Abstract
BACKGROUND
Ibrutinib is an irreversible inhibitor of Bruton’s tyrosine kinase (BTK) and is effective in chronic lymphocytic leukemia (CLL). Resistance to irreversible kinase inhibitors and resistance associated with BTK inhibition have not been characterized. Although only a small proportion of patients have had a relapse during ibrutinib therapy, an understanding of resistance mechanisms is important. We evaluated patients with relapsed disease to identify mutations that may mediate ibrutinib resistance.
METHODS
We performed whole-exome sequencing at baseline and the time of relapse on samples from six patients with acquired resistance to ibrutinib therapy. We then performed functional analysis of identified mutations. In addition, we performed Ion Torrent sequencing for identified resistance mutations on samples from nine patients with prolonged lymphocytosis.
RESULTS
We identified a cysteine-to-serine mutation in BTK at the binding site of ibrutinib in five patients and identified three distinct mutations in PLCγ2 in two patients. Functional analysis showed that the C481S mutation of BTK results in a protein that is only reversibly inhibited by ibrutinib. The R665W and L845F mutations in PLCγ2 are both potentially gain-of-function mutations that lead to autonomous B-cell–receptor activity. These mutations were not found in any of the patients with prolonged lymphocytosis who were taking ibrutinib.
CONCLUSIONS
Resistance to the irreversible BTK inhibitor ibrutinib often involves mutation of a cysteine residue where ibrutinib binding occurs. This finding, combined with two additional mutations in PLCγ2 that are immediately downstream of BTK, underscores the importance of the B-cell–receptor pathway in the mechanism of action of ibrutinib in CLL. (Funded by the National Cancer Institute and others.)
The development of B-cell–receptor antagonists has been a therapeutic advance in chronic lymphocytic leukemia (CLL). Although B-cell–receptor ligation in normal cells induces proliferation, apoptosis, or anergy,1 pathway dysregulation in CLL results in the propagation of proliferative and prosurvival signals.2,3 Several agents targeting the B-cell–receptor pathway are in development, including the Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib. Although BTK is not recurrently mutated in CLL,4,5 it is up-regulated at the transcript level and is constitutively active.6,7 Ibrutinib irreversibly binds BTK at the C481 residue, rendering it kinase-inactive, inducing modest CLL-cell apoptosis, and abolishing proliferation and B-cell–receptor signaling in vitro.6,8 Ibrutinib has been shown to have clinically significant activity in patients with relapsed CLL, with 71% of patients having an objective complete or partial response and an additional 15 to 20% of patients having a partial response with persistent lymphocytosis. At 26 months, the estimated progression-free survival rate among patients treated with ibrutinib is 75%.9 Few patients have had a relapse, but as more patients are treated with ibrutinib, it becomes increasingly important to identify mechanisms of acquired resistance in order to offer effective salvage therapies. In addition, determining whether persistent lymphocytosis has similar resistant features could affect treatment choices for patients with prolonged lymphocytosis during ibrutinib therapy.
The model for kinase inhibition in hematologic cancers is the BCR-ABL inhibitor imatinib, which transformed therapy for chronic myeloid leukemia.10 The most common mechanisms of acquired resistance to imatinib are point mutations in the kinase domain of ABL. Although the T315I mutation is the most common,11,12 more than 100 resistance mutations have been identified that prevent imatinib binding through binding-site alteration or destabilization of the inactive conformation of ABL.13 Because BTK has not been identified as a mutated gene in CLL, whereas BCR-ABL has been shown to be a mutational hot spot,14 it is uncertain whether the type of resistance seen with imatinib will be relevant to CLL. In addition, ibrutinib is an irreversible inhibitor of BTK through its ability to bind to the C481 site, distinguishing it from imatinib and other reversible kinase inhibitors that have been studied in cancer to date. How cancer cells, including CLL cells, develop resistance to ibrutinib or other irreversible inhibitors is still unknown. The development of mutations in genes that reactivate downstream B-cell–receptor signaling or other pathways is certainly possible, because clonal evolution is common in previously treated CLL.15 We evaluated patients who had CLL and acquired resistance to ibrutinib for mutations that may mediate resistance.
