Abstract
WWOX is a putative tumor suppressor gene that spans approximately a 1 Mb genomic region and is the site for the second most common chromosomal fragile site, FRA16D at 16q23. Various studies have focused on the expression of WWOX in human cancer mostly at the RNA level, but little is known about the normal pattern of WWOX protein expression in nonneoplastic tissues. In this study, a comprehensive analysis of WWOX protein expression in normal tissues was performed by means of immunohistochemistry utilizing a very specific anti-WWOX polyclonal antibody. We analyzed tissue cores of human samples representing more than 30 organs, using various tissue microarray (TMA) slides. Due to the potential role of WWOX in sex-steroid metabolism, whole sections from hormonally regulated organs like breast, ovaries, testes and prostate were also analyzed. The results from our study indicate that WWOX is preferentially highly expressed in secretory epithelial cells of reproductive, endocrine and exocrine organs, as well as in ductal epithelial cells from specific segments of the urinary system. Interestingly, we also observed significant WWOX protein expression in various cell types of neural origin including neurons, ependymal cells and astrocytes. No expression of WWOX was detected in adipose, connective, and lymphoid tissues, myelinized structures and blood vessels. By better defining the topographic distribution of WWOX in normal tissues this study provides some insight on the potential physiological role of this novel protein.
Keywords: WWOX, Protein expression, Tissue Microarrays, Tumor suppressor, FRA16D
Introduction
We originally cloned the WWOX gene, spanning a large genomic region on the long arm of human chromosome 16 at band q23 (Bednarek et al. 2000a). It was concluded that the region in which WWOX resides is the same as that of the second most common chromosomal fragile site, FRA16D (Bednarek et al. 2000a, b). This chromosomal region is frequently affected by loss of heterozygosity and homozygous deletions in various neoplasias including breast, prostate, ovarian and liver cancer (O’keefe and Richards 2006). Subsequently, cloning by other laboratories was later reported as well, confirming our original observations (Paige et al. 2000a). We also observed that ectopic WWOX expression was able to inhibit anchorage independent growth and in vivo tumorigenicity of highly aggressive breast carcinoma lines, suggesting a putative tumor suppressor role for this novel protein (Bednarek et al. 2000b; Paige et al. 2001b). Alterations of WWOX at the genomic and expression level have been reported in numerous neoplasias including, breast, ovarian, esophageal, stomach, liver, pancreas, lung, oral mucosa and multiple myeloma (Krummel et al. 2000; Kuroki et al. 2002; Park et al. 2004; Gourly et al. 2005; Guler et al. 2005; Iliopoulos et al. 2006b; Nunez et al. 2005a, b; Pimenta et al. 2006; Pluciennik et al. 2006). It was also reported that epigenetic mechanisms could also play a role in loss of WWOX expression in leukemia, lung and bladder cancer (Ishii et al. 2003; Iliopoulos et al. 2005a). Nevertheless, still remains unclear whether and how, total or partial loss of WWOX, contributes to cancer etiology or progression.
WWOX encodes a 46 kDa protein (414 aminoacids), which contains two WW domains at the NH2 terminus and a short chain oxidoreductase (SDR) central domain (Bednarek et al. 2000a). The first WW domain is involved in protein–protein interactions by binding the specific proline rich motif PPXY of other proteins (Ludes-Meyers et al. 2004b; Aqeilan et al. 2005) Within the SDR, the presence of amino acid residues, serine 281 and 293- YNRS K-297, makes up a catalytic signature motif conserved in short steroid dehydrogenases (Duax and Ghosh 1997). Even thought the biochemical function of WWOX remains unknown, due to the aforementioned aminoacid signature, is likely to be involved in steroid metabolism (Kallberg et al. 2002). We developed a highly specific anti-WWOX antibody that has been useful for determining protein expression by immunoblots and immunohistochemistry (IHC) (Nunez et al. 2005a, b) in this paper. Due to the paucity of information on the baseline pattern of distribution of WWOX protein expression in normal human tissues, in this study, we performed Western blot in a panel of organs and a detailed organ and cell type immunohistological analysis, utilizing whole tissue sections and multiple TMAs representative of a large variety of human organs.
Material and methods
Anti-WWOX antibody
The antibody was raised against a GST fusion protein harboring the WW domains of the WWOX protein. We have previously reported on the specificity of the WWOX antibody used for the current study (Nunez et al. 2005a, b), this paper demonstrated specificity by using Peo1 extracts as negative control. Only full length WWOX (i.e. the 46 kDa product) is detected as expressed, and we have no evidence that any other of the putative RNA alternative spliced WWOX forms are able to generate stable viable protein products (Ludes-Meyers et al. 2003a).
Western blot
Western blotting of human tissue lysates was performed using INSTA-Blot™ membranes (#IMB-103, IMGENEX, San Diego, CA). Anti-actin monoclonal antibody (#A1978, SIGMA-ALDRICH, St. Louis, MO) was used at a dilution of 1:5,000. Anti-WWOX antibody was used at a dilution of 1:2000. Secondary antibodies were horse radish peroxidase (HRP) conjugated anti-rabbit and anti-mouse (KPL, Gaithersburg, MD) and were used at a dilution of 1:2,000. Primary and secondary antibodies were diluted in TBS-Tween (0.1%) containing 5% non-fat dry milk and incubated with the membrane for 1 h at room temperature. Membranes were washed with TBS-Tween (0.1%) three times for five minutes each followed one 10-min wash followed by detection of HRP using Enhanced Chemiluminescent reagents from KPL, Gaithersburg, MD.
Immunohistochemistry
Anti-WWOX immunostaining and automated evaluation of staining intensity was performed as previously reported (Nunez et al. 2005a, b). We also performed a manual evaluation defining levels of staining as negative (absence), mild, moderate and strong. Manual and ACIS® final scale: negative (−) = < 59 arbitrary units (au), mild (+) = 60–68 au, moderate (++) = 69–79 au, strong (+++) = > 80 au (Table 1). The ovarian surface epithelium (OSE) was used as positive control.
Table 1.
Summary of organs showing the distribution of WWOX positive staining according to tissue and cell type
| Organs | WWOX staining cell/tissue specificity | WWOX intensity |
|---|---|---|
| Breast | Resting ductal luminal & myoepithelial cells | ++/+++ |
| Secretion | ++ | |
| Epithelial cells lactation changes | +++ | |
| Ovary | Ovarian surface epithelial cells (OSE) | +++ |
| Inclusion cyst | +++ | |
| Walthard nest | ++ | |
| Stromal luteinized cells | ++ patchy | |
| Stromal cells | − | |
| Fallopian tube | Endoluminal epithelial cells | +++ |
| Tubal stromal cells | − | |
| Placenta | Immature Villi, Cytotrophoblast | ++ |
| Hofbauer cells | ++ | |
| Mature Villi, Syncytiotrophoblast | − | |
| Uterus | Proliferative & secretory phase endometrial glands | +++ |
| Endometrial stromal cells | − | |
| Myometrium | − | |
| Uterine | Mucus secreting cells of endocervix | ++/+ |
| Cervix | Subcolumnar endocervical cells | +++ |
| Squamous epithelium from exocervix | − | |
| Bronchus | Mucus secreting cells | +++ |
| Omentum | Cubic mesothelial cells | + |
| Adipose tissue | − | |
| Pituitary gland | Basophilic cells nests/cords | +++/++ |
| Acidophilic cells | ++ | |
| Chromophobic cells | +/− | |
| Pituitary stalk glial cells and Herring bodies | ++ | |
| Adrenal | Cortex, zona glomerulosa, zona fasciculate and zona reticularis | +++ |
| Medulla, Pheocromocytes adrenal medulla | +++ | |
| Liver | Hepatocytes | +++ |
| Intrahepatic biliar ducts | ++ | |
| Connective tissue septum | − | |
| Blood vessels walls | − | |
| Gall bladder | Epithelial cells lining mucosa | ++ |
| Thyroid | Cuboideal follicular cells | ++ |
| Flat follicular cells | − | |
| Para follicular cell (C cells) | ++ | |
| Parathyroid | Inactive chief cells cords/sheets/pseudofollicules | +++ variable |
| Oxyphil cells nests/cords | +++ | |
| Pancreas | Epithelial cells in acini | +++ |
| Ducts | ++ | |
| Langerhans nests | ++ | |
| Salivary gland | Serous acinar epithelial cells | +++ |
| Mucous acinar cells | − | |
| Luminal ductal and myoepithelial cells | ++ | |
| Epithelial cells in interlobular ducts | ++ | |
| Esophagus | Squamous epithelium | − |
| Submucosal esophageal glands | − | |
| Stomach (fundal mucosa) | Parietal cells in the isthmus and neck of the gland | ++ |
| Chief cells in the base of the gland | ++ | |
| Tall mucus secreting epithelial cells in surface and pit | − | |
| Small bowel | Duodenum enterocytes | ++ |
| Jejunum and ileum enterocytes | − | |
| Paneth cells, granular supranuclear | ++ | |
| Enteroendocrine cells, granular infranuclear | +++ | |
| Goblet cells | − | |
| Colon | Goblet cells in crypts | − |
| Enteroendocrine cells | +++ | |
| Cerebellum (Folia) | Purkinje cells layer | +++/++ |
| Molecular layer | ++ | |
| Internal granular cells neurons | +++ | |
| Cerebrum | Frontal, occipital, limbic cortex and nucleus caudate: Soma and dendrites of large neurons | +++ |
| Soma and processes of astrocytes | +++ | |
| Neuropil of gray matter | ++ | |
| Parietal cortex, temporal cortex and substantia nigra | +/− | |
| Pons and medulla | Neurons | ++ |
| Peripheral nerve | Schwann cells | – |
| Urinary bladder | Transitional epithelium | +++/++ |
| Kidney | Proximal collector ducts (CD) and distal convolute duct (DCD) | +++ |
| Proximal convoluted duct (PCD) | ++ | |
| Parietal layer of Bowman capsule | − | |
| Intraglomerular mesangial cells | − | |
| Apherent and epherent arterioles in glomeruli (vascular tuft) | − | |
| Prostate | Luminal cubic cells in active glands | ++ |
| Flat cells of glands with cystic atrophy | + | |
| Basal cells | +++ | |
| Prostatic urethra transitional epithelium | +++ | |
| Testis | Leydig cells in interstitium | +++ |
| Spermatogonias and sertoli cells in seminiferous tubules | ++/+ | |
| Epidydimus, rete testis and vas deferens | Basal cells and luminal epithelial cells | +++ |
| Basal membrane | − | |
| Smooth muscle in vas deferens | − | |
| Skin | Keratinocytes | ++ patchy |
| Hair follicle | ++ | |
| Sebocytes | +++ | |
| Sweat glands myoepithelial cells | ++ |
Manual and ACIS® final scale: negative (−) = < 59, mild (+) = 60–68, moderate (++) = 69–79, strong (+++) = > 80
Tissue microarrays and whole tissue sections
Normal adult human tissues were obtained from commercial sources. The TMAs used in this study were obtained from the following commercial sources; Ambion, Inc (Austin, TX, USA) Innogenex, (San Ramon, CA, USA) and Zymed (South San Francisco, CA, USA). Thus, by combining two serial sections of each TMA, we obtained duplicate representation of 30 organs displayed in either high density TMA with 200 elements per slide with 0.2 and 0.6 mm in diameter cores or low density TMA with approximately 50 elements per slide with 1.5 mm in diameter cores. The nervous system was analyzed in a separate Neuro-trial TMA from Chemicon International (Temecula, CA, USA). This specific TMA carried two cores representing each of frontal, occipital, parietal, limbic and temporal cortex, nucleus caudate, pons, nuclei olivaris of medulla, substantia nigra and cerebellum. TMA representative of adjacent sections from each tissue core were also stained in parallel for quality and diagnostic controls with H&E. Whole formalin fixed and paraffin embedded tissue sections representing breast, ovaries, testes and prostate were evaluated by standard H&E and IHC.
Results
A summary of all studied WWOX positive organs and cells is shown in Table 1.
WWOX expression in female reproductive system
Resting and lactating female breast
In the resting female breast both, the ductal luminal cells and myoepithelial cells were consistently positive for WWOX protein expression (Nunez et al. 2005a). The lactating breast epithelium was strongly positive for WWOX expression in both ductal and acinar cells. The secretory fluid and detritus observed within the lumen were also strongly positive. This raises the possibility that the WWOX protein could be a component of breast epithelial secretory vacuoles. Intra and interlobular connective tissue was negative.
Ovary and Fallopian tubes
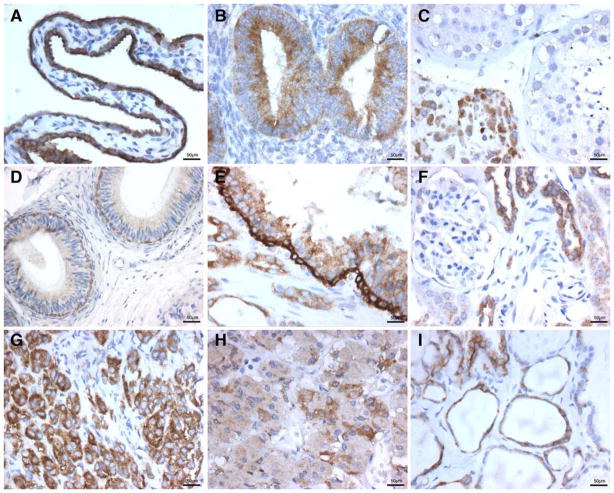
Because of WWOX’s putative role in sex steroid metabolism and in ovarian carcinogenesis we analyzed TMA cores as well as whole ovarian sections. We have already reported that WWOX is particularly highly expressed in ovarian surface epithelial cells (OSE) and inclusion cysts (Nunez et al. 2005b); we also observed a moderate to mild staining in patchy areas of ovarian stromal like cells. Interestingly, we detected positive WWOX staining, albeit moderate, in groups of luteinized cells surrounding corpi albicans. All these observations are in agreement with the putative role of WWOX in steroid metabolism, since all these various ovarian cell types are active in sex steroid hormones metabolic functions (Kallberg et al. 2002). They also confirm previous determinations by means of Western and Northern analyses, indicating that ovary is an organ with high WWOX expression. We were also able to detect at least one Walthard nest at the ovarian hilium displaying moderate WWOX staining. The endoluminal epithelial cells, lining the finger like projections of the Fallopian tubes fimbria, (Fig. 2A) showed the strongest positivity for WWOX protein expression seen in the female genital tract.
Fig. 2.
Immunohistology of WWOX protein expression in A: fallopian tube, B: endometrium, C: testes, D: epidydimus, E: prostate, F: kidney, G: adrenal, H: pituitary, I: Thyroid. All images 400 tm. Scale bar = 50 μm
Uterine corpus and cervix
The epithelial cells lining the endometrial mucosa showed strong apical and basal staining in samples representative of proliferative phase (Fig. 2 B) while secretory phase was moderate. In contrast, the endometrial stromal cells and the smooth muscle myometral cells were negative for WWOX protein expression. The mature squamous epithelium from exocervix showed negative staining. The mucus secreting cells from endocervical epithelium were mildly positive. Interestingly, the subcolumnar reserve cells, normally inconspicuous, became evident with this antibody and showed strong WWOX staining. It is worth mentioning that these cells have shown to be positive for estrogen receptor. Areas with immature squamous metaplasia stained moderately positive for WWOX.
Placenta
Avascular villi in immature placenta showed moderate staining mostly in the cytotrophoblast. On the other hand, vascular mature villi showed no staining in the syncytiotrophoblast and positivity only in Hofbauer cells in lamina propia.
WWOX expression in male reproductive system
Testes and excretory duct system
We analyzed WWOX staining in testes using both TMA cores and whole tissue sections. In seminiferous tubules staining was variable, we observed a gradual decrease in staining intensity with increasing stages of spermeogenesis, going from moderate-strong staining in spermatogonia at the basal compartment to mild staining of mature forms at the adluminal compartment. Sertoli cells were moderately positive. We observed very strong positive cytoplasmic staining in clusters of Leydig cells within the interstitium (Fig. 2C). This observation is compatible with a role for WWOX in sex steroid metabolism, as previously speculated, since the highest expression is observed in the hormone producing Leydig cells. In the excretory ducts, the epydidimus displayed a strong cytoplasmic staining in cells from the basal layer (Fig. 2D) while the luminal cells were moderately midly positive. In samples from rete testis, efferent ducts and proximal vas deferens the pseudostratified columnar epithelium was also strongly positive.
Prostate
We observed moderate positive staining in luminal cells from active glands, while the epithelial staining was observed to be milder in prostate samples with glands undergoing cystic changes. The basal cell layer was strongly positive regardless the functional status of the glands (Fig. 2E). Interestingly, prostate epithelial cells are rich in androgen receptors again supporting the speculation of WWOX and sex hormones being somehow associated. The transitional epithelium lining the prostatic urethra was strongly positive.
WWOX expression in urinary system
Kidney
In the analysis of structures in the cortex, the corpuscles of Malpighi showed almost undetectable positive stain for the parietal layer of Bowman’s capsule and intraglomerular mesangial cells, while the endothelium from glomerular vascular tuft was completely negative (Fig. 2F). Moderate staining was observed in epithelial cells from the proximal convoluted tubule. The loop of Henle, represented in samples from the medulla, was mildly positive for the thick ascending segment. On the other hand, we observed strong cytoplasmic WWOX expression in the distal convoluted tubules and proximal collector ducts. This remarkable difference in staining at different levels of kidney’s excretory apparatus is very intriguing. Protein detection in kidney by Western blot showed strong signal (Fig. 1, lane 2).
Fig. 1.
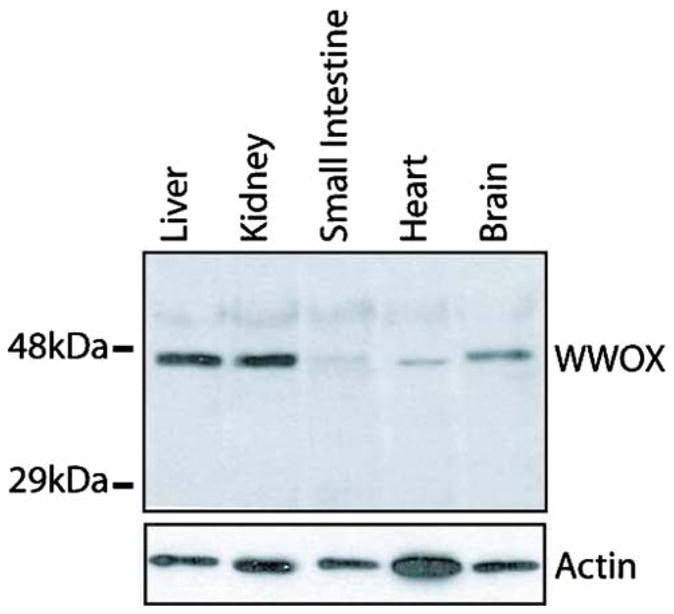
Immunoblot detection of WWOX protein in human tissues. Top panel: 20 μg of total protein lysates from each tissue were resolved onto an Immobilon membrane analyzed with anti-WWOX antibody. Full length WWOX protein is ~46 kDa. Bottom panel: Detection of β-actin was performed to determine equal loading of protein from each tissue. The membrane was stripped after WWOX detection and washed extensively before immunodetection with anti-actin antibody
Urinary bladder
The transitional epithelial cells were strongly positive for WWOX expression. The smooth muscle from the wall was negative.
WWOX expression in the endocrine system
Adrenal glands
Cells from all three cortical layers of the adrenal glands: glomerular, fasciculate and reticular, were strongly positive for WWOX expression (Fig. 2G). The spongiocytes from the fasciculate layer that synthesize mainly glucocorticoids displayed a clear cytoplasm due to the presence of microvacuoles. Interestingly, when using an oil immersion high power objective, we could determine that WWOX staining was stronger in between rather than inside spongyocytes’ microvacuoles. On the other hand, cells from the reticularis layer, cells in charge of sex hormones synthesis, demonstrated a strong homogeneous positive cytoplasmic staining. Pheochromocytes in the medulla (preganglionic sympathetic neurons epinephrine/norepinephrine producers), displayed strong to mild cytoplasmic staining. Interestingly, cells in the cortex derived from coelomic mesoderm and cells in the medulla derived from neural crests, both, were positive for WWOX protein expression regardless of their different embryologic cell origin.
Pituitary gland
We studied tissue cores from the anterior lobe of the adeno-hypophysis as well as pituitary stalk. Basophilic cells nests/cords were strongly to moderately reactive to WWOX antibodies (Fig. 2H), while acidophilic cells reacted mildly and chromophobic cells were negative for WWOX expression. The pituitary stalk showed moderate positive staining of glial cells and scattered Herring bodies were also positive.
Thyroid and parathyroid glands
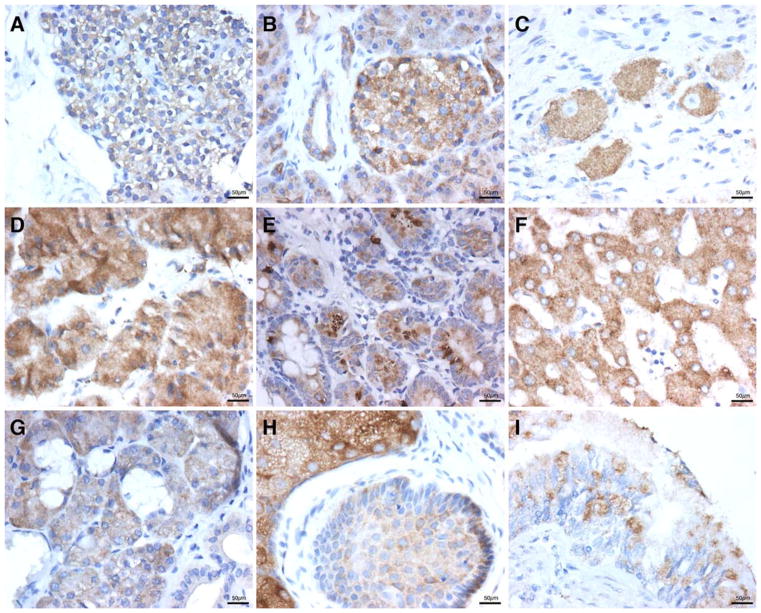
The thyroid gland showed strong positive cytoplasmic staining for WWOX in the cuboideal follicular cells and in parafollicular cells (C cells) (Fig. 2I). However no staining was observed in thyroid samples representative of inactive glandular activity that displayed mostly flat follicular cells. The parathyroid gland showed mostly strong positive staining among Chief cells arranged as cords, sheets (Fig. 3A) and pseudofollicules. Also, strong staining was seen in occasional cords of oxyphil cells.
Fig. 3.
Immunohistology of WWOX protein expression in A: parathyroid, B: pancreas, C: autonomic ganglia, D: gastric, E: small intestine, F: liver, G: salivary gland, H: hair, I: bronchus. All images 400 tm. Scale bar = 50 μm
Endocrine and exocrine pancreas
The endocrine pancreas showed homogeneous intense staining in all cell types of Langerhans nests (Fig. 3B). We observed a strong positive anti-WWOX staining in epithelial cells lining acini and of the exocrine pancreas while the intercalated ducts were only moderate (Fig. 3B).
WWOX expression in the nervous system
Brain
WWOX expression was evaluated both at the histological and topographical levels. Topography was included in this evaluation due to intriguing differential WWOX expression observed in specific anatomical areas of the CNS. The brain was preferentially positive in pyramidal neurons and astrocytes from the frontal cortex, in astrocytes and neurons of the occipital cortex and in neurons of the nucleus caudatus, pons and nuclei olivaris of medulla. The neuropil, constituted by dendritic branches, sub branches and astrocyte processes, was also positive. The ependymal cells from a lateral cerebral ventricle were moderately positive for staining in their scant cytoplasm. The subependymal glia was mildly reactive and small neurons were strongly positive. Interestingly, the parietal, limbic and temporal cortexes as well as the substantia nigra were negative for anti-WWOX staining. Oligodendrocytes showed no WWOX immunoreactivity.
Cerebellum
The folia of cerebellum showed strong positive staining in, small neurons in internal granular cell layer and the molecular layer, while bulbous Purkinje cells stained moderately.
Autonomic ganglia
The neurons at autonomic ganglia showed strong positive diffuse cytoplasmic anti-WWOX staining while, in sharp contrast, the aligned nerve processes and Schwann cells were negative (Fig. 3C). Noteworthy, cortical neurons derived from neural tube and the ones in autonomic ganglia derived from neural crest, both, were positive for WWOX protein expression.
WWOX expression in the digestive tract and accessory glands
Esophagus and stomach
The esophagus showed negative immunoreactivity on squamous epithelium as well in epithelial cells of submucosal esophageal glands, few ducts stained very midly. The stomach was represented by samples from fundal mucosa. Immunoreactivity showed a zonation pattern. Parietal cells from isthmus and neck of gastric glands and chief cells at their base, all showed moderate positive anti -WWOX cytoplasmic staining (Fig. 3D). In sharp contrast to the positive staining pattern of parietal cells, the tall columnar mucus secreting epithelial cells from the surface and pit were consistently negative for WWOX expression. Interestingly, a recent report by (Jin et al. 2006) presented evidence that ezrin is one of the many potential WWOX’s interacting partners. These authors described that the ezrin–WWOX interaction takes place at the apical membrane of gastric parietal cells and that this interaction appears regulated by Protein Kinase A via phosphorylation of ezrin’s Ser 66. Ezrin is known to be a protein implicated in cell shape, adhesion, motility, survival (Bretscher et al. 2002) and apical polarity in epithelial cells (Jin et al. 2006) demonstrated that WWOX, H-K-ATPase and ezrin biochemically interact and co-localize to the apical membrane of parietal cells. These observations are consistent with our findings of WWOX being predominantly expressed in epithelial secretory cell types.
Small intestine and colon
The small intestine showed positive staining in three of the four major cell types in the mucosa. Enterocytes on villi of duodenum were moderately positive (Fig. 3E). In other small bowel areas, characteristic moderate positive staining was observed in supranuclear granules in Paneth’s cells at Crypts of Lieberkühn. Surprisingly, WWOX immunostaining showed striking granular positive signal in isolated cells, along the villi and at crypts. We speculate that such positive cells are of the neuroendocrine type and their perinuclear granular positive staining reinforces the idea of Golgi intracellular location. The cytoplasm of Goblet cells was vacant for WWOX immunostaining and was the only WWOX negative cell type in small intestine mucosa. Interestingly, the ganglion cells at Meissner’s plexus just beneath the muscularis mucosae were strongly positive. The submucosa and external muscular layers were negative. The colonic mucosa showed crypts where the isolated putative neuroendocrine cells were the only type demonstrating positive staining. Goblet cells remained consistently negative all along the bowel.
Liver and gall bladder
The liver parenchyma displayed a strong staining for WWOX protein expression in all analyzed tissue cores as we have previously described (Park et al. 2004). The hepatocytes showed a strong granular and diffuse cytoplasmic staining compatible with cytoplasmic vacuolar structures (Fig. 3F). Strong signal was detected by Western blot (Fig. 1, lane 1). The cubic cells lining intrahepatic biliar ducts stained moderately midly. On the other hand, the connective tissue in the septums and the blood vessels were consistently negative. Moderate to strong WWOX cytoplasmic staining was also observed in gallbladder epithelial mucosal cells. This organ was the only showing mild staining in smooth muscle from the wall.
Salivary glands
Salivary glands samples showed also strong positive staining in luminal epithelial cells from serous acini and moderately positive in epithelial cells from intercalated and striated ducts. The mucinous acini were clearly negative (Fig. 3G). The myoepithelial cells ensheathing individual acini were also positive for WWOX.
WWOX expression in the skin
Whole sections of skin were analyzed, the basal cell layer was observed to be moderately positive while the keratinized multilayer squamous epithelium displayed a patchy weakly positive staining. The hair follicles sheaths were moderately but always positive while sebocytes were strongly positive (Fig. 3H). In the few sweat glands excretory ducts analyzed exclusively the myoepithelial cells were observed to be strongly positive for WWOX protein expression. The deep adenomatous component was not represented in the sample.
WWOX expression in the respiratory system
The pseudostratified cylindrical and ciliated bronchial epithelium was strongly positive for WWOX protein expression in cytoplasm of mucus secreting cells showing sharp perinuclear staining (Fig. 3I). No WWOX protein expression was detected on type I and II pneumocytes on distal lung parenchyma. Intraalveolar macrophages were mildly positive.
Miscellaneous tissues negative for WWOX staining
Immunology related tissues such as spleen; lymph node and thymus were negative for WWOX staining (Table 2). Despite of the fact that it has been reported high WWOX expression by Northern blot in peripheral lymphocytes, only scattered patches of plasmocytes in inflammatory infiltrates have shown positive staining by IHC in our hands. Only one sample from bone marrow displayed moderate positivity for scattered megakaryocytes. The Aorta was negative as well as all components of blood vessels walls (endothelium, muscular, adventitia). Capillaries were consistently negative in all the organs analyzed. The Schwann cells and myelinized structures were negative for WWOX staining (Fig. 3C). No WWOX expression was detected in connective tissue from any of the described organs. Adipose tissue from all locations was also negative for WWOX expression. Observations on the few skeletal muscle samples examined were inconclusive. Specific negative cell types from all studied organs are shown in Table 2.
Table 2.
Summary of organs negatives for WWOX protein expression
| Organs | WWOX staining cell/tissue specificity | WWOX intensity |
|---|---|---|
| Thymus | Cortical thymocytes | – |
| Lymphocytes | – | |
| Lymph node | Cortex and medulla | – |
| Spleen | Red pulp and White pulp | – |
| Lung | Alveolar lining epithelial cells | – |
| Interalveolar septum | – | |
| Aorta | Intima, muscular and adventitia | – |
| Soft tissue | Adipose tissue and connective tissue | – |
Discussion
In summary, to the best of our knowledge, this is the first study to examine in detail the cell type and topographic distribution of WWOX protein expression in non-neoplastic adult human tissues. Only a cytoplasmic pattern of staining was demonstrated in all samples analyzed. The cytoplasmic staining pattern observed was usually granular, predominantly perinuclear and in some cases homogeneously diffuse. These observations are compatible with our previous findings, suggesting that WWOX is a cytoplasmic protein, predominantly localized to the Golgi apparatus and perhaps late endosomes (Bednarek et al. 2000a). We did not detect any instance of WWOX nuclear staining (Watanabe et al. 2003). The organs that expressed the highest levels of WWOX protein were: fallopian tubes, ovaries, mammary epithelium, endometrium, prostate glands, testes and genital ducts, liver, stomach, salivary glands, adrenal gland, thyroid, parathyroid, adenohypofisis, distal nephron segments, prostatic urethra, autonomic ganglia, and specific cerebellum and brain cells. Some of these observations are compatible with previous findings from our laboratory using Northern and Western blot methods for expression analysis (Bednarek et al. 2000a, b). Interestingly we also observed that isolated putative neuroendocrine cells in the gastrointestinal tract were distinctively and strongly immunoreactive to the anti-WWOX antibody. The positive staining in these GI isolated cells, in pheocromocytes and in C cells from the thyroid gland suggests a possible role of WWOX in the neuroendocrine system. It is also intriguing that WWOX is ubiquitously expressed in many epithelial cell types, in particular secretory epithelia, and in neural cells. No WWOX protein expression was detected in lymph node, thymus, spleen, aorta, adipose, vascular and connective tissues.
Even though WWOX is a widely express protein found in a multitude of tissues and cell types, it appears to exist a distinctive pattern of organ and cell type specificity. It remains to be determined the underpinning for such cell type specificity but it appear to be directly link, at least in part, to the fact that WWOX is an enzyme highly likely to be involved in sex steroid metabolism. Thus, the organs consistently strongly positive, are either steroid targets or related with steroids production/metabolism. Whether high abundance or just minimal amounts of this protein are sufficient to function normally as a tumor suppressor remains to be determined. However, the consistently high levels of WWOX protein expression normally observed in sex-hormone related tissues plus our previous studies in breast and ovarian cancer in which we observed high percentage of tumors with a notable marked reduction in expression (Nunez et al. 2005a, b), suggests that perhaps reduced expression and not necessarily complete lack of expression, is of significance in carcinogenesis. Further studies are necessary to elucidate the specific physiologic role of the WWOX proteins and its enzymatic substrate/s; this would obviously pave the way to understand both the intriguing expression pattern of this protein as well as its cell type specificity.
Acknowledgments
The authors gratefully acknowledge Nancy W. Abbey for technical assistance, Joi Holcomb for the elaboration of photo composites, Dr. Mario A. Luna and Dr. Daniel G. Rosen for critical reading. RO1 CA 102444 supported this project.
Contributor Information
Maria I. Nunez, Department of Carcinogenesis, The University of Texas, M.D. Anderson Cancer Center, Science Park-Research Division, P.O. Box 389, Smithville, TX 78957, USA
John Ludes-Meyers, Department of Carcinogenesis, The University of Texas, M.D. Anderson Cancer Center, Science Park-Research Division, P.O. Box 389, Smithville, TX 78957, USA.
C. Marcelo Aldaz, Email: maaldaz@mdanderson.org, Department of Carcinogenesis, The University of Texas, M.D. Anderson Cancer Center, Science Park-Research Division, P.O. Box 389, Smithville, TX 78957, USA.
References
- Aqeilan RI, Donati V, Palamarchuk A, Trapasso F, Kaou M, Pekarsky Y, Sudol M, Croce CM. WW domain-containing proteins, WWOX and YAP, compete for interaction with ErbB-4 and modulate its transcriptional function. Cancer Res. 2005 Aug 1;65 (15):6764–6772. doi: 10.1158/0008-5472.CAN-05-1150. [DOI] [PubMed] [Google Scholar]
- Bednarek AK, Laflin KJ, Daniel RL, Liao Q, Hawkins KA, Aldaz CM. WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3–24.1, a region frequently affected in breast cancer. Cancer Res. 2000a;60:2140–2145. [PubMed] [Google Scholar]
- Bednarek AK, Keck-Waggoner CL, Daniel RL, Laflin KJ, Bergsagel PL, Kiguchi K, Brenner AJ, Aldaz CM. WWOX, the FRA16D gene, behaves as a suppressor of tumor growth. Cancer Res. 2001b;61:8068–8073. [PubMed] [Google Scholar]
- Duax WL, Ghosh D. Structure and function of steroid dehydrogenases involved in hypertension, fertility, and cancer. Steroids. 1997;62:95–100. doi: 10.1016/s0039-128x(96)00166-3. [DOI] [PubMed] [Google Scholar]
- Gourley C, Paige AJ, Taylor KJ, Scott D, Francis NJ, Rush R, Aldaz CM, Smyth JF, Gabra H. WWOX mRNA expression profile in epithelial ovarian cancer supports the role of WWOX variant 1 as a tumour suppressor, although the role of variant 4 remains unclear. Int J Oncol. 2005 Jun;26(6):1681–1689. doi: 10.3892/ijo.26.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler G, Uner A, Guler N, Han SY, Iliopoulos D, McCue P, Huebner K. Concordant loss of fragile gene expression early in breast cancer development. Pathol Int. 2005 Aug;55(8):471–478. doi: 10.1111/j.1440-1827.2005.01855.x. [DOI] [PubMed] [Google Scholar]
- Iliopoulos D, Guler G, Han SY, Johnston D, Druck T, Mc-Corkell KA, Palazzo J, McCue PA, Baffa R, Huebner K. Fragile genes as biomarkers: epigenetic control of WWOX and FHIT in lung, breast and bladder cancer. Oncogene. 2005a Feb 24;24(9):1625–1633. doi: 10.1038/sj.onc.1208398. [DOI] [PubMed] [Google Scholar]
- Iliopoulos D, Guler G, Han SY, Druck T, Ottey M, McCorkell KA, Huebner K. Roles of FHIT and WWOX fragile genes in cancer. Cancer Lett. 2006b Jan 28;232(1):27–36. doi: 10.1016/j.canlet.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Ishii H, Vecchione A, Furukawa Y, Sutheesophon K, Han SY, Druck T, Kuroki T, Trapasso F, Nishimura M, Saito Y, Ozawa K, Croce CM, Huebner K. Expression of FRA16D/WWOX and FRA3B/FHIT genes in hematopoietic malignancies. Mol Cancer Res. 2003;1:940–947. [PubMed] [Google Scholar]
- Jin C, Ge L, Ding X, Chen Y, Zhu H, Ward T, Wu F, Cao X, Wang Q, Yao X. PKA-mediated protein phosphorylation regulates ezrin-WWOX interaction. Biochem Biophys Res Commun. 2006 Mar 17;341(3):784–791. doi: 10.1016/j.bbrc.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Kallberg Y, Oppermann U, Jornvall H, Persson B. Short-chain dehydrogenase/reductase (SDR) relationships: a large family with eight clusters common to human, animal, and plant genomes. Protein Sci. 2002 Mar;11(3):636–641. doi: 10.1110/ps.26902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel KA, Roberts LR, Kawakami M, Glover TW, Smith DI. the characterization of the common fragile site FRA16D and its involvement in multiple myeloma translocations. Genomics. 2000;69:37–46. doi: 10.1006/geno.2000.6321. [DOI] [PubMed] [Google Scholar]
- Kuroki T, Trapasso F, Shiraishi T, Alder H, Mimori K, Mori M, Croce CM. Genetic alterations of the tumor suppressor gene WWOX in esophageal squamous cell Carcinoma. Cancer Res. 2002;62:2258–2260. [PubMed] [Google Scholar]
- Ludes-Meyers JH, Bednarek AK, Popescu NC, Bedford M, Aldaz CM. WWOX, the common chromosomal fragile site, FRA16D, cancer gene. Cytogenet Genome Res. 2003a;100:101–110. doi: 10.1159/000072844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludes-Meyers JH, Kil H, Bednarek AK, Drake J, Bedford MT, Aldaz CM. WWOX binds the specific proline-rich ligand PPXY: identification of candidate interacting proteins. Oncogene. 2004b Jun 24;23(29):5049–5055. doi: 10.1038/sj.onc.1207680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez MI, Ludes-Meyers J, Abba MC, Kil H, Abbey NW, Page RE, Sahin A, Klein-Szanto A, Aldaz CM. Frequent loss of WWOX expression in breast cancer: correlation with estrogen receptor status. Breast Cancer Res Treat. 2005a;89(2):99–105. doi: 10.1007/s10549-004-1474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez MI, Rosen DG, Ludes-Meyers JH, Abba MC, Kil H, Page R, Klein-Szanto AJ, Godwin AK, Liu J, Mills GB, Aldaz CM. WWOX protein expression varies among ovarian carcinoma histotypes and correlates with less favorable outcome. BMC Cancer. 2005b Jun 27;5(1):64. doi: 10.1186/1471-2407-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’keefe LV, Richards RI. Common chromosomal fragile sites and cancer: focus on FRA16D. Cancer Lett. 2006 Jan 28;232(1):37–47. doi: 10.1016/j.canlet.2005.07.041. [DOI] [PubMed] [Google Scholar]
- Paige AJ, Taylor KJ, Stewart A, Sgouros JG, Gabra H, Sellar GC, Smyth JF, Porteous DJ, Watson JE. A 700-kb physical map of a region of 16q23.2 homozygously deleted in multiple cancers and spanning the common fragile site FRA16D. Cancer Res. 2000a;60:1690–1697. [PubMed] [Google Scholar]
- Paige AJ, Taylor KJ, Taylor C, Hillier SG, Farrington S, Scott D, Porteous DJ, Smyth JF, Gabra H, Watson JE. WWOX: a candidate tumor suppressor gene involved in multiple tumor types. Proc Natl Acad Sci U S A. 2001b;98:11417–11422. doi: 10.1073/pnas.191175898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Ludes-Meyers J, Zimonjic DB, Durkin ME, Popescu NC, Aldaz CM. Frequent downregulation and loss of WWOX gene expression in human hepatocellular carcinoma. Br J Cancer. 2004;91:753–759. doi: 10.1038/sj.bjc.6602023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenta FJ, Gomes DA, Perdigao PF, Barbosa AA, Romano-Silva MA, Gomez MV, Aldaz CM, De Marco L, Gomez RS. Characterization of the tumor suppressor gene WWOX in primary human oral squamous cell carcinomas. Int J Cancer. 2006 Mar 1;118(5):1154–1158. doi: 10.1002/ijc.21446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluciennik E, Kusinska R, Potemski P, Kubiak R, Kordek R, Bednarek AK. WWOX-the FRA16D cancer gene: expression correlation with breast cancer progression and prognosis. Eur J Surg Oncol. 2006 Mar;32(2):153–157. doi: 10.1016/j.ejso.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Smith DI, Zhu Y, McAvoy S, Kuhn R. Common fragile sites, extremely large genes, neural development and cancer. Cancer Lett. 2006 Jan 28;232(1):48–57. doi: 10.1016/j.canlet.2005.06.049. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Hippo Y, Taniguchi H, Iwanari H, Yashiro M, Hirakawa K, Kodama T, Aburatani H. An opposing view on WWOX protein function as a tumor suppressor. CancerRes. 2003;63:8629–8633. [PubMed] [Google Scholar]