Abstract
Macrophages form a heterogeneous group of hematopoietic cells that reside in tissues, where they are required to maintain organ integrity. Tissue macrophages contribute to tissue formation, metabolism, homeostasis, and repair. They have a unique ability to sense and respond to tissue damage. They serve as the first line of defense during infection and help promote immune tolerance in the steady state. Although most tissue macrophages share a high phagocytic and degradative potential, they are heterogeneous in origin, as well as in homeostatic function and response to insults. Here, we will discuss recent developments in our understanding of the origin of tissue macrophages and their functional specialization in tissues.
Introduction
Macrophages form a heterogeneous group of tissue-resident hematopoietic cells of myeloid origin that share a superior phagocytic potential and an ability to recognize and respond to tissue damage and infection. Macrophages populate all tissues of the body and play a key role in the maintenance of tissue integrity and repair (1, 2). Global transcriptome analysis of purified tissue macrophage populations by the Immunological Genome Project has contributed to a better understanding of the macrophage lineage and has revealed considerable transcriptional diversity between macrophages from different organs (3), emphasizing their specialized role in different tissues. The development of engineered mouse models to trace myeloid progenitors, quantify macrophage repopulation in situ and probe macrophage regulation in vivo has also revealed novel developmental and regulatory control of the macrophage lineage. In this review, we will discuss our current understanding on the regulation of macrophage development and function that has emerged from these studies.
Macrophage Phenotype
Macrophages are characterized by specific phenotypic features and by the expression of particular markers, none of which is entirely restricted to the cell type. In mice, macrophages express the hematopoietic lineage marker CD45 and lack lineage markers of other immune cells (including Gr-1, CD3, and CD20). They express the receptor for macrophage colony-stimulating factor (M-CSF, renamed Csf1), the integrin CD11b, Fcγ receptor 1 (FcγRI) CD64, and the receptor tyrosine kinase MerTK. The latter two markers are particularly indicative of the unique function of macrophages as scavengers of foreign antigens and apoptotic cells. In certain tissues, particularly in locations where antigen presentation is critical, such as the microbial coated intestine, macrophages constitutively express cell surface MHC class II and the integrin CD11c, two molecules shared with dendritic cells (4). In humans, macrophage markers include the Csf1 receptor CD115, the FcγRI CD64, the FcγRIII CD16, and the scavenger receptors CD68 and CD163. In both mice and humans, these markers need to be used in combination to define macrophages, as none is cell type specific (see Fig. 1).
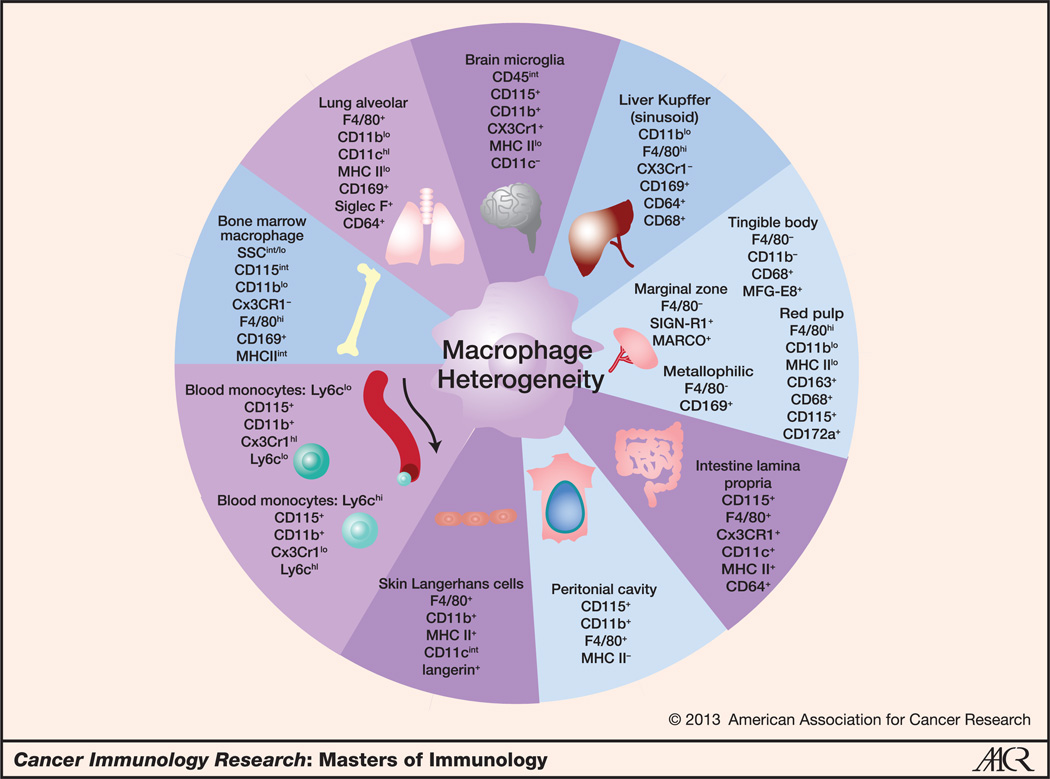
Figure 1.