METHODS
DNA SEQUENCING
We obtained blood samples from patients enrolled in institutional review board–approved trials of ibrutinib. One of the patients (Patient 1) is described extensively in the Journal by Furman et al.16 Tumor DNA was isolated from blood mononuclear cells with the use of the AllPrep DNA/RNA Mini Kit (Qiagen). Sample preparation and whole-exome sequencing with the use of Agilent SureSelect Human All Exon V4 and Illumina HiSeq 2000 technology were performed by Expression Analysis.
DATA-ANALYSIS WORKFLOW
The exome-sequencing analysis pipeline is shown in Figure 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org. Sequencing reads were aligned to the human reference genome (1000 Genomes Project human assembly GRCh37) with the use of Burrows– Wheeler Aligner, version 0.7.5.17 After potential polymerase-chain-reaction or optical duplicates had been marked with the use of Picard, version 1.94 (http://picard.sourceforge.net), local realignment around indels was performed by means of the Genome Analysis Toolkit (GATK), version 2.8.1,18 and relapse-specific single point mutations and indels were detected with the use of MuTect, version 1.1.4,19 and GATK Somatic Indel Detector, respectively. Variants previously reported in the dbSNP database, build 137, were filtered out, and the remaining variants were annotated and their potential mutational effects predicted with the use of SnpEff, version 3.4.20 Finally, newly acquired, relapse-specific, nonsynonymous mutations with high-quality reads were verified by means of Sanger or Ion Torrent sequencing.
ION TORRENT ANALYSIS
DNA was extracted from cryopreserved cells with the use of the QIAamp DNA Mini Kit (Qiagen). BTK and PLCγ2 were analyzed with the use of the Ion Torrent platform from Life Technologies. Details of sample preparation, library generation, and sequence analysis are provided in the Supplementary Appendix.
DNA CONSTRUCTS AND CELL CULTURE
The PiggyBac Transposon Vector System (System Biosciences) was used to generate DNA constructs of BTK with both green-fluorescent-protein and puromycin selection markers, and protein expression was controlled with the use of chicken β-actin promoter. See the Supplementary Appendix for details.
IN VITRO KINASE ASSAY
Full-length His6- and Strep-tagged BTK and BTK C481S were expressed in mammalian HEK-EBNA cells and purified by means of affinity chromatography. Purified proteins (100 ng per milliliter) were used in a LANCE Ultra kinase assay (PerkinElmer), with 100 nM ULight-poly GT (PerkinElmer) as a substrate and 200 µM ATP. Compounds were preincubated at room temperature with enzyme before the kinase reaction. After 45 minutes of kinase reaction, excess EDTA was added, and a europium-labeled anti–poly-GT antibody was incubated before signal measurement in a dual-laser EnVision plate reader (PerkinElmer), set at an excitation wavelength of 320 nm and an emission wavelength of 615 to 665 nm.
FLOW-CYTOMETRIC AND IMMUNOBLOT ASSAYS
HEK 293T cells were transiently transfected with the indicated expression constructs, treated with ibrutinib for 1 hour, and fixed with paraformaldehyde or washed into fresh media and then fixed. Cells were permeabilized, stained, and analyzed on a BD FACSCanto II analyzer (BD Biosciences).
For immunoblot assays, whole-cell lysates were prepared and equivalent amounts of protein were separated on polyacrylamide gels and transferred onto nitrocellulose membranes. After antibody incubation, proteins were detected with the use of a chemiluminescent substrate (Super-Signal, Pierce). Details on the antibodies used are provided in the Supplementary Appendix.
STATISTICAL ANALYSIS
The statistical methods for individual experiments are detailed in the Supplementary Appendix.
RESULTS
MUTATIONS IN BTK AND PLCγ2 REVEALED BY WHOLE-EXOME SEQUENCING
Peripheral-blood samples were available at baseline and at the time of relapse from six patients with acquired resistance to ibrutinib manifested by progressive CLL. Whole-exome sequencing was performed on each sample. Clinical characteristics and new mutations identified at the time of relapse in the patients with matched samples are shown in Table 1, and Table 1 in the Supplementary Appendix. Alignment statistics are shown in Table 2 in the Supplementary Appendix. Copynumber analysis was performed to ensure that identified variants were not a result of potential copy-number alterations (Table 3 and Fig. 2 in the Supplementary Appendix). All patients had highrisk cytogenetic features, such as del(11q22.3), del(17p13.1), or a complex karyotype.
Table 1.
Characteristics of Six Patients with Resistance to Ibrutinib.
| Patient No. |
Age yr |
Prior Therapies no. |
Baseline Cytogenetic Features* |
Study Treatment and Daily Dose† |
Duration of Ibrutinib Treatment days |
Best Response |
Time to First Response days |
Identified Mutations of Interest‡ |
|---|---|---|---|---|---|---|---|---|
| 1 | 59 | 5 | del(17p13.1), trisomy 12 | Ibrutinib, 560 mg | 621 | Partial | 70 | C481S mutation in BTK |
| 2 | 59 | 3 | del(11q22.3) | Bendamustine–rituximab for 6 cycles; ibrutinib, 420 mg | 388 | Complete | 70 | C481S mutation in BTK |
| 3 | 51 | 2 | complex karyotype | Ofatumumab for 24 wk; ibrutinib, 420 mg | 674 | Complete | 85 | C481S mutation in BTK |
| 4 | 69 | 9 | del(17p13.1), complex karyotype | Ibrutinib, 840 mg | 868 | Partial | 133 | C481S mutation in BTK |
| 5 | 61 | 4 | del(17p13.1), complex karyotype | Ofatumumab for 24 wk; ibrutinib, 420 mg | 505 | Partial | 85 | L845F, R665W, and S707Y mutations in PLCγ2 and C481S mutation in BTK |
| 6 | 75 | 2 | del(17p13.1), complex karyotype | Ibrutinib, 420 mg | 673 | Partial | 159 | R665W mutation in PLCγ2 |
We used fluorescence in situ hybridization to detect del(17p13.1), del(11q22.3), centromere 12, and del(13q14.3) and metaphase analysis of stimulated G-banded cells to determine complexity.
Doses are given for ibrutinib only.
All functional mutations that were detected only at the time of relapse are listed in Table 1 in the Supplementary Appendix.
In Patients 1 through 5, sequencing of the relapse sample revealed a cysteine-to-serine mutation in BTK at position 481 (C481S) (Fig. 3A in the Supplementary Appendix). In Patient 6, the relapse sample had an arginine-to-tryptophan mutation in PLCγ2 at position 665 (R665W) (Fig. 3B in the Supplementary Appendix). One patient with a low-frequency C481S mutation in BTK also had three distinct PLCγ2 mutations: the R665W mutation, a leucine-to-phenylalanine mutation at position 845 (L845F) (Fig. 3C in the Supplementary Appendix), and a serine-to-tyrosine mutation at position 707 that was previously reported as an activating mutation in a dominantly inherited inflammatory disease.21 In this patient, BTK C481S and PLCγ2 L845F were identified by means of whole-exome sequencing, and the other mutations were identified by means of Ion Torrent sequencing. To verify these clones, Ion Torrent sequencing was performed again on samples obtained 1 month after relapse, and all previously identified mutations were still present (Table 4 in the Supplementary Appendix). Mutations identified by whole-exome sequencing were confirmed by Sanger sequencing, Ion Torrent deep sequencing, or both.
At baseline, no patient had evidence of mutations in either BTK or PLCγ2 on the basis of whole-exome sequencing. In Patients 3, 5, and 6, Ion Torrent sequencing was performed, and no mutation was identified in more than 0.2% of reads (Table 5 in the Supplementary Appendix). At relapse, none of the patients had mutations in other kinases containing a cysteine residue homologous to C481 in BTK that are irreversibly inhibited by ibrutinib (data on ITK, BMX, TEC, EGFR, JAK3, HER2, HER4, and BLK not shown).7,22 No other recurrent mutation was noted in any of these six patients at the time of relapse.
REVERSIBLE INHIBITION OF MUTANT BTK BY IBRUTINIB IN VITRO AND IN PATIENTS
We performed functional characterization of non-mutant BTK and BTK with the C481S mutation. The intrinsic affinity of ibrutinib for nonmutant BTK was significantly higher than for mutant BTK (dissociation constant, 0.2 nM vs. 10.4 nM) (Table 6 in the Supplementary Appendix). Although both nonmutