholar]
- 7.Furze RC, Rankin SM. The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse. FASEB J Off Publ Fed Am Soc Exp Biol. 2008;22:3111–3119. doi: 10.1096/fj.08-109876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordy C, Pua H, Sempowski GD, He Y-W. Regulation of steady-state neutrophil homeostasis by macrophages. Blood. 2011;117:618–629. doi: 10.1182/blood-2010-01-265959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waskow C, Liu K, Darrasse-Jèze G, Guermonprez P, Ginhoux F, Merad M, Shengelia T, Yao K, Nussenzweig M. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008;9:676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35:323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 12.McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, Maliszewski CR, Lynch DH, Smith J, Pulendran B, Roux ER, Teepe M, Lyman SD, Peschon JJ. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–3497. [PubMed] [Google Scholar]
- 13.Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, Price J, Yin N, Bromberg J, Lira SA, Stanley ER, Nussenzweig M, Merad M. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sathaliyawala T, O’Gorman WE, Greter M, Bogunovic M, Konjufca V, Hou ZE, Nolan GP, Miller MJ, Merad M, Reizis B. Mammalian target of rapamycin controls dendritic cell development downstream of Flt3 ligand signaling. Immunity. 2010;33:597–606. doi: 10.1016/j.immuni.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu D, Hattori H, Jo H, Jia Y, Subramanian KK, Loison F, You J, Le Y, Honczarenko M, Silberstein L, Luo HR. Deactivation of phosphatidylinositol 3,4,5-trisphosphate/Akt signaling mediates neutrophil spontaneous death. Proc Natl Acad Sci U S A. 2006;103:14836–14841. doi: 10.1073/pnas.0605722103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 17.Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17:413–423. doi: 10.1016/s1074-7613(02)00424-7. [DOI] [PubMed] [Google Scholar]
- 18.Cai XY, Gommoll CP, Jr, Justice L, Narula SK, Fine JS. Regulation of granulocyte colony-stimulating factor gene expression by interleukin-17. Immunol Lett. 1998;62:51–58. doi: 10.1016/s0165-2478(98)00027-3. [DOI] [PubMed] [Google Scholar]
- 19.Ritzman AM, Hughes-Hanks JM, Blaho VA, Wax LE, Mitchell WJ, Brown CR. The chemokine receptor CXCR2 ligand KC (CXCL1) mediates neutrophil recruitment and is critical for development of experimental Lyme arthritis and carditis. Infect Immun. 2010;78:4593–4600. doi: 10.1128/IAI.00798-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hierholzer C, Kelly E, Lyons V, Roedling E, Davies P, Billiar TR, Tweardy DJ. G-CSF instillation into rat lungs mediates neutrophil recruitment, pulmonary edema, and hypoxia. J Leukoc Biol. 1998;63:169–174. doi: 10.1002/jlb.63.2.169. [DOI] [PubMed] [Google Scholar]
- 21.Balamayooran G, Batra S, Balamayooran T, Cai S, Jeyaseelan S. Monocyte chemoattractant protein 1 regulates pulmonary host defense via neutrophil recruitment during Escherichia coli infection. Infect Immun. 2011;79:2567–2577. doi: 10.1128/IAI.00067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michalec L, Choudhury BK, Postlethwait E, Wild JS, Alam R, Lett-Brown M, Sur S. CCL7 and CXCL10 orchestrate oxidative stress-induced neutrophilic lung inflammation. J Immunol Baltim Md 1950. 2002;168:846–852. doi: 10.4049/jimmunol.168.2.846. [DOI] [PubMed] [Google Scholar]
- 23.McDonald B, Kubes P. Cellular and molecular choreography of neutrophil recruitment to sites of sterile inflammation. J Mol Med Berl Ger. 2011;89:1079–1088. doi: 10.1007/s00109-011-0784-9. [DOI] [PubMed] [Google Scholar]
- 24.Reichel CA, Rehberg M, Lerchenberger M, Berberich N, Bihari P, Khandoga AG, Zahler S, Krombach F. Ccl2 and Ccl3 mediate neutrophil recruitment via induction of protein synthesis and generation of lipid mediators. Arterioscler Thromb Vasc Biol. 2009;29:1787–1793. doi: 10.1161/ATVBAHA.109.193268. [DOI] [PubMed] [Google Scholar]
- 25.Roth J, Vogl T, Sorg C, Sunderkötter C. Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol. 2003;24:155–158. doi: 10.1016/s1471-4906(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 26.Probst HC, Tschannen K, Odermatt B, Schwendener R, Zinkernagel RM, Van Den Broek M. Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells. Clin Exp Immunol. 2005;141:398–404. doi: 10.1111/j.1365-2249.2005.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birnberg T, Bar-On L, Sapoznikov A, Caton ML, Cervantes-Barragán L, Makia D, Krauthgamer R, Brenner O, Ludewig B, Brockschnieder D, Riethmacher D, Reizis B, Jung S. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity. 2008;29:986–997. doi: 10.1016/j.immuni.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Ohnmacht C, Pullner A, King SBS, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Huang G, Vogel P, Neale G, Reizis B, Chi H. Transforming growth factor beta-activated kinase 1 (TAK1)-dependent checkpoint in the survival of dendritic cells promotes immune homeostasis and function. Proc Natl Acad Sci U S A. 2012;109:E343–352. doi: 10.1073/pnas.1115635109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aliberti J, Schulz O, Pennington DJ, Tsujimura H, Reis e Sousa C, Ozato K, Sher A. Essential role for ICSBP in the in vivo development of murine CD8alpha + dendritic cells. Blood. 2003;101:305–310. doi: 10.1182/blood-2002-04-1088. [DOI] [PubMed] [Google Scholar]
- 31.Holtschke T, Löhler J, Kanno Y, Fehr T, Giese N, Rosenbauer F, Lou J, Knobeloch KP, Gabriele L, Waring JF, Bachmann MF, Zinkernagel RM, Morse HC, 3rd, Ozato K, Horak I. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell. 1996;87:307–317. doi: 10.1016/s0092-8674(00)81348-3. [DOI] [PubMed] [Google Scholar]
- 32.Meredith MM, Liu K, Darrasse-Jeze G, Kamphorst AO, Schreiber HA, Guermonprez P, Idoyaga J, Cheong C, Yao K-H, Niec RE, Nussenzweig MC. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J Exp Med. 2012;209:1153–1165. doi: 10.1084/jem.20112675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hambleton S, Salem S, Bustamante J, Bigley V, Boisson-Dupuis S, Azevedo J, Fortin A, Haniffa M, Ceron-Gutierrez L, Bacon CM, Menon G, Trouillet C, McDonald D, Carey P, Ginhoux F, Alsina L, Zumwalt TJ, Kong X-F, Kumararatne D, Butler K, Hubeau M, Feinberg J, Al-Muhsen S, Cant A, Abel L, Chaussabel D, Doffinger R, Talesnik E, Grumach A, Duarte A, Abarca K, Moraes-Vasconcelos D, Burk D, Berghuis A, Geissmann F, Collin M, Casanova J-L, Gros P. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med. 2011;365:127–138. doi: 10.1056/NEJMoa1100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33:955–966. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fahlén-Yrlid L, Gustafsson T, Westlund J, Holmberg A, Strömbeck A, Blomquist M, MacPherson GG, Holmgren J, Yrlid U. CD11c(high)dendritic cells are essential for activation of CD4+ T cells and generation of specific antibodies following mucosal immunization. J Immunol Baltim Md 1950. 2009;183:5032–5041. doi: 10.4049/jimmunol.0803992. [DOI] [PubMed] [Google Scholar]
- 36.Tittel AP, Heuser C, Ohliger C, Llanto C, Yona S, Hämmerling GJ, Engel DR, Garbi N, Kurts C. Functionally relevant neutrophilia in CD11c diphtheria toxin receptor transgenic mice. Nat Methods. 2012;9:385–390. doi: 10.1038/nmeth.1905. [DOI] [PubMed] [Google Scholar]
- 37.Fiévez L, Desmet C, Henry E, Pajak B, Hegenbarth S, Garzé V, Bex F, Jaspar F, Boutet P, Gillet L, Vanderplasschen A, Knolle PA, Leo O, Moser M, Lekeux P, Bureau F. STAT5 is an ambivalent regulator of neutrophil homeostasis. PloS One. 2007;2:e727. doi: 10.1371/journal.pone.0000727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Autenrieth SE, Warnke P, Wabnitz GH, Lucero Estrada C, Pasquevich KA, Drechsler D, Günter M, Hochweller K, Novakovic A, Beer-Hammer S, Samstag Y, Hämmerling GJ, Garbi N, Autenrieth IB. Depletion of dendritic cells enhances innate anti-bacterial host defense through modulation of phagocyte homeostasis. PLoS Pathog. 2012;8:e1002552. doi: 10.1371/journal.ppat.1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, Stanley ER, Nussenzweig M, Lira SA, Randolph GJ, Merad M. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