Macrophage heterogeneity. Macrophages are a heterogeneous population of tissue-resident hematopoietic cells. This figure illustrates key cell surface markers of mouse macrophage and monocyte populations. Macrophages can be identified by a combination of cell surface markers, including the hematopoietic lineage marker CD45, the integrin CD11b, and the GPCR F4/80, among other markers. However, macrophages in different organs have different compositions and expression levels of these cell surface proteins, reflecting their inherent diversity. CD169, sialoadhesin, is an important marker in certain macrophage populations such as the bone marrow, spleen, and lung. CX3CR1 helps to identify macrophages in the intestine and differentiate the blood monocyte subsets.
Macrophage Ontogeny during Steady State and in Inflamed State
The mononuclear phagocyte system
In 1908, Elie Metchnikoff (1845–1916) was awarded the Nobel Prize in Physiology or Medicine for the discovery of phagocytosis (5). Phagocytosis, derived from the Greek word phago meaning "to devour," refers to the process of engulfment of large particles by phagocytes. In mice and humans, phagocytes include mononuclear phagocytes and neutrophils, which are also called polymorphonuclear phagocytes due to the segmented shape of their nuclei. Mononuclear phagocytes include blood-circulating monocytes, tissue-resident macrophages, and dendritic cells. A foundational dogma in immunology suggests that monocytes and macrophages are part of a continuum that form the mononuclear phagocyte system, in which blood monocytes are the circulating link between bone marrow–derived myeloid precursors and tissues macrophages (6).
Recent studies have revealed that commitment to the mononuclear phagocyte lineage is determined at the stage of the macrophage–dendritic cell progenitor (MDP), at which point, erythroid, megakaryocyte, lymphoid, and granulocyte fates have been precluded (7, 8). MDP cells, phenotypically defined as lineage− c-kit+ CX3CR1+ CD115+ , give rise to common dendritic cell progenitors (CDP), which differentiate into circulating pre–dendritic cell precursors that leave the bone marrow to repopulate the short-lived tissue dendritic cell pool (9–11). In parallel, MDP differentiate through the recently described common monocyte precursor (cMoP; ref. 12) into two subsets of monocytes that are distinguished on the basis of the expression of the lymphoid 6c (Ly6C) antigens. Ly6Chi monocytes are short-lived cells that extravasate in inflamed tissues in response to injury signals to differentiate into inflammatory dendritic cells and macrophages. Ly6Clo monocytes have distinct homing and functional properties compared with Ly6Chi monocytes (13). In contrast to Ly6Chi monocytes, Ly6Clo monocytes cannot infiltrate tissues; they patrol the endothelium and contribute to the maintenance of endothelial cell integrity (14). Recent data revealed that circulating Ly6Clo monocytes in fact likely arise from Ly6Chi monocytes, and these cells form a steady state continuum [see Fig. 2 (13)].
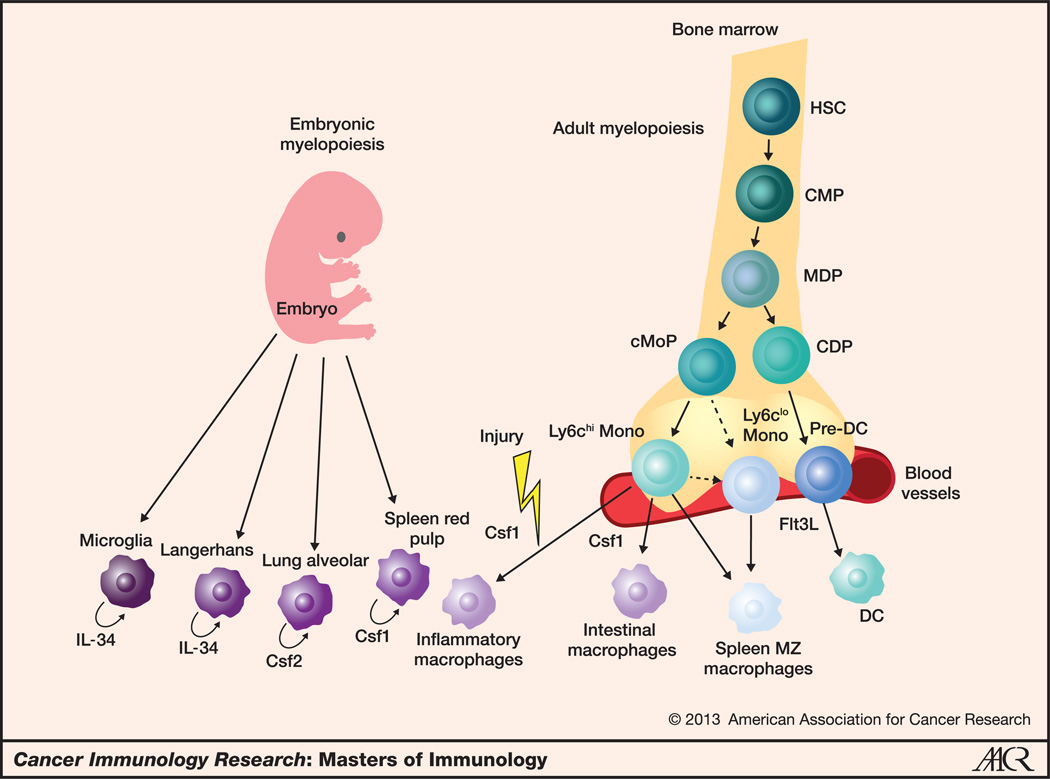
Figure 2.
Regulation of macrophage development and homeostasis in mice. This figure summarizes the current model of the pathways to the development of nonlymphoid tissue-resident macrophages. Most tissue-resident macrophages in the steady state arise from embryonic precursors that are recruited to tissues before birth with the exception of intestinal and spleen marginal zone macrophages that derive from circulating monocytes. Embryonic precursor-derived macrophages receive trophic signals that depend upon their particular locations. Microglia and Langerhans cells require interleukin (IL)-34, alveolar macrophages depend on CSF2, and splenic red pulp macrophages use CSF1 for tissue maintenance. The successive steps that give rise to circulating monocytes in the adult bone marrow have been characterized. Adult bone marrow hematopoietic stem cells (HSC) give rise to the common myeloid precursors (CMP), which subsequently give rise to the MDP. MDP produce both the cMoP and the CDP. CDP differentiate in response to Flt3 ligand into pre-dendritic cells (pre-DC), which migrate through the blood to peripheral tissues and differentiate locally into dendritic cells. cMoP give rise to circulating monocytes that survey the blood for the presence of foreign pathogens and damaged cells. Ly6Chi monocytes differentiate into Ly6Clo cells, which have been shown to control vessel integrity and to give rise to splenic marginal zone (MZ) macrophages. Ly6Chi monocytes also give rise to intestinal macrophages in the steady state and differentiate into inflammatory macrophages in most injured tissues.
Tissue-resident macrophages
All tissues have resident macrophages that perform local homeostatic functions in the steady state (16). In vivo radio-isotope-labeling studies in mice exposed to inflammatory injuries and radiation chimera experiments led to the dogma that circulating monocytes are constantly maintaining the tissue macrophage pool (17, 18). However, many observations conflict with the supposed monocyte origin of tissue-resident macrophages in the steady state. For example, the first macrophages, called primitive macrophages, appear embryonically before the development of monocytes (19). In addition, monocytopenic animals have normal tissue macrophage density (20, 21). Recently, novel fate-mapping models in the mouse have revealed that, unlike most hematopoietic cells that are constantly replenished from the bone marrow, macrophages are unique in that they are embedded in the tissue during embryonic life.
In the embryos, hematopoiesis begins in an extra-embryonic structure called the yolk sac around embryonic age E7.5. Yolk sac hematopoiesis wanes with time and is slowly replaced by new waves of hematopoiesis initiated in the embryo proper, first in the aorta–gonads–mesonephros region and later in the fetal liver (22–24). Hematopoiesis initiated in the embryo proper is called definitive hematopoiesis as opposed to yolk sac–derived primitive hematopoiesis. Fate-mapping analysis of primitive and definitive hematopoietic precursors in the embryo (25) revealed that yolk sac macrophages seed most tissue rudiment in the embryos but differentially contribute to the adult macrophage pool (F. Ginhoux and M. Merad; unpublished data). Primitive macrophages that arise in the yolk sac can maintain the homeostasis of adult brain macrophages, also called microglia, with minimal contribution from hematopoiesis that arises after E7.5 (26). Langerhans cells, the macrophage-like dendritic cells of the epidermis, are seeded initially by yolk sac macrophages. However, a second wave of fetal liver monocytes infiltrate the skin rudiment around E14.5 and almost entirely replace the yolk sac–derived macrophages for the maintenance of epidermal Langerhans cells in the adult (27). A small fraction of macrophages that derive from yolk sac precursors have also been found in several tissues of the adult mouse (28). Other tissue macrophages may be replenished by subsequent waves from the fetal liver monocytes as well, although this has yet to be shown in a fate-mapping model. Nonetheless, most macrophages in the spleen, peritoneum, liver, and lung seem to be maintained by embryonic precursors that take residence in tissues before birth, independently of adult hematopoiesis (15, 28, 29).
Similarly in humans, numerous studies have reported that tissue macrophages can still form despite strongly reduced levels of circulating monocytes (30–33). For example, in four patients with a syndrome of monocytopenia and deficiencies in dendritic cells, B lymphocytes, and natural killer cells, cutaneous macrophages were found unaffected (30). Similarly, a patient with a null mutation of the IFN response factor 8 (irf8) gene was found to have a block in monocyte and dendritic cell differentiation, but despite profound peripheral monocytopenia, macrophages were present in the lymph nodes and in the bone marrow (32). The normalcy of macrophages in these monocytopenic patients further suggests that tissue macrophages can develop in the absence of monocytes in humans.
Some populations of tissue-resident macrophages do arise from circulating bone marrow precursors. Mouse intestinal macrophages are replenished by bone marrow–derived Ly6Chi monocytes (34, 35). The kidney and uterine macrophages may also in part arise from bone marrow precursors (2, 28). The marginal zone macrophages located at the interface between splenic red and white pulp, where most arterial blood enters the spleen, also derive from circulating monocytes in response to liver X receptor (LXR) nuclear receptor signaling (36). It is unclear why some macrophages need to be maintained by circulating precursors and some do not, and whether inflammatory cues expressed in these specific sites contribute to their steady state turnover. These data emphasize the heterogeneity of the macrophage lineage and the limitations of the current classification of the mononuclear phagocyte system. A new conceptualization of the mononuclear phagocytes should include the diverse origins of tissue macrophages.
Maintenance of tissue-resident macrophages in the steady state
Local maintenance of tissue-resident macrophages is likely due to their prolonged half-life in tissues and their ability to repopulate locally. Local proliferation and survival of tissue-resident macrophages are dependent on growth factors that differ between tissues. Most tissue macrophages are dependent on Csf1 (37). However, some tissue macrophage populations remain unaffected in the Csf1op/op mouse that carries a natural null mutation of the Csf1 gene (37). Epidermal Langerhans cells and microglia among others persist in the Csf1op/op mouse and yet these cells are dependent on the receptor for Csf1 (26, 38). An alternate ligand for the Csf1 receptor, called interleukin (IL)-34, is expressed locally in the skin and brain tissues and was found recently to be critical for maintenance of these cells (39, 40). In addition to Csf1 and IL-34, granulocyte macrophage colony-stimulating factor (GM-CSF; recently renamed Csf2) is another cytokine that controls local macrophage maintenance playing an essential role in the repopulation of alveolar macrophages in the steady state (29). The cellular steps that control local macrophage repopulation remain unclear although recent studies from our laboratories revealed that tissue macrophage proliferation is not restricted to a specific compartment, suggesting that macrophages should be able to proliferate locally without relying on a dedicated progenitor population (29).
Inflammatory macrophages
In contrast with resident macrophages, infiltrating or inflammatory macrophages are found only in injured tissues, and they derive from circulating monocytes. Most tissue injuries lead to the recruitment of large numbers of monocytes and induce their local differentiation into inflammatory macrophages. Circulating monocytes provide a source of infiltrating macrophages in many pathologic settings, such as cancer (41), atherosclerosis (42), and metabolic disease (43). The interplay of bone marrow–derived inflammatory macrophages and tissue-resident macrophages has only recently started to be explored, and it seems to vary with the nature of the injury. For example, parasitic infection leads to IL-4– dependent expansion of tissue-resident macrophages (44), and influenza viral infection (29) leads not only to the depletion and subsequent repopulation of tissue-resident macrophages but also to the recruitment of infiltrating macrophages. Ionized irradiation, on the other hand, ablates tissue-resident macrophages and promotes their replacement by adult bone marrow–derived macrophages. However, even in this case, tissue-resident macrophages can be triggered to repopulate locally if adult bone marrow–derived precursors are impaired in their ability to differentiate into macrophages. X-ray irradiation does not definitively eliminate the tissue-resident macrophage pool but likely delays its repopulation potential, providing a competitive advantage to adult bone marrow–derived macrophages (29). Detailed ontogeny studies have not yet been carried out in chronic inflammatory conditions, but it is likely that a mixture of tissue-resident and monocyte-derived macrophages accumulate in chronically inflamed tissues such as in solid tumors (45). The exact contribution of tissue-resident and -infiltrating macrophages to inflammation and repair remains to be examined. Another important question will be to examine whether infiltrating macrophages can engraft in tissues and acquire resident macrophage function or whether similar to inflammatory dendritic cells they disappear once the inflammation resolves (4).
Macrophages in Homeostasis and Disease
Macrophages play a key role in organ integrity through their contribution to tissue development, homeostasis, and repair. Macrophages also take on specialized roles integral to the organ in which they reside, and their unique functions are reflected in the diversity of the tissue macrophage transcriptome.
Homeostasis
Tissue-resident macrophages play a key role in tissue formation and remodeling during development. Macrophages are especially important in ductal formation and bone structure, and mice with a mutation in Csf1 or Csf1 receptor have developmental deformities (46–48). Macrophages remove the wave of apoptotic cells during the formation of digits (49). They assist in angiogenesis and regulate blood vessel formation (50, 51).
In the adult, resident macrophages continue to play a critical role for many homeostatic functions that differ between tissues, which may explain why tissue macrophage expression profile varies between tissues (3, 52). Osteoclasts maintain bone development by constant bone resorption, and the predominant phenotype in mice that lack Csf1 or its receptor is osteopetrosis (53). Lung macrophages clear surfactant proteins in the lung, and in the absence of Csf2, which is required for macrophage maintenance and function, both mice and humans develop alveolar proteinosis (29, 54, 55). In the spleen, phagocytosis of red blood cells by red pulp macrophages controls iron levels and erythrocyte turnover (56). Microglia are important for neuronal development, and during the first few weeks of life they are involved in synaptic pruning (57, 58). In the mature brain, microglia constantly survey the surrounding area, making transient synaptic connections with their long processes so that they are able to respond rapidly to injury and ischemia (59, 60). Intestinal macrophages constantly sense commensal microbes and sample luminal antigens by projecting their dendrites through intestinal epithelial cells (61, 62). They produce anti-inflammatory cytokines (63–65) critical for the local expansion of T-regulatory cells (66). Macrophages also clear bacteria that penetrate the epithelial barrier (67) and help contain pathogens locally by preventing their systemic dissemination (68).
Developmental niches rely on macrophages for maintenance. In the bone marrow, CD169+ macrophages maintain functional erythroblasts and contribute to the maintenance of late erythroid development (69). Bone marrow macrophages expressing VCAM1 are critical for effective recovery from irradiation and bone marrow transplantation as well as acute blood loss or hemolytic anemia. Their depletion conversely helps reduce hyperactive erythron production in a polycythemia vera mouse model (69, 70). Bone marrow macrophages also maintain hematopoietic stem progenitor cell (HSPC) niches. Upon macrophage depletion, Nestin+ stromal cells that surround HSPC showed loss of many genes, such as CXCL12 and VCAM1, which are critical for HSPC maintenance and retention in the bone marrow leading to HSPC egress to the blood circulation (71). An important role may exist for macrophages in signaling and maintenance of other local stem cells niches such as in intestinal crypts (72, 73), although this has yet to be shown.
