Abstract
Purpose/Aim
Klebsiella pneumoniae causes a blinding infection called endogenous endophthalmitis. The role of innate immune recognition of K. pneumoniae in the eye during infection is not known. We hypothesized that intraocular recognition of K. pneumoniae was mediated by TLR4 and may be dependent on MagA-regulated hypermucoviscosity.
Materials and Methods
Experimental endophthalmitis was induced in C57BL/6J or TLR4−/− mice by intravitreal injection of 100 CFU of wild type or ΔmagA K. pneumoniae. Infection and inflammation were quantified by determining viable K. pneumoniae per eye, retinal responses via electroretinography, myeloperoxidase activity of infiltrating neutrophils, and the proinflammatory cytokine and chemokine response.
Results
C57BL/6J and TLR4−/− mice could not control intraocular wild type K. pneumoniae growth. TLR4−/− mice were less able than C57BL/6J to control the intraocular growth of ΔmagA K. pneumoniae. Retinal function testing suggested that infection with ΔmagA K. pneumoniae resulted in less retinal function loss. There was a TLR4-dependent delay in initial neutrophil recruitment, regardless of the infecting organism. The proinflammatory cytokine/chemokine data supported these results. These findings were not due to an inability of TLR4−/− neutrophils to recognize or kill K. pneumoniae.
Conclusions
These studies suggest that TLR4 is important in the early intraocular recognition and host response to K. pneumoniae. However, the role of MagA in TLR4-mediated intraocular recognition and subsequent inflammation is less clear.
Keywords: Toll-like receptor 4 (TLR4), Klebsiella pneumoniae, endophthalmitis, hypermucoviscosity, eye
INTRODUCTION
Klebsiella pneumoniae is a Gram negative opportunistic pathogen capable of infecting various tissues, including the eye, liver, central nervous system (CNS), bladder, and lungs [1]. In the Far East, K. pneumoniae is recognized as the major etiologic agent of community acquired liver abscesses [2] which may progress into metastatic infections such as meningitis or endophthalmitis [3]. K. pneumoniae isolates of the K1 capsule type cause invasive disease/liver abscesses and have a hypermucoviscous phenotype (HMV). This phenotype is associated with the putative K1 capsule polymerase, mucoviscosity associated gene A (MagA/WzyKpK1) [4], which is presumably responsible for joining precursor carbohydrates and forming higher ordered capsule polymers. Strains which have mutated or non-functional magA demonstrate decreased resistance to serum killing and phagocytosis, rendering these strains highly attenuated in virulence [4].
Endogenous endophthalmitis is a particularly devastating, invasive sequelae of K. pneumoniae liver abscesses. Although the mechanisms are unclear, the bacteria likely circumvent immune and ocular barriers and infect the posterior segment of the eye. The consequences of K. pneumoniae endophthalmitis are severe, as the majority of infected eyes lose significant vision [5, 6]. Despite aggressive antibiotic and anti-inflammatory treatment, blindness is not uncommon in endogenous K. pneumoniae endophthalmitis. The most frequently reported underlying disease states associated with K. pneumoniae liver abscess are biliary tract disease and diabetes [7]. Septic spread to ocular and CNS tissues has been reported to occur in as many as 13% of K. pneumoniae liver abscess patients [7]. Additionally, the septic spread to the eye occurs without ocular surgery or underlying trauma. Recent reports of complete preservation of vision following early intervention in patients with ocular symptoms and suspected liver abscess highlight the necessity for prompt identification and aggressive treatment in such cases [8].
We have demonstrated that in experimental endophthalmitis, K. pneumoniae is highly inflammatory and causes near complete visual function loss within 24 hours [9, 10]. Unlike other endophthalmitis pathogens such as Staphylococcus aureus [12], Enterococcus faecalis [12], or Bacillus cereus [13], which also cause significant loss of vision, K. pneumoniae does not produce membrane damaging toxins such as hemolysins, proteases, or lipases. However, K. pneumoniae produces lipopolysaccharide (LPS), which can be sensed by the innate immune receptor Toll-like receptor 4 (TLR4). In disease models, TLR4 deficiency renders the host less able to respond to infection by many pathogens [14–16]. However, it has recently been reported that HMV+ K. pneumoniae are masked from TLR4 recognition by their capsule [17]. The implications of this phenomenon in vivo are unclear, especially in the ocular environment which is classically described as immune-privileged. Functional TLR4 has been described in human cultured retinal pigment epithelium [18] and others have implicated loss of TLR4 in protection from inflammation in retinal ischemia/reperfusion injury [19]. We found it interesting that a ΔmagA strain was more inflammatory in experimental endophthalmitis than its parental wild type strain, but at much lower bacterial loads [9] and hypothesized that this observation may be due to differential sensing of the two bacterial phenotypes by TLR4. Thus, we investigated the role of TLR4 in experimental K. pneumoniae endophthalmitis.
MATERIALS AND METHODS
Ethics Statement
These experiments involved the use of mice. All procedures were carried out in strict accordance with the recommendations in the Guide for Use of Laboratory Animals of the National Institutes of Health, institutional guidelines set forth by the University of Oklahoma Health Sciences Center IACUC (approved protocol 10-154-I), and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Bacterial Strains and Infection
K. pneumoniae clinical isolate NTUHK2044 and the isogenic ΔmagA mutant have been previously described [4] and have been used to initiate experimental K. pneumoniae endophthalmitis [9]. Although K. pneumoniae endophthalmitis is typically of endogenous origin, this direct intravitreal injection model reduces potentially confounding factors associated with systemic infection or other underlying disease [20], facilitating the direct and reproducible evaluation of infection once the organisms have reached the intraocular environment.
Animals
Toll-like receptor 4 deficient (TLR4−/−) mice were a generous gift from Dr. Eric Pearlman (Department of Ophthalmology and Visual Sciences, Case Western Reserve University, Cleveland, Ohio) and were used with the permission of Dr. Shizou Akira [21] (Department of Biochemistry, Hyogo College of Medicine, Hyogo, Japan). C57BL/6J Mice were purchased from commercially available colonies (Stock No. 000664, The Jackson Laboratory, Bar Harbor, Maine). Following rederivation, TLR4−/− mice were bred on the C57BL/6J background and maintained in-house on a 12 hour on/12 hour off light cycle under microisolation conditions. All animals were acclimated to conventional housing after arrival/weaning for at least 2 weeks and were used in experiments at 8–10 weeks of age.
Bacterial Quantitation of Ocular Samples
Whole globes were harvested and placed in 400 μl of PBS containing 1mm glass beads (Propper Manufacturing Co. Inc., Long Island City, New York) and homogenized in a mini-beadbeater (BioSpec Products, Inc., Bartlesville, Oklahoma) at approximately 2,800 oscillations/minute for one minute. Samples were kept on ice until bacterial colony forming units (CFU) were quantified by serial dilution [9].
Quantitation of Myeloperoxidase in Ocular Samples
Samples from the above CFU determination were stored at −80°C until myeloperoxidase (MPO) abundance was quantified by ELISA, according to the manufacturer’s recommendations (Hycult Biotech, Uden, The Netherlands) [10]. The lower limit of detection for this assay was 1.6 ng/eye.
Electroretinography
Scotopic electroretinography was performed to determine the extent of retinal response to a light stimulus, as described previously [9, 10]. Briefly, mice were dark adapted for at least 6 hours, thus only mice that were infected for 6 or more hours could be assessed by electroretinography (ERG). Mice were anesthetized as referenced above, and pupils were dilated with topical phenylephrine (10%; Akorn, Inc., Buffalo Grove, Illinois). Gold wire electrodes recorded voltage differences between each cornea and an oral reference electrode in response to a 10 msec flash of white light. The average of 5 flashes (spaced 60 seconds apart) was used for analysis. Retinal function was reported as percent of function in the infected eye compared to the contralateral non-infected or mock injected eye, as previously described [9, 10]. The A-wave amplitude was calculated as the absolute value of the difference between baseline voltage and the trough of A-wave voltage. The B-wave amplitude was calculated as the absolute value of the difference between the trough of the A-wave and maximum B-wave voltages [9, 10].
Histology of Ocular Samples
Whole globes were harvested and fixed in Excalibur’s Alcoholic Z-Fix for 24 hours then embedded in paraffin. Sections were cut and stained with hematoxylin/eosin (Excalibur Pathology, Moore, Oklahoma), as described previously [9, 10].
Neutrophil Phagocytosis and Killing Assay
Murine neutrophils were isolated essentially as described by Luo and Dorf [22], except that Lymphoprep was substituted as the density separating medium. A single assay was used to measure phagocytosis and bacterial killing [23]. Bacterial strains were incubated in PBS containing 10% heat-inactivated C57BL/6J mouse serum (Valley Biomedical Products, Winchester, Virginia) at 37°C with rotation (12 rpm) for 20 minutes. Neutrophils were suspended in PBS + 10% serum and incubated at 37°C for 10 minutes. The bacteria and neutrophil suspensions were mixed (1:1). At various time points, samples were removed from the reaction and placed on ice. Samples were centrifuged and pellets washed 3 times with ice cold PBS. All wash supernatants were pooled. Neutrophil pellets were lysed with ice cold PBS + 0.5% saponin (Sigma-Aldrich, St. Louis, Missouri). Bacteria were enumerated and compared to the input to determine the percent of live extracellular or cell-associated bacteria.
Measurement of Cytokines and Chemokines by ELISA
Ocular homogenates (see above) were subjected to ELISA. The concentrations of keratinocyte chemoattractant (KC/CXCL1), macrophage inflammatory protein 1 alpha (MIP1α), and tumor necrosis factor alpha (TNFα) were measured in homogenates according to the manufacturer’s instructions (R&D Systems, Minneapolis, Minnesota) [24]. The lower limits of detection for each assay were: KC, 0.8 pg/eye; MIP1α, 1.5 pg/eye; TNFα, 0.75 pg/eye.
Real Time of TLR4
Real time PCR was performed on RNA isolated from the retinas of C57BL/6J mice infected with K. pneumoniae using standard procedures [24]. Data was collected using a BioRad C1000 Touch Thermal Cycler, CFX96 Real-Time System using primers for TLR4 (TLR4F: CTG CGT GAG ACC AGA AAG C, TLR4R: ATT AAG GTA GAG AGG TGG CTT AGG) and beta-actin (BAF: CTT CTA CAA TGA GGC TGC GTG TG, BAR: TTG AAG GTC TCA AAC ATG ATC TGG). Each sample was normalized to beta-actin expression and compared to the amount of TLR4 transcript at 0 hours to PBS control injected eyes. Data is reported as fold difference (2ΔΔCT) for n=3 eyes per group per time point.
Statistics and Estimation of Values below the Limit of Detection
For all in vitro and in vivo analyses, two-tailed, two-sample t-tests assuming equal variance were used to statistically compare groups. A p value of ≤ 0.05 was considered significant. For ELISAs, when undiluted samples were below the limit of detection (LOD) for a given cytokine assay, the values were replaced using the formula: [25].
RESULTS
Containing Intraocular Growth of K. pneumoniae
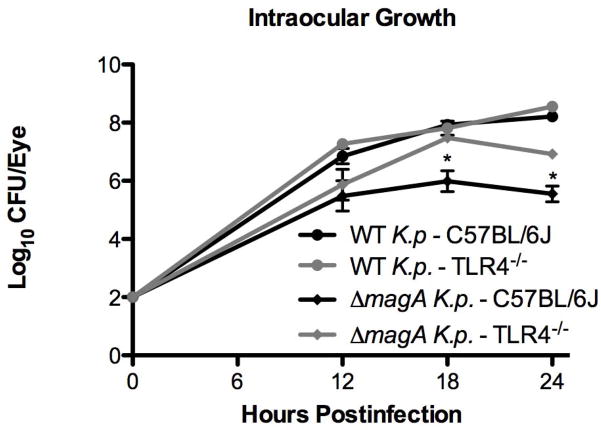
Wild type C57BL/6J or TLR4−/− mice were infected with wild type or ΔmagA K. pneumoniae by intravitreal injection. In our previous study [9], significant inflammation, as measured by detection of myeloperoxidase, was not detected in ΔmagA infected eyes of C57BL/6J mice until 12 hours postinfection. Additionally, histology showed that at 12 hours postinfection, eyes of C57BL/6J mice infected with any Klebsiella strain displayed minimal, if any, signs of inflammation. Therefore, 12 hours was set as the earliest time point for this study. At 12 hours postinfection, wild type K. pneumoniae grew to 6.85 ± 0.26 log10 CFU/eye in wild type mice and to a density of 7.27 ± 0.08 log10 CFU/eye in TLR4−/− mice (Figure 1). However, this was not a statistically significant difference (p = 0.20). This trend continued at 18 hours when bacterial loads in the eye were 7.93 ± 0.03 and 7.81 ± 0.24 log10 CFU/eye for wild type and TLR4−/− mice, respectively (p = 0.60) (Figure 1). At 24 hours, bacteria had grown to 8.21 ± 0.08 log10 CFU/eye in wild type mice and 8.55 ± 0.13 log10 CFU/eye in TLR4−/− mice (p=0.16). As the bacterial loads in the eyes of wild type and TLR4−/− mice were not statistically different at any time point, the data suggest that the loss of TLR4 signaling did not affect the growth of wild type K. pneumoniae in the eye.
Figure 1. Comparison of Intraocular Bacterial Growth in C57BL/6J and TLR4-Deficient Mice.
C57BL/6J or TLR4−/− mice were infected with either wild type or ΔmagA K. pneumoniae. At the indicated time points, eyes were harvested for bacterial counts. n≥5 eyes per time point. *;p<0.01 for C57BL/6J ΔmagA vs. TLR4−/− ΔmagA.
When mice were infected with the ΔmagA strain, there was no difference in the intraocular bacterial loads at 12 hours between C57BL/6J and TLR4−/− mice (Figure 1) (5.48 ± 0.52 vs. 5.87 ± 0.53 log10 CFU/eye, respectively [p= 0.61]). At 18 hours postinfection, there was a significant difference in bacterial loads, with bacteria in C57BL/6J mice growing to 5.99 ± 0.36 log10 CFU/eye while TLR4−/− mice allowed the bacteria to reach 7.48 ± 0.16 log10 CFU/eye (p = 0.002). While there was a decline in the number of ΔmagA bacteria recovered from the eyes in both mouse strains between 18 and 24 hours, this difference in bacterial loads between mouse strains was maintained at 24 hours postinfection (5.55 ± 0.27 vs. 6.92 ± 0.06 log10 CFU/eye for wild type and TLR4−/− mice, respectively (p = 5.10×10−5). In contrast to what was seen above for wild type bacteria, ΔmagA bacteria grew to a higher density in the eye when the host was deficient in TLR4. These results suggest that TLR4−/− mice were less able to control the growth of ΔmagA bacteria in the vitreous compared to wild type C57BL/6J mice.
Loss of Retinal Response is Independent of TLR4
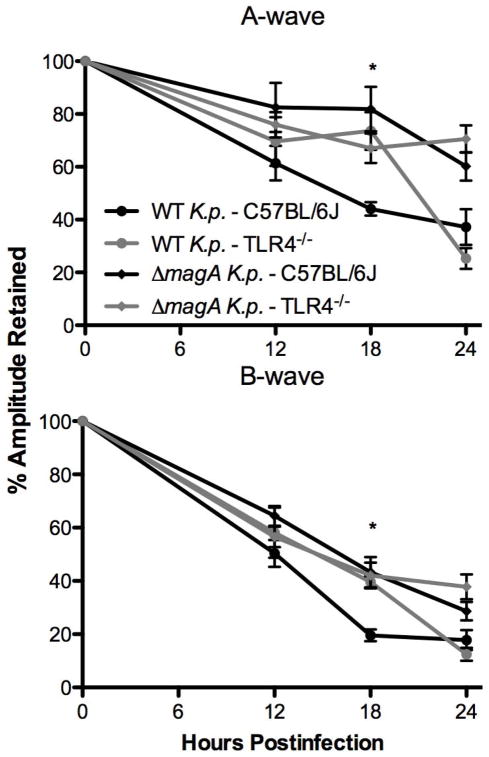
A-Wave
The retinal responses of mice were evaluated by electroretinography. When mice were infected by wild type K. pneumoniae, there was no difference in the percent of A-wave amplitude retained at 12 hours between C57BL/6J or TLR4−/− mice (Figure 2) (61.4% vs. 69.6%, respectively [p = 0.47]). At 18 hours postinfection there was a significant difference in retained A-wave amplitudes. Wild type mice retained 44.1% A-wave amplitude, while TLR4−/− mice retained 73.6% A-wave amplitude (p = 0.003). By 24 hours, the A-wave amplitudes of TLR4−/− mice had significantly deteriorated, resulting in no difference in retained amplitudes (37.2% vs. 25.3% for wild type vs. TLR4−/−, respectively [p = 0.13]). However when ΔmagA bacteria were used to infect mice, there was no difference in the amount of retained A-wave amplitude at any time point, regardless of the presence of functional TLR4 in the host (Figure 2).
Figure 2. Evaluation of Retinal Function.
After infection with K. pneumoniae, mice were dark adapted for at least 6 hours. At the indicated time points, mice were anesthetized as described above and subjected to electroretinography. The average of n>6 eyes ± SEM is shown for each time point. *;p≤0.01 for C57BL/6J –wild type K. pneumoniae. vs. TLR4−/−–wild type K. pneumoniae
B-Wave
In general, percent B-wave amplitudes declined faster than did A-wave amplitudes regardless of mouse or bacterial strain. The phenomenon of the B-wave declining at a greater rate than the A-wave has been described before in B. cereus [27] and K. pneumoniae endophthalmitis [9, 10]. Retinal B-wave amplitudes of mice infected with wild type bacteria declined to 50.4% and 58.1% for C57BL/6J and TLR4−/−, respectively by 12 hours postinfection (p = 0.45) (Figure 2). However, at 18 hours, there was a significant difference between B-wave amplitudes retained in the C57BL/6J and TLR4−/− groups. B-wave amplitudes declined to 19.6% in wild type mice whereas the TLR4−/− mice retained 39.5% B-wave amplitude (p = 0.01). At 24 hours postinfection, there was significant loss of B-wave amplitude in eyes of TLR4−/− mice, resulting in no difference in retained B-wave in TLR4−/− mice compared to C57BL/6J mice (17.8% vs. 12.45%; wild type vs. TLR4−/−, respectively [p = 0.23]). Just as for the A-wave, ERGs of mice which were infected with the ΔmagA strain declined at similar rates that were not statistically different, independent of the mouse strain (Figure 2). Taken together, these results agreed with previous findings [10] that infection with ΔmagA K. pneumoniae resulted in less retinal function loss. However, retinal function loss from infection with wild type or ΔmagA K. pneumoniae was independent of TLR4.
TLR4-Dependent Delay in Inflammation
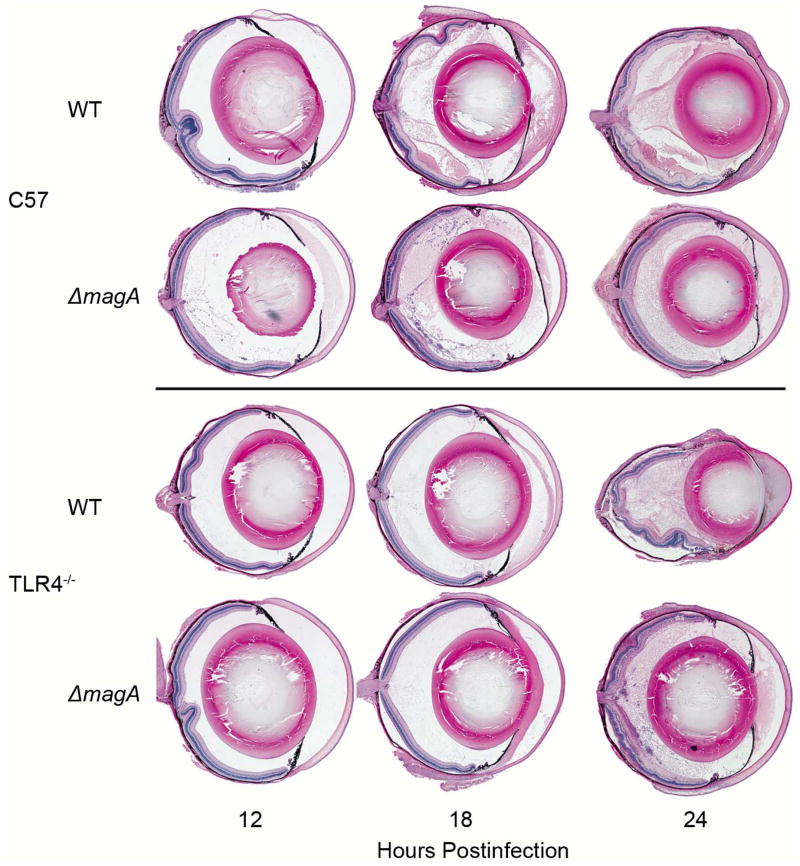
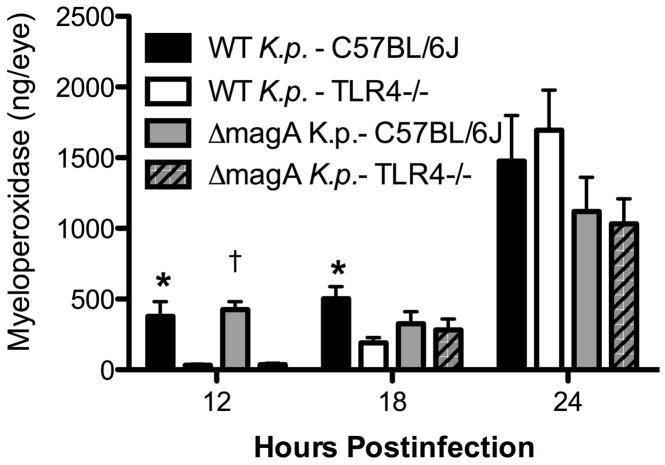
Whole eye histology is shown in Figure 3. As noted in the previous study [9], C57BL/6J mice infected with either wild type or ΔmagA bacteria do not exhibit significant inflammation until between 12 and 18 hours. C57BL/6J mice infected with the ΔmagA strain exhibited minimal fibrin deposition and cellular infiltrates in the vitreous at 12 hours which increased over the next 12 hours. However, the structure of the retina remained largely intact and uniform. Similarly, C57BL/6J mice infected with the wild type strain showed minimal fibrin and cellular infiltration into the vitreous at 12 hours, an effect which increased until 24 hours. Unlike ΔmagA-infected eyes, wild type K. pneumoniae-infected eyes exhibited significant inflammation of the retina, which was depicted by retinal folding and edema (24 hours) (Figures 3 and 4). Myeloperoxidase (MPO) was readily detected in the homogenates of infected eyes (Figure 5). Eyes from TLR4−/− mice contained lower levels of MPO in response to wild type K. pneumoniae than C57BL/6J mice at 12 hours (377.7 ± 105.5 ng/eye C57 vs. 32.2 ± 8.7 ng/eye TLR4−/−; p=0.03092) and 18 hours (503.7 ± 83.8 ng/eye C57 vs. 191.5 ± 37.6 ng/eye TLR4−/−; p=0.01146) postinfection. However, there was no difference in the amount of MPO detected between the two mouse strains at 24 hours postinfection (1475.9 ± 322.6 ng/eye C57 vs. 1695.8 ± 281.5 ng/eye TLR4−/−; p=0.9774). When eyes were infected with ΔmagA K. pneumoniae, TLR4−/− eyes contained lower levels of MPO at 12 hours (426.4 ± 56.8 ng/eye C57 vs. 36.2 ± 10.1 ng/eye TLR4−/−; p<0.0001). There was no difference in the levels of MPO in TLR4−/− or C57BL/6J eyes at 18 hours (326.7 ± 84.7 ng/eye C57 vs. 283.0 ± 76.8 ng/eye TLR4−/−; p=0.8544) or 24 hours (1119.7 ± 241.5 ng/eye C57 vs. 1032.4 ± 176.2 ng/eye TLR4−/−; p=0.5506). This suggested that neutrophil recruitment was a TLR4-dependent, MagA-independent process. There was an approximately 6-hour delay in TLR4-dependent recruitment of neutrophils when mice were infected with either strain of K. pneumoniae. Cells with multi-lobed nuclei could be seen in the vitreous and retina, indicating that these cells had indeed infiltrated the eye and were not merely circulating neutrophils in the retinal and/or choroidal vasculature (Figure 4).
Figure 3. Ocular Histology of C57BL/6J and TLR4−/− Mice during Experimental K. pneumoniae Endophthalmitis.
At the indicated time points postinfection, mice were euthanized and whole globes were harvested, fixed, sectioned and stained with hematoxylin and eosin as described above. Each eye is representative of an n≥3, except the 24 hour time point for wild type K. pneumoniae in C57BL/6J mice. At this time point only one eye had not undergone pthisis and could be removed intact.
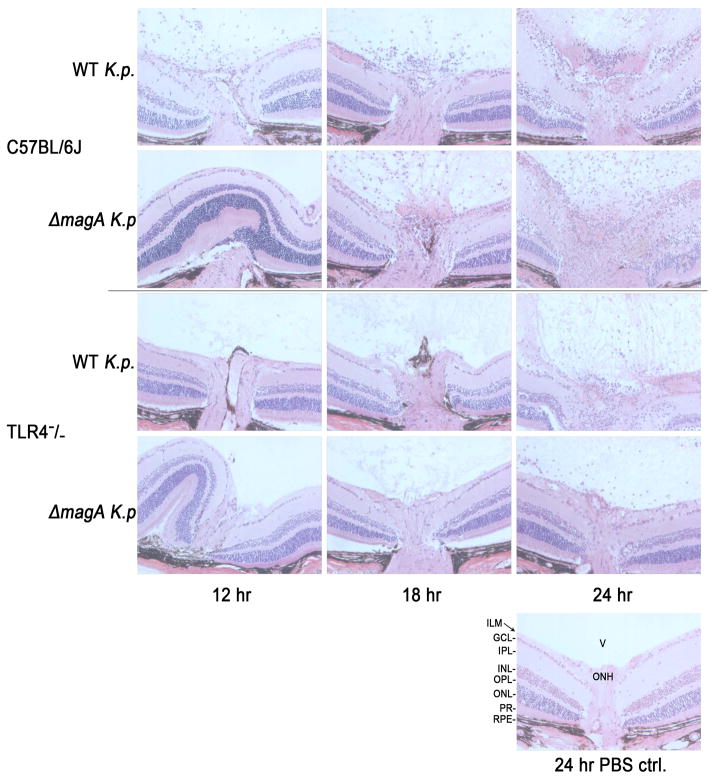
Figure 4. Comparison of Retinal Histology During Experimental K. pneumoniae Endophthalmitis.
Sections of C57BL/6J and TLR4−/− eyes infected with wild type or ΔmagA K. pneumoniae were stained with hematoxylin and eosin. There was a marked delay in the infiltration of immune cells into the vitreous of C57BL/6J mice infected with the ΔmagA strain as well as in TLR4−/− mice infected with either strain. Retinal folding and thickening of the inner plexiform layer can be seen in wild type-infected retinas. RPE; retinal pigment epithelium, PR; photoreceptors, ONL; outer nuclear layer, OPL; outer plexiform layer, INL; inner nuclear layer, IPL; inner plexiform layer, GCL; ganglion cell layer, ILM; inner limiting membrane. All sections are 20x magnification.
Figure 5. Neutrophil Recruitment to the Eye is Delayed in TLR4−/− mice.
At the indicated time points postinfection, mice were euthanized and whole globes were harvested, homogenized in PBS and used to determine the amount MPO present in the sample by ELISA. *;p≤0.05.
When TLR4−/− mice were infected, there was a noticeable difference in the timing of cellular infiltration into the eye (Figure 4). Whereas many cellular infiltrates can be seen in the eyes of C57BL/6J mice at 12 and 18 hours, little to no infiltrating cells were seen at this time point in TLR4−/− infected mouse eyes. Between 18 and 24 hours, TLR4−/− infected eyes began to show significant infiltration of cells into the vitreous. Again, multi-lobed cells were observed in both the retina and vitreous of infected eyes (Figure 4). However, these cells were not observed in great numbers until 24 hours (Figure 4). These results indicate that in TLR4−/− mice, inflammatory cell recruitment was delayed. No pathology was observed in the eyes of mice mock-injected with PBS (Figure 4).
TLR4-Dependent Delay in Cytokine/Chemokine Production
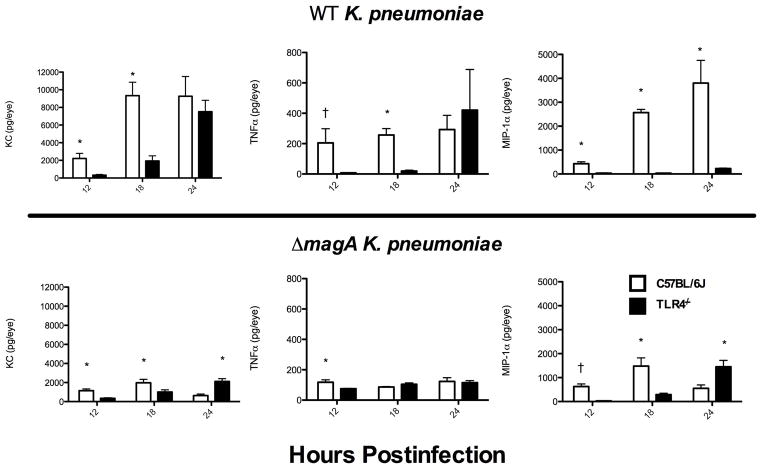
To investigate the possibility that differential expression of inflammatory cytokines may be responsible for recruiting neutrophils to the vitreous after infection, wild type and ΔmagA K. pneumoniae were used to infect C57BL/6J and TLR4−/− mice. The concentration of KC (CXCL1) and TNFα were assayed by ELISA (Figure 6). Eyes from C57BL/6J mice infected with wild type bacteria contained approximately 6.7 times more KC at 12 hours and 4.8 times more KC at 18 hours than TLR4−/− mice (2219 ± 578 pg/eye vs. 328 ± 81 pg/eye [p=0.000217] and 9,336 ± 1,523 pg/eye vs. 1,937 ± 573 pg/eye [p=0.00264] for C57BL/6J and TLR4−/− at 12 and 18 hours, respectively). By 24 hours, preliminary data suggests that there was no difference in the amount of KC present in eyes of C57BL/6J and TLR4−/− mice (9,268 pg/eye vs. 7,516 ± 1,290 pg/eye). While there were not enough intact C57BL/6J eyes at 24 hours to assay for KC and compare with statistics, there did not appear to be an increase over the levels seen at 18 hours. Additionally, when the C57BL/6J 18 hour levels are compared to TLR4−/− 24 hour levels, there was no statistical difference in the amount of KC present (p=0.10). This suggested that TLR4−/− mice exhibited approximately an 8 hour lag in the “maximum KC response” that was seen in C57BL/6J mice. When mice were infected with ΔmagA K. pneumoniae, a similar trend toward a diminished response in the TLR4−/− animals was noticed, although the responses were less than that observed in mice challenged with wild type bacteria (1,136 ± 180 pg/eye vs. 343 ± 58 pg/eye [p=0.0016] and 1,962 ± 364 pg/eye vs. 1,010 ± 225 pg/eye [p=0.042] for C57BL/6J vs. TLR4−/− at 12 and 18 hours, respectively) (Figure 6). However, at 24 hours, there was significantly more KC in the eyes of TLR4−/− (621 ± 166 pg/eye vs. 2,119 ± 281 pg/eye [p=0.0007]), indicating that lack of TLR4 did not prevent robust expression of KC at later times postinfection.
Figure 6. Delay in Proinflammatory Cytokine Production in TLR4−/− Mice.
C57BL/6J or TLR4−/− mice were infected with wild type or ΔmagA K. pneumoniae and eyes were assayed for KC, TNFα, or MIP-1α by ELISA. n≥5 eyes except for KC in C57BL/6J mice at 24 hours where n=3. Error bars represent standard deviation, *; p≤0.01, †; p<0.01 when values below the LOD were estimated by. .
Similar to what was seen for KC, TNFα levels rose with faster kinetics in C57BL/6J mice than TLR4−/− mice. At 12 hours TNFα levels were 205 ± 93 pg/eye in C57BL/6J mice but only 7.71 pg/eye in TLR4−/− [p=0.0019] (Figure 6). At 18 hours there was a statistical difference in the amount of TNFα in C57BL/6J and TLR4−/− mice (257 ± 42 pg/eye vs. 19.6 ± 6.15 pg/eye [p=4.47×10−6]). This represented an approximately 13-fold difference. At 24 hours no difference was observed in TNFα levels (293 ± 93 pg/eye vs. 421 ± 267 pg/eye [p=0.378] forC57BL/6J and TLR4−/− mice, respectively) (Figure 6). However, mice infected with the ΔmagA strain had much lower levels of TNFα. At 12 hours there was a small, but significant difference in TNFα levels between C57BL/6J and TLR4−/− mice (117 ± 15 pg/eye vs. 75 ± 40 pg/eye [p=0.02]) (Figure 6). There was no difference in TNFα levels at 18 hours (86 ± 3 pg/eye vs. 104 ± 9 pg/eye [p=0.11]) or 24 hours (122 ± 24 pg/eye vs. 115 ± 13 pg/eye [p=0.78]).
In addition to KC and TNFα, the levels of MIP-1α (CCL3) in C57BL/6J and TLR4−/− mice infected with wild type K. pneumoniae were assayed. As seen for KC and TNFα, there was a delay in the production of MIP-1α in TLR4−/− mice. At 12 hours there was 430 ± 79 pg/eye in C57BL/6J mice, but only 32.8 ± 13.6 pg/eye in TLR4−/− mice (p=6.2×10−5) (Figure 6). This difference continued at both 18 hours (2,569 ± 134 pg/eye vs. 30 ± 9 pg/eye [p=0.00011] and 24 hours (3,802 ± 949 pg/eye vs. 224 ± 21 pg/eye [p=0.013]). When mice were infected with ΔmagA bacteria, results were similar to that seen with KC. There was significantly more MIP-1α in C57BL/6J mice at 12 and 18 hours. However, at 24 hours there was significantly more MIP-1α detected in the eyes of TLR4−/− mice.
In general, these data agree with the inflammation data above, with greater inflammatory cell influx, MPO, and proinflammatory cytokine/chemokine synthesis in C57BL/6J eyes than in TLR4−/− eyes infected with wild type K. pneumoniae during the earlier stages of endophthalmitis. Differences in C57BL/6J and TLR4−/− eyes infected with ΔmagA K. pneumoniae were similar to that of eyes infected with wild type K. pneumoniae, with more gross inflammation, MPO, and proinflammatory cytokine/chemokine synthesis during the earlier stages of infection in eyes of C57BL/6J mice. At 24 hours postinfection, these differences became less clear as ERG responses were uniformly poor in the infected eyes of both mouse strains at this time point, gross inflammation and MPO activity was significant in all groups, and proinflammatory cytokine/chemokine synthesis varied depending on the molecule analyzed. Taken together, these results suggest that the delay in inflammation in TLR4−/− infected eyes may be ameliorated by 24 hours postinfection in the absence of sterilizing therapy, and that there are TLR4-independent pathways for inflammation in the eye in response to K. pneumoniae.
Neutrophil Phagocytosis of K. pneumoniae is Independent of TLR4
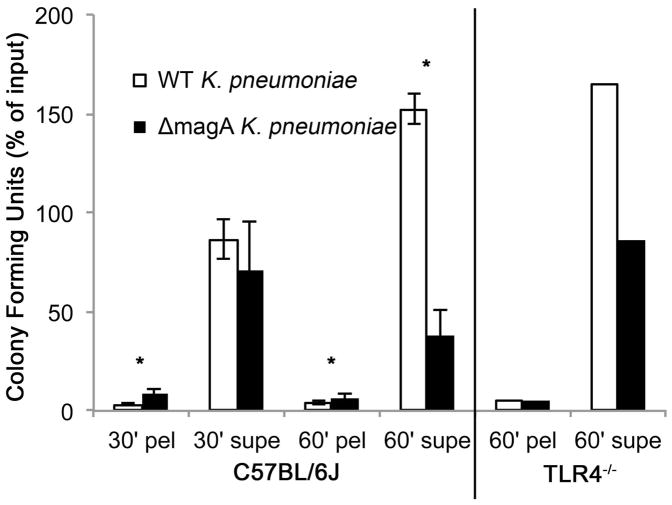
During acute endophthalmitis, neutrophils are the predominant immune cell infiltrating into the eye [26]. In infected TLR4−/− mice, there was a delay in the recruitment of neutrophils to the eye. To determine if neutrophils were capable of effectively killing bacteria, casein-elicited peritoneal neutrophils were purified and used in a phagocytosis assay. Results are shown in Figure 7. Significantly more ΔmagA bacteria were associated with neutrophils at 30 minutes, indicating that neutrophils were better able to engulf the capsule-deficient strain than the wild type strain. There was no difference in the number of bacteria recovered in the supernatant fraction 30 minutes. At 60 minutes, fewer wild type bacteria were associated with the pellet fraction compared to the number of ΔmagA associated with the pellet fraction. There were also fewer ΔmagA bacteria in the pellet fraction at 60 minutes compared to that recovered from the pellet fraction at 30 minutes (13.4% vs. 7.4%, respectively. p=0.036). When bacteria in supernatant fractions were counted at 60 minutes, it became clear that the C57BL/6J neutrophils could not control the growth of wild type bacteria. These fractions contained 153.7% more bacteria than the inoculum input into the reaction, indicating that wild type bacteria were able to actively proliferate in the presence of 10% serum and neutrophils. However, only 61.3% of ΔmagA bacteria were detected in the supernatant at 60 minutes. When the pellet and supernatant fraction are summed, only 68.8% of the original input of ΔmagA bacteria were recovered in the assay. The difference (31.2%) was counted as intracellular or extracellular killed bacteria. The rate of killing for this strain by neutrophils was approximately 1.3×104 bacteria/minute.
Figure 7. Killing of K. pneumoniae by Neutrophils.
Casein-elicited peritoneal neutrophils were purified as described in the Methods. Cells and bacteria were mixed in a 1:1 ratio and incubated at 37°C for the indicated times when cell pellets were washed and supernatants pooled. Data in A is displayed as percent of input bacteria recovered ± standard deviation and represents an n= 3 repeated on separate days (total of n=6). A, wild type; B, TLR4−/− mice. Pel; pellet, supe; supernatant. *, p<0.01.
Neutrophils were also purified from TLR4−/− mice to determine if TLR4 was required for efficient recognition and killing of bacteria. TLR4−/− mice yielded much fewer neutrophils than C57BL/6J mice (5.875×105 cells/mouse vs. 7.84×106/mouse, respectively). The ability of TLR4−/− neutrophils to kill bacteria was not different from that of C57BL/6J neutrophils (Figure 7). At 60 minutes, a similar number of bacteria were recovered from the pellet and supernatant fractions of TLR4−/− neutrophils. These results suggested that neutrophils in TLR4−/− mice may not have been recruited to the site of infection with the same kinetics as in C57BL/6J mice, but their ability to recognize and kill K. pneumoniae was not hampered. This also indicated that encapsulated bacteria were consistently more resistant to killing by neutrophils regardless of the donor strain of the neutrophils.
Taken together, these results indicate that TLR4 is important for the host to elicit an early response to bacteria in the eye, as there was a 6 hour delay in pathology and retinal function loss in TLR4−/− mice. This delay in pathology was mirrored by the inflammatory markers that were assayed. The early recognition of and response to infection in C57BL/6J mice came at a cost of vision loss earlier in the time course, which may have been due to bystander damage by infiltrating immune cells. This is supported by the fact that TLR4−/− neutrophils were not less able than wild type neutrophils to kill bacteria or to control bacterial growth in vitro when the requirement for chemotaxis to the site of infection was removed.
Retinal TLR4 mRNA During Infection
Because there was a more robust inflammatory response in C57BL/6J mice to wild type K. pneumoniae than the ΔmagA strain, we investigated the expression of retinal TLR4 to determine if there was also differential upregulation of the receptor during infection. Wild type mice were intraocularly infected as before, except time points were focused on the early phase of the infection. While there was a trend toward greater TLR4 message in retinas of infected versus mock infected eyes at the zero hour timepoint (i.e. immediately following injection), only eyes infected with the ΔmagA strain reached significance (3.5 fold) compared to PBS-injected eyes (control set at 1, p<0.001). At subsequent time points (3, 6, 9, and 12 hours postinfection), the TLR4 message levels in all groups decreased to less than 1-fold compared to PBS at 0 hours and were not statistically different from each other.
DISCUSSION
Wu et al. [17] reported that TLR-mediated LPS recognition in vitro was masked by the hypermucviscosity of K. pneumoniae, mediated by MagA. In that report, cells deficient in TLR4 were unable to secrete TNF-α when stimulated with UV-inactivated magA+ bacteria. A TLR4-competent cell line secreted more TNF-α when treated with heat-killed bacteria than when treated with UV-inactivated bacteria, suggesting that the integrity of the capsule was essential for evading TLR4 recognition. In contrast, Yang et al. [27] reported that purified K1 capsule can directly interact with and activate TLR4. We previously demonstrated that an equivalent amount of MPO was elicited in C57BL/6J eyes by wild type and ΔmagA K. pneumoniae at 12 and 18 hours postinfection [9]. At these time points, 1.5–3.0 logs less bacteria were recovered from eyes infected with the ΔmagA strain. This suggested that the lack of MagA may have exposed this strain’s underlying LPS to TLR4, causing ΔmagA to be more inflammogenic, and agreeing with previous the findings of Wu et al. [17]. It is possible that TLR4-dependent recruitment of immune cells into the eye after infection with Gram-negative bacteria could exacerbate visual outcome by causing bystander damage to nearby retinal neurons. To test this, we sought to determine the role of TLR4 in experimental endophthalmitis and assess whether TLR4 recognition was dependent upon MagA.
TLR4 is expressed in the human eye [28] and is involved in inflammation during experimental bacterial corneal infections [29–31], but its role in inflammation and the clearance of Gram-negative bacteria during endophthalmitis has not been analyzed. In the current study, when wild type bacteria were inoculated into eyes of C57BL/6J or TLR4−/− mice, there was no difference in intraocular bacterial loads, suggesting that there was no direct effect on the growth of wild type bacteria in the absence of TLR4. However, when mice were infected with the ΔmagA strain, TLR4−/− eyes had significantly greater number of bacteria, suggesting that the loss of hypermucoviscosity made these bacteria sensitive to control by C57BL/6J mice. Histological data suggested a delay in the recruitment of inflammatory cells to the eye in TLR4−/− mice, a difference supported by the MPO data. The results of in vitro phagocytosis assays suggested that there was no TLR4-dependent difference in phagocytosis or bacterial killing. Because there were different kinetics of neutrophil recruitment in C57BL/6J and TLR4−/− mice, the phenomenon may be explained by a difference in inflammatory cytokine production. Therefore, the production of KC, TNFα, and MIP-1α in the eyes of infected mice was measured.
KC was detected at much lower concentrations in TLR4−/− mice at 12 and 18 hours postinfection. This result is similar to what has been reported for B. cereus endophthalmitis [24], as TLR2−/− mice were also delayed in the production of wild type levels of KC and other proinflammatory cytokines. KC, the functional homolog of human IL8, is a small secreted protein containing a CXC motif that binds the chemokine receptor CXCR2 and stimulates a potent neutrophil response [32]. KC has been implicated in various ocular inflammatory disease models. KC is upregulated in hyperoxia-induced retinopathy [33], though growth of bacteria in the eye may mimic a hypoxic rather than hyperoxic state. CD40 has been shown to be required for expression of KC and other inflammatory cytokines as well as recruitment of neutrophils in a model of retinal ischemia [34]. Chintakuntlawar et al. [35] demonstrated that KC-deficient mice were delayed in neutrophil recruitment in the cornea following adenoviral infection. However, to our knowledge, no studies have yet analyzed the role of KC in neutrophil recruitment during experimental endophthalmitis.
Similar to what was observed with KC, TNFα was detected at very low levels at 12 and 18 hours postinfection in TLR4−/− mice. Eventually, at 24 hours, TNFα levels in TLR4−/− mice rose to levels that were similar to those detected in C57BL/6J animals. The levels of TNFα followed the trend of neutrophil migration into the eye. When there was no difference in MPO levels between C57BL/6J and TLR4−/− mice, there was also no difference in the levels of TNFα. TNFα is a member of the TNF superfamily of cytokines and is important for many cellular processes including cell survival, inflammation, and apoptosis. TNFα is also a strong chemoattractant of neutrophils. During experimental B. cereus endophthalmitis, TNFα upregulation paralleled the recruitment of neutrophils to the vitreous [26] and fewer neutrophils were recruited into infected eyes in TNFα-deficient mice [36]. The direct injection of TNFα into the vitreous led to blood retinal barrier permeability and cellular infiltration [37]. It is important to note that during endophthalmitis, a loss of TNFα was associated with fewer infiltrating immune cells but a worse disease outcome, likely due to uncontrolled bacterial replication and toxin production [36]. Taken together, these findings reinforce the need for a multifaceted approach to the treatment of endophthalmitis that includes infection and inflammation control to achieve a good visual prognosis.
Macrophage inflammatory protein 1-alpha (MIP-1α) was measured at 12 hours postinfection and levels were significantly lower in eyes of TLR4−/− mice compared to C57BL/6J mice. This is further evidence that TLR4 is important for the early inflammatory response to K. pneumoniae in the vitreous. MIP-1α is a CC motif chemokine produced by activated macrophages. In mice, administration of MIP-1α has been shown to contribute to increased neutrophil rolling, adhesion and extravasation into tissue [38]. In the retina, resident microglial cells produce MIP-1α in response to ischemia/reperfusion injury [39]. Interestingly, loss of TLR4 in retinal ischemia/reperfusion injury resulted in a decrease in inflammatory gene transcription, including TNFα and intracellular adhesion molecules important for immune cell recruitment [19]. Loss of TLR4 was protective in myocardial ischemia/reperfusion injury as well [40]. These data suggest that during the oxidative stress induced by ischemia, endogenous TLR4 ligands are expressed and/or released from cells, causing activation of glial cells, production of proinflammatory molecules and recruitment of immune cells.
In the retinal ischemia/reperfusion model, only the inner retina is hypoxic, while the outer retina receives oxygen from the choroidal vasculature via the RPE cells [41]. During endophthalmitis, bacteria actively growing in the vitreous likely consume a great deal of oxygen, which could put an oxidative stress on the inner retina similar to ischemic injury. If so, this may represent a novel mechanism whereby TLRs may mediate inflammation in the eye during endophthalmitis in the absence of an exogenous ligand. Bacterial growth-induced oxidative stress could also account for the B-wave loss that occurs at a faster rate than A-wave loss, as cells responsible for the B-wave are located in the inner retina and are closer to the bacterial stress in the vitreous. However, further studies will need to be undertaken to confirm this.
Currently, the literature is conflicting as to the role of capsule and TLR4 dependent recognition of bacteria. Our data does not clarify the role of MagA in the ability of TLR4 to recognize capsule and initiate intraocular recognition of K. pneumoniae. A question remains as to why the presence or absence of TLR4 did not result in a difference in overall infection outcome in ΔmagA infections. If the absence of MagA unmasked LPS for TLR4 recognition of ΔmagA bacteria, one would expect less inflammation in TLR4−/− eyes than in eyes from wild type mice. Yeh et al. [42] reported that an absence of MagA does not affect LPS (O-antigen) synthesis, so a ΔmagA mutant should have the same amount of LPS on its surface as its wild type parental strain. Mag A does, however, affect K1 CPS biosynthesis [43], so the ΔmagA mutant may be altered in K1 CPS. If the CPS interaction with TLR4 is altered in the ΔmagA mutant, the presence/absence of TLR4 may not be relevant for recognition of ΔmagA bacteria.
Regardless of whether capsule is directly recognized by TLR4 or is masking LPS recognition, one would expect some amount of LPS to be liberated from actively growing bacteria, thus initiating the inflammatory cascade. Here we report that the loss of TLR4 resulted in a delay in robust inflammation in response to intraocular infection with K. pneumoniae. Mice deficient in TLR4 eventually exhibited similar, and in some cases greater, amounts of inflammation and retinal function loss, albeit there was approximately a 6 hour delay. The possibility that there are other factors of the innate immune response partially compensating for loss of TLR4 cannot be excluded as additional triggers for the inflammatory response. Other components of the innate immune response, such as TLR2, TLR9, and NOD1, are reported to be important to Klebsiella recognition in other infection models [44–46]. Additionally, endogenous ligands may be released in response to bacterial growth and oxidative stress in the vitreous. This study reinforces the idea that the immune system is a double-edged sword, as TLR4 signaling in C57BL/6J mice likely led to earlier detection and response to intraocular infection, but the result was significant loss of retinal function early in the time course. TLR4−/− mice enjoyed a delay in robust inflammation, however, this delay was eventually overcome, which resulted in similar pathology by the end of the time course.
Acknowledgments
FUNDING
This study was funded by a Lew R. Wasserman Award from Research to Prevent Blindness (to MCC). Our research is also supported in part by NIH Grants R01EY012985 (to MCC), R21EY022466 (to MCC), P30EY12191 (NIH CORE grant to Robert E. Anderson, OUHSC), P20RR17702 (NCRR COBRE grant to Robert E. Anderson, OUHSC), and an unrestricted grant to the Dean A. McGee Eye Institute from Research to Prevent Blindness.
The authors thank Phil Coburn, Ph.D. (University of Oklahoma Health Sciences Center) for helpful discussion, Mark Dittmar (Dean A. McGee Eye Institute Animal Research Facility) and Elizabeth B. Christy (Dean A. McGee Eye Institute) for their invaluable technical assistance, and Paula Pierce (Excalibur Pathology, Moore, OK) for histology expertise.
Footnotes
DECLARATION OF INTERESTS
None.
References
- 1.Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11:589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang JH, Liu YC, Lee SS, Yen MY, Chen YS, Wang JH, et al. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis. 1998;26:1434–1438. doi: 10.1086/516369. [DOI] [PubMed] [Google Scholar]
- 3.Han SH. Review of hepatic abscess from Klebsiella pneumoniae. An association with diabetes mellitus and septic endophthalmitis. West J Med. 1995;162:220–224. [PMC free article] [PubMed] [Google Scholar]
- 4.Fang CT, Chuang YP, Shun CT, Chang SC, Wang JT. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J Exp Med. 2004;199:697–705. doi: 10.1084/jem.20030857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ang LP, Lee HM, Au Eong KG, Yap EY, Lim AT. Endogenous Klebsiella endophthalmitis. Eye. 2000;14:855–860. doi: 10.1038/eye.2000.236. [DOI] [PubMed] [Google Scholar]
- 6.Chee SP, Ang CL. Endogenous Klebsiella endophthalmitis--a case series. Ann Acad Med Singapore. 1995;24:473–478. [PubMed] [Google Scholar]
- 7.Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL, Chang SC. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis. 2007;45:284–293. doi: 10.1086/519262. [DOI] [PubMed] [Google Scholar]
- 8.Ishii K, Hiraoka T, Kaji Y, Sakata N, Motoyama Y, Oshika T. Successful treatment of endogenous Klebsiella pneumoniae endophthalmitis: a case report. Int Ophthalmol. 2011;31:29–31. doi: 10.1007/s10792-010-9387-7. [DOI] [PubMed] [Google Scholar]
- 9.Hunt JJ, Wang JT, Callegan MC. Contribution of mucoviscosity-associated gene A (magA) to virulence in experimental Klebsiella pneumoniae endophthalmitis. Investig Ophthalmol Vis Sci. 2011;52:6860–6866. doi: 10.1167/iovs.11-7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiskur BJ, Hunt JJ, Callegan MC. Hypermucoviscosity as a virulence factor in experimental Klebsiella pneumoniae endophthalmitis. Investig Ophthalmol Vis Sci. 2008;49:4931–4938. doi: 10.1167/iovs.08-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Booth MC, Cheung AL, Hatter KL, Jett BD, Callegan MC, Gilmore MS. Staphylococcal accessory regulator (sar) in conjunction with agr contributes to Staphylococcus aureus virulence in endophthalmitis. Infect Immun. 1997;65:1550–1556. doi: 10.1128/iai.65.4.1550-1556.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelbert M, Mylonakis E, Ausubel FM, Calderwood SB, Gilmore MS. Contribution of gelatinase, serine protease, and fsr to the pathogenesis of Enterococcus faecalis endophthalmitis. Infect Immun. 2004;72:3628–3633. doi: 10.1128/IAI.72.6.3628-3633.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callegan MC, Cochran DC, Kane ST, Gilmore MS, Gominet M, Lereclus D. Contribution of membrane-damaging toxins to Bacillus endophthalmitis pathogenesis. Infect Immun. 2002;70:5381–5389. doi: 10.1128/IAI.70.10.5381-5389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan A, Lynch M, Smith SM, Amu S, Nel HJ, McCoy CE, et al. A role for TLR4 in Clostridium difficile infection and the recognition of surface layer proteins. PLoS Pathog. 2011;7:e1002076. doi: 10.1371/journal.ppat.1002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez N, Wantia N, Fend F, Durr S, Wagner H, Miethke T. Differential involvement of TLR2 and TLR4 in host survival during pulmonary infection with Chlamydia pneumoniae. Eur J Immunol. 2006;36:1145–1155. doi: 10.1002/eji.200535152. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien AD, Rosenstreich DL, Scher I, Campbell GH, MacDermott RP, Formal SB. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J Immunol. 1980;124:20–24. [PubMed] [Google Scholar]
- 17.Wu MF, Yang CY, Lin TL, Wang JT, Yang FL, Wu SH, et al. Humoral immunity against capsule polysaccharide protects the host from magA+ Klebsiella pneumoniae-induced lethal disease by evading Toll-like receptor 4 signaling. Infect Immun. 2009;77:615–621. doi: 10.1128/IAI.00931-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elner SG, Petty HR, Elner VM, Yoshida A, Bian ZM, Yang D, et al. TLR4 mediates human retinal pigment epithelial endotoxin binding and cytokine expression. Trans Am Ophthalmol Soc. 2005;103:126–135. [PMC free article] [PubMed] [Google Scholar]
- 19.Dvoriantchikova G, Barakat DJ, Hernandez E, Shestopalov VI, Ivanov D. Toll-like receptor 4 contributes to retinal ischemia/reperfusion injury. Mol Vis. 2010;16:1907–1912. [PMC free article] [PubMed] [Google Scholar]
- 20.Coburn PS, Wiskur BJ, Christy E, Callegan MC. The diabetic ocular environment facilitates the development of endogenous bacterial endophthalmitis. Investig Ophthalmol Vis Sci. 2012;53:7426–7431. doi: 10.1167/iovs.12-10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 22.Luo Y, Dorf ME. Isolation of mouse neutrophils. Curr Protoc Immunol. 2001;Chapter 3(Unit 3):20. doi: 10.1002/0471142735.im0320s22. [DOI] [PubMed] [Google Scholar]
- 23.Hampton MB, Vissers MC, Winterbourn CC. A single assay for measuring the rates of phagocytosis and bacterial killing by neutrophils. J Leukoc Biol. 1994;55:147–152. doi: 10.1002/jlb.55.2.147. [DOI] [PubMed] [Google Scholar]
- 24.Novosad BD, Astley RA, Callegan MC. Role of Toll-Like Receptor (TLR) 2 in Experimental Bacillus cereus Endophthalmitis. PLoS One. 2011;6:e28619. doi: 10.1371/journal.pone.0028619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Croghan CaEPP. Methods of Dealing with Values Below the Limit of Detection using SAS. Presented at Souther SAS User Group; St. Petersburg, FL. 2003. [Google Scholar]
- 26.Ramadan RT, Ramirez R, Novosad BD, Callegan MC. Acute inflammation and loss of retinal architecture and function during experimental Bacillus endophthalmitis. Curr Eye Res. 2006;31:955–965. doi: 10.1080/02713680600976925. [DOI] [PubMed] [Google Scholar]
- 27.Yang FL, Yang YL, Liao PC, Chou JC, Tsai KC, Yang AS, et al. Structure and immunological characterization of the capsular polysaccharide of a pyrogenic liver abscess caused by Klebsiella pneumoniae: activation of macrophages through Toll-like receptor 4. J Biol Chem. 2011;286:21041–21051. doi: 10.1074/jbc.M111.222091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang JH, McCluskey P, Wakefield D. Expression of toll-like receptor 4 and its associated lipopolysaccharide receptor complex by resident antigen-presenting cells in the human uvea. Invest Ophthalmol Vis Sci. 2004;45:1871–1878. doi: 10.1167/iovs.03-1113. [DOI] [PubMed] [Google Scholar]
- 29.Tullos NA, Thompson HW, Taylor SD, Sanders M, Norcross EW, Tolo I, et al. Modulation of Immune Signaling, Bacterial Clearance, and Corneal Integrity by Toll-like Receptors during Streptococcus pneumoniae Keratitis. Curr Eye Res. 2013;38:1036–1048. doi: 10.3109/02713683.2013.804094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karthikeyan RS, Priya JL, Leal SM, Jr, Toska J, Rietsch A, Prajna V, et al. Host response and bacterial virulence factor expression in Pseudomonas aeruginosa and Streptococcus pneumoniae corneal ulcers. PLoS One. 2013;8:e64867. doi: 10.1371/journal.pone.0064867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou R, Zhang R, Sun Y, Platt S, Szczotka-Flynn L, Pearlman E. Innate immune regulation of Serratia marcescens-induced corneal inflammation and infection. Investig Ophthalmol Vis Sci. 2012;53:7382–7388. doi: 10.1167/iovs.12-10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J, Cacalano G, Camerato T, Toy K, Moore MW, Wood WI. Chemokine binding and activities mediated by the mouse IL-8 receptor. J Immunol. 1995;155:2158–2164. [PubMed] [Google Scholar]
- 33.Powers MR, Davies MH, Eubanks JP. Increased expression of chemokine KC, an interleukin-8 homologue, in a model of oxygen-induced retinopathy. Curr Eye Res. 2005;30:299–307. doi: 10.1080/02713680590923276. [DOI] [PubMed] [Google Scholar]
- 34.Portillo JA, Van Grol J, Zheng L, Okenka G, Gentil K, Garland A, et al. CD40 mediates retinal inflammation and neurovascular degeneration. J Immunol. 2008;181:8719–8726. doi: 10.4049/jimmunol.181.12.8719. [DOI] [PubMed] [Google Scholar]
- 35.Chintakuntlawar AV, Chodosh J. Chemokine CXCL1/KC and its receptor CXCR2 are responsible for neutrophil chemotaxis in adenoviral keratitis. J Interferon Cytokine Res. 2009;29:657–666. doi: 10.1089/jir.2009.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramadan RT, Moyer AL, Callegan MC. A role for tumor necrosis factor-alpha in experimental Bacillus cereus endophthalmitis pathogenesis. Investig Ophthalmol Vis Sci. 2008;49:4482–4489. doi: 10.1167/iovs.08-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luna JD, Chan CC, Derevjanik NL, Mahlow J, Chiu C, Peng B, et al. Blood-retinal barrier (BRB) breakdown in experimental autoimmune uveoretinitis: comparison with vascular endothelial growth factor, tumor necrosis factor alpha, and interleukin-1beta-mediated breakdown. J Neurosci Res. 1997;49:268–280. doi: 10.1002/(sici)1097-4547(19970801)49:3<268::aid-jnr2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 38.Wan MX, Wang Y, Liu Q, Schramm R, Thorlacius H. CC chemokines induce P-selectin-dependent neutrophil rolling and recruitment in vivo: intermediary role of mast cells. Br J Pharmacol. 2003;138:698–706. doi: 10.1038/sj.bjp.0705094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida S, Yoshida A, Ishibashi T, Elner SG, Elner VM. Role of MCP-1 and MIP-1alpha in retinal neovascularization during postischemic inflammation in a mouse model of retinal neovascularization. J Leukoc Biol. 2003;73:137–144. doi: 10.1189/jlb.0302117. [DOI] [PubMed] [Google Scholar]
- 40.Oyama J, Blais C, Liu XL, Pu M, Kobzik L, Kelly RA, et al. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation. 2004;109:784–789. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 41.Ozaki H, Yu AY, Della N, Luna JD, Yamada H, Hackett SF, et al. Hypoxia inducible factor-1alpha is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Investig Ophthalmol Vis Sci. 1999;40:182–189. [PubMed] [Google Scholar]
- 42.Yeh KM, Lin JC, Yin FY, Fung CP, Hung HC, Siu LK, Chang FY. Revisiting the importance of virulence determinant magA and its surrounding genes in Klebsiella pneumoniae causing pyogenic liver abscesses: exact role in serotype K1 capsule formation. J Infect Dis. 2010;201:1259–1267. doi: 10.1086/606010. [DOI] [PubMed] [Google Scholar]
- 43.Lin TL, Yang FL, Yang AS, Peng HP, Li TL, Tsai MD, Wu SH, Wang JT. Amino acid substitutions of MagA in Klebsiella pneumoniae affect the biosynthesis of the capsular polysaccharide. PLoS One. 2012;7:e46783. doi: 10.1371/journal.pone.0046783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wieland CW, van Lieshout MH, Hoogendijk AJ, van der Poll T. Host defence during Klebsiella pneumonia relies on haematopoietic-expressed Toll-like receptors 4 and 2. Eur Respir J. 2011;37:848–857. doi: 10.1183/09031936.00076510. [DOI] [PubMed] [Google Scholar]
- 45.Bhan U, Ballinger MN, Zeng X, Newstead MJ, Cornicelli MD, Standiford TJ. Cooperative interactions between TLR4 and TLR9 regulate interleukin 23 and 17 production in a murine model of gram negative bacterial pneumonia. PLoS One. 2010;5:e9896. doi: 10.1371/journal.pone.0009896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.March C, Moranta D, Regueiro V, Llobet E, Tomás A, Garmendia J, Bengoechea JA. Klebsiella pneumoniae outer membrane protein A is required to prevent the activation of airway epithelial cells. J Biol Chem. 2011;286:9956–967. doi: 10.1074/jbc.M110.181008. [DOI] [PMC free article] [PubMed] [Google Scholar]