Macrophages express a wide range of cell surface and cytosolic receptors that allow them to detect tissue damage or infection (74). In the steady state, macrophages phagocytose and clear dying cells, and expression of oxidized phosphati-dylserine on the surface of apoptotic cells constitutes a major signal for phagocyte engulfment. Macrophages produce opsonins such as milk fat globule EGF 8 (MFG-E8), Del-1, and growth arrest–specific gene 6 (Gas6) that bind to phosphati-dylserine and promote engulfment through engagement of integrins or the Tyro3-Axl-Mer receptor tyrosine kinases (75–77). MFG-E8 release and the engagement of the Tyro3-Axl-Mer family receptor tyrosine kinases inhibit the induction of an innate immune response against self-antigens. Conversely, mice deficient in these pathways develop autoimmunity and persistent inflammation (77, 78). Another mechanism of regulation of macrophage phagocytosis is through CD47, the ligand of the inhibitory Sirp1a receptor on macrophages. Macrophages phagocytose red blood cells and foreign bodies that lack or downregulate this ligand (79).
Macrophages in the steady state maintain homeostasis by a wide array of housekeeping functions that clear unwanted debris and maintain the critical balance of inflammatory and tolerant signals.
Disease
Macrophages play a key role in acute inflammation following infection or tissue injury, as the classic signs of acute inflammation (rubor, calor, dolor, and tumor) can be attributed to processes they initiate (80). Macrophages recognize foreign antigens through a range of pathogen sensors that lead to the production of inflammatory cytokines and antimicrobial mediators (81). Inflammatory cytokines such as TNF-α, IL-6, and IL-1β lead to increased endothelium permeability and promote early recruitment of innate immune cells such as neutrophils and monocytes that differentiate locally into inflammatory macrophages. In parallel, resident dendritic cells leave the tissue and migrate in large numbers to the draining lymph node to initiate adaptive immune responses (4). Macrophages can also load extracellular antigen on MHC class II compartments and contribute to the differentiation of regulatory and effector CD4+ T cells. In addition to their contribution to effector immunity, macrophages are also essential in resolving inflammation by undergoing apoptosis, inducing an anti-inflammatory response, and facilitating wound-healing and repair mechanisms (16, 82).
Macrophages in cancer
As an emerging hallmark of cancer, inflammation paves the way for tumor growth, and chronic inflammation can give rise to tumors (83, 84). Solid tumors are infiltrated by a large number of immune cells among which macrophages represent the predominant population. Initially thought to play a role in antitumor immunity, studies in mice and humans have revealed the major protumorigenic role played by tumor-associated macrophages (TAM; refs. 85, 86). The protumorigenic roles of TAM result from macrophages attempting to restore tissue integrity by the promotion of an angiogenic program for tissue remodeling that also favors tumor growth, progression, and metastasis, and for the induction of immunosuppressive microenvironments that inhibit antitumor cytotoxic T-cell responses (2, 86, 87).
Macrophage phagocytosis is also important in regulating tumor progression. Despite their protumorigenic roles, deletion of macrophages before tumor induction leads to unchecked primary tumor growth and decreased survival (88, 89). Tumors upregulate CD47, the "don't eat me" signal, to inhibit phagocytosis of macrophages expressing SIRPα (90, 91), and inhibiting CD47 can increase phagocytosis of tumor cells (92). Macrophages are thus critically involved in both tumor regression and tumor spread.
Macrophage heterogeneity in tumors
The use of nonspecific cell markers as surrogates of macrophage function likely contributed to some of the confusion in our understanding of macrophage contribution to tumor outcome. Thus, it is important to avoid using single cell surface markers to identify macrophage populations and to interpret with caution those studies using suboptimal macrophage markers. In the tumor environment, in particular, precise identification of macrophage populations is necessary as many immune cells accumulate at the tumor site and in the tumor, and they can express heterogeneous markers. Identifying macrophages by CD68 alone has led to mixed assessment of the accumulation and the role of macrophage in tumors. Many studies show CD68 as correlating with tumor growth and decreased survival (85, 93). Other studies of human tumors, such as non–small cell lung carcinoma and colorectal cancer, show CD68+ cell accumulation as a good prognostic sign and correlated with increased survival (94, 95).
In addition to the heterogeneity of the population identified by using a single cell marker, the identification of a mix of pro-and anti-inflammatory signatures within the tumor microenvironment (96, 97) may reflect a spectrum of macrophage activation and responses by different macrophage populations that accumulate within the tumor. Macrophages at different stages in tumor development may act differently; the resident macrophages and monocyte-derived inflammatory macrophages may play diverse functional roles, and the nature of the tumor tissue likely drives different types of macrophage effector function locally. Better identification of macrophages at the tumor site through the use of multiple surface markers and differentiating subpopulations based on ontogeny will help clarify the roles of macrophages in cancer.
Macrophages and cancer therapy
The increased understanding of the key role played by Csf1 and its receptor in the promotion of macrophage survival and function has led to the development of novel drug targets that inhibit Csf1 receptor or its signaling in vivo. Both a small-molecule inhibitor of Csf1r phosphorylation and monoclonal antibodies to Csf1r inhibit accumulation of infiltrating macrophages in the tumor (98, 99) and in combination with VEGF receptor-2 (VEGFR-2) blockade (100), chemotherapy (101), or radiotherapy (102) help reduce tumor growth in mice (103). Studies of macrophage phagocytic receptors have led to the development of high-affinity SIRPα variant monomers that inhibit CD47 and together with tumor-specific monoclonal antibodies showed an increase in survival and tumor regression (104). Similarly, blockade of the MFG-E8 cooperates with antitumor therapy to increase apoptosis and decrease tumor size (105). The effects of blocking recruitment of infiltrating monocytes to the tumor has also been explored as a therapeutic option by many mechanisms including Ccl2 blockade in breast cancer cell lines (87), VCAM1 inhibition (106), the use of GM-CSF antagonists in a genetic model of pancreatic tumors (107, 108), and a small-molecule inhibitor in neurofibroma (109). These results suggest that modulation of the macrophage and monocyte compartment can become an important component of an antitumor therapeutic regimen.
Regulation of macrophage function
The tumor model, alongside other inflammatory processes, drives macrophage action to both tolerance and activation. Macrophages can display a spectrum of phenotypes (110), and the transcriptional response to activation is controlled by different transcription factors and epigenetics (111). During macrophage development, master transcription factors such as Pu.1 bind to many regions throughout the genome along with the more specific CAAT/enhancer binding protein α (C/EBPα). These transcription factors open the chromatin, acting as master transcription factors that determine lineage specificity and remain stable in the presence of stimuli (112– 114). These factors are important for cell type–specific differentiation; recently, Trib1 was shown to be important for the differentiation of splenic red pulp and inflammatory adipose tissue macrophages, likely mediated through altering the expression of CEBPα (115). A second level of transcription factors responds to the environment and further shapes the chromatin state. Although many genes are marked by master transcription factors as poised for activation, not all of them are transcribed. Inflammatory genes are kept in check in the steady state by binding of the transcriptional repressor Bcl-6 (116), the repressors NCoR and SMRT (117), and are associated with repressive histone marks such as H4K20me3 (118). Toll-like receptor activation induces the transcription factor NF-κB to bind to these regions and activates an inflammatory profile. However, the response to the stimuli is set during development and is cell type specific (119); for example, in fibroblasts exposed to the same stimuli as macrophages, the IL-12 locus instead of being activated remains inhibited and repressive methylation increases (120). A complex process of regulation controls the heterogeneous action of macrophages in development, inflammation, and tumor. Understanding the epigenetic level of regulation will provide further insights into how ontogeny and the environment shape the macrophage response.
Conclusions
Innate immunity comprises immune cells that are able to recognize a wide variety of stimuli and respond only after the triggering of robust inflammatory signals. Macrophages exemplify the balance that is required between tolerance and inflammation. In tumors, the dichotomy is particularly evident as self-tissue gone awry. Macrophages at these locations exhibit a combination of inflammatory and tolerance-inducing phenotypes. Abetter understanding of cellular ontogeny, tissue regulation, and epigenetics can clarify the function and response of different cell types. Ideally, this will lead to the development of more specific and effective therapeutics.
Acknowledgment of Financial or Other Support
This activity does not receive commercial support.
Footnotes
Authors' Contributions
Conception and design: Y. Lavin, M. Merad
Writing, review, and/or revision of the manuscript: Y. Lavin, M. Merad
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
CME Staff Planners' Disclosures
The members of the planning committee have no real or apparent conflict of interest to disclose.
Learning Objectives
Upon completion of this activity, the participant should acquire a basic knowledge of the heterogeneity of macrophages, their roles in homeostasis, infection, and in the maintenance of organ integrity. A better understanding of the cellular ontogeny and tissue regulation of this group of circulating and tissue-resident hematopoietic cells of myeloid origin, how they balance between promoting immune tolerance during steady state, and responding to tissue damage and inflammation during infection will lead to the development of more specific and effective therapeutics.
References
- 1.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 2.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaufmann SH. Immunology's foundation: the 100-year anniversary of the Nobel Prize to Paul Ehrlich and Elie Metchnikoff. Nat Immunol. 2008;9:705–712. doi: 10.1038/ni0708-705. [DOI] [PubMed] [Google Scholar]
- 6.van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46:845–852. [PMC free article] [PubMed] [Google Scholar]
- 7.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 8.askow C, Liu K, Darrasse-Jeze G, Guermonprez P, Ginhoux F, Merad M, et al. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008;9:676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, et al. In vivo analysis of dendritic cell development and homeo-stasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol. 2007;8:1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- 11.Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- 12.Hettinger J, Richards DM, Hansson J, Barra MM, Joschko AC, Krijgsveld J, et al. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol. 2013;14:821–830. doi: 10.1038/ni.2638. [DOI] [PubMed] [Google Scholar]
- 13.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–92. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 14.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, et al. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153:362–375. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray PJ, Wynn TA. Protective and pathogenic functions of mac-rophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Virolainen M. Hematopoietic origin of macrophages as studied by chromosome markers in mice. J Ex Med. 1968;127:943–954. doi: 10.1084/jem.127.5.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi K, Yamamura F, Naito M. Differentiation, maturation, and proliferation of macrophages in the mouse yolk sac: a light-microscopic, enzyme-cytochemical, immunohistochemical, and ultra-structural study. J Leukoc Biol. 1989;45:87–96. doi: 10.1002/jlb.45.2.87. [DOI] [PubMed] [Google Scholar]
- 20.Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, et al. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci U S A. 1997;94:12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi K. Development and differentiation of macrophages and related cells: historical review and current concepts. J Clin Exp Hematop. 2001;41:1–31. [Google Scholar]
- 22.Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DYR, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cumano A, Godin I. Ontogeny of the hematopoietic system. Annu Rev Immunol. 2007;25:745–785. doi: 10.1146/annurev.immunol.25.022106.141538. [DOI] [PubMed] [Google Scholar]
- 24.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446:1056–1061. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- 26.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. 2012;209:1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bigley V, Haniffa M, Doulatov S, Wang XN, Dickinson R, McGovern N, et al. The human syndrome of dendritic cell, monocyte, B and NK lymphoid deficiency. J Exp Med. 2011;208:227–234. doi: 10.1084/jem.20101459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emile JF, Geissmann F, Martin OC, Radford-Weiss I, Lepelletier Y, Heymer B, et al. Langerhans cell deficiency in reticular dysgenesis. Blood. 2000;96:58–62. [PubMed] [Google Scholar]
- 32.Hambleton S, Salem S, Bustamante J, Bigley V, Boisson-Dupuis S, Azevedo J, et al. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med. 2011;365:127–138. doi: 10.1056/NEJMoa1100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinh DC, Patel SY, Uzel G, Anderson VL, Freeman AF, Olivier KN, et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. 2010;115:1519–1529. doi: 10.1182/blood-2009-03-208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 36.A-Gonzalez N, Guillen JA, Gallardo G, Diaz M, de la Rosa JV, Hernandez IH, et al. The nuclear receptor LXRalpha controls the functional specialization of splenic macrophages. Nat Immunol. 2013;14:831–839. doi: 10.1038/ni.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cecchini MG, Dominguez MG, Mocci S, Wetterwald A, Felix R, Fleisch H, et al. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- 38.Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, et al. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity. 2012;37:1050–1060. doi: 10.1016/j.immuni.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ingersoll MA, Platt AM, Potteaux S, Randolph GJ. Monocyte trafficking in acute and chronic inflammation. Trends Immunol. 2011;32:470–477. doi: 10.1016/j.it.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sawanobori Y, Ueha S, Kurachi M, Shimaoka T, Talmadge JE, Abe J, et al. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood. 2008;111:5457–5466. doi: 10.1182/blood-2008-01-136895. [DOI] [PubMed] [Google Scholar]
- 46.Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- 47.Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–2282. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- 48.Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW, Jr, Ahmed-Ansari A, Sell KW, Pollard JW, et al. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990;87:4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hopkinson-Woolley J, Hughes D, Gordon S, Martin P. Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. J Cell Sci. 1994;107(Pt 5):1159–1167. doi: 10.1242/jcs.107.5.1159. [DOI] [PubMed] [Google Scholar]
- 50.Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, et al. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437:417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stefater JAIII, Lewkowich I, Rao S, Mariggi G, Carpenter AC, Burr AR, et al. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature. 2011;474:511–515. doi: 10.1038/nature10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35:323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, et al. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 54.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, et al. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci U S A. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med. 2003;349:2527–2539. doi: 10.1056/NEJMra023226. [DOI] [PubMed] [Google Scholar]
- 56.Kohyama M, Ise W, Edelson BT, Wilker PR, Hildner K, Mejia C, et al. Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature. 2009;457:318–321. doi: 10.1038/nature07472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 58.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 60.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 62.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 63.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 64.Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37:1076–1090. doi: 10.1016/j.immuni.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 66.Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, et al. Intestinal tolerance requires gut homing and expansion of FoxP3þ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 67.Hapfelmeier S, Muller AJ, Stecher B, Kaiser P, Barthel M, Endt K, et al. Microbe sampling by mucosal dendritic cells is a discrete, MyD88-independent step in DeltainvG S. Typhimurium colitis. J Exp Med. 2008;205:437–450. doi: 10.1084/jem.20070633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diehl GE, Longman RS, Zhang JX, Breart B, Galan C, Cuesta A, et al. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature. 2013;494:116–120. doi: 10.1038/nature11809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chow A, Huggins M, Ahmed J, Hashimoto D, Lucas D, Kunisaki Y, et al. CD169(+) macrophages provide a niche promoting erythro-poiesis under homeostasis and stress. Nat Med. 2013;19:429–436. doi: 10.1038/nm.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramos P, Casu C, Gardenghi S, Breda L, Crielaard BJ, Guy E, et al. Macrophages support pathological erythropoiesis in polycythemia vera and beta-thalassemia. Nat Med. 2013;19:437–445. doi: 10.1038/nm.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, et al. Bone marrow CD169 +macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci U S A. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 75.Hanayama R, Tanaka M, Miwa K, Nagata S. Expression of developmental endothelial locus-1 in a subset of macrophages for engulf-ment of apoptotic cells. J Immunol. 2004a;172:3876–3882. doi: 10.4049/jimmunol.172.6.3876. [DOI] [PubMed] [Google Scholar]
- 76.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 77.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004b;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 79.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 80.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 81.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 82.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 83.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 84.Mantovani A. Cancer: inflaming metastasis. Nature. 2009;457:36–37. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 85.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 86.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dace DS, Chen PW, Niederkorn JY. CD4+ T-cell-dependent tumour rejection in an immune-privileged environment requires macro-phages. Immunology. 2008;123:367–377. doi: 10.1111/j.1365-2567.2007.02700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oosterling SJ, van der Bij GJ, Meijer GA, Tuk CW, van Garderen E, van Rooijen N, et al. Macrophages direct tumour histology and clinical outcome in a colon cancer model. J Pathol. 2005;207:147–155. doi: 10.1002/path.1830. [DOI] [PubMed] [Google Scholar]
- 90.Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med. 2010;362:875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13:1472–1479. doi: 10.1158/1078-0432.CCR-06-2073. [DOI] [PubMed] [Google Scholar]
- 95.Kim DW, Min HS, Lee KH, Kim YJ, Oh DY, Jeon YK, et al. High tumour islet macrophage infiltration correlates with improved patient survival but not with EGFR mutations, gene copy number or protein expression in resected non-small cell lung cancer. Br J Cancer. 2008;98:1118–1124. doi: 10.1038/sj.bjc.6604256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation) Blood. 2006;107:2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 97.Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 98.MacDonald KP, Palmer JS, Cronau S, Seppanen E, Olver S, Raffelt NC, et al. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood. 2010;116:3955–3963. doi: 10.1182/blood-2010-02-266296. [DOI] [PubMed] [Google Scholar]
- 99.Priceman SJ, Sung JL, Shaposhnik Z, Burton JB, Torres-Collado AX, Moughon DL, et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood. 2010;115:1461–1471. doi: 10.1182/blood-2009-08-237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M, et al. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med. 2009;206:1089–1102. doi: 10.1084/jem.20081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu J, Escamilla J, Mok S, David J, Priceman S, West B, et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weiskopf K, Ring AM, Ho CC, Volkmer JP, Levin AM, Volkmer AK, et al. Engineered SIRPalpha variants as immunotherapeutic adjuvants to anticancer antibodies. Science. 2013;341:88–91. doi: 10.1126/science.1238856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jinushi M, Sato M, Kanamoto A, Itoh A, Nagai S, Koyasu S, et al. Milk fat globule epidermal growth factor-8 blockade triggers tumor destruction through coordinated cell-autonomous and immune-mediated mechanisms. J Exp Med. 2009;206:1317–1326. doi: 10.1084/jem.20082614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ferjancic S, Gil-Bernabe AM, Hill SA, Allen PD, Richardson P, Sparey T, et al. VCAM-1 and VAP-1 recruit myeloid cells that promote pulmonary metastasis in mice. Blood. 2013;121:3289–3297. doi: 10.1182/blood-2012-08-449819. [DOI] [PubMed] [Google Scholar]
- 107.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822–835. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836–847. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Prada CE, Jousma E, Rizvi TA, Wu J, Dunn RS, Mayes DA, et al. Neurofibroma-associated macrophages play roles in tumor growth and response to pharmacological inhibition. Acta Neuropathol. 2013;125:159–168. doi: 10.1007/s00401-012-1056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Smale ST. Selective transcription in response to an inflammatory stimulus. Cell. 2010;140:833–844. doi: 10.1016/j.cell.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Garber M, Yosef N, Goren A, Raychowdhury R, Thielke A, Guttman M, et al. A high-throughput chromatin immunoprecipitation approach reveals principles of dynamic gene regulation in mammals. Mol Cell. 2012;47:810–822. doi: 10.1016/j.molcel.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ghisletti S, Barozzi I, Mietton F, Polletti S, De Santa F, Venturini E, et al. Identification and characterization of enhancers controlling the inflammatory gene expression program in macrophages. Immunity. 2010;32:317–328. doi: 10.1016/j.immuni.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 114.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, et al. Simple combinations of lineage-determining transcription factors prime cisregulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Satoh T, Kidoya H, Naito H, Yamamoto M, Takemura N, Nakagawa K, et al. Critical role of Trib1 in differentiation of tissue-resident M2-like macrophages. Nature. 2013;495:524–528. doi: 10.1038/nature11930. [DOI] [PubMed] [Google Scholar]
- 116.Barish GD, Yu RT, Karunasiri M, Ocampo CB, Dixon J, Benner C, et al. Bcl-6 and NF-kappa B cistromes mediate opposing regulation of the innate immune response. Genes Dev. 2010;24:2760–2765. doi: 10.1101/gad.1998010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ghisletti S, Huang W, Jepsen K, Benner C, Hardiman G, Rosenfeld MG, et al. Cooperative NCoR/SMRT interactions establish a core-pressor-based strategy for integration of inflammatory and anti-inflammatory signaling pathways. Genes Dev. 2009;23:681–693. doi: 10.1101/gad.1773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stender JD, Pascual G, Liu W, Kaikkonen MU, Do K, Spann NJ, et al. Control of proinflammatory gene programs by regulated trimethylation and demethylation of histone H4K20. Mol Cell. 2012;48:28–38. doi: 10.1016/j.molcel.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Natoli G. Maintaining cell identity through global control of genomic organization. Immunity. 2010;33:12–24. doi: 10.1016/j.immuni.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 120.Zhu Y, van Essen D, Saccani S. Cell-type-specific control of enhancer activity by H3K9 trimethylation. Mol Cell. 2012;46:408–423. doi: 10.1016/j.molcel.2012.05.011. [DOI] [PubMed] [Google Scholar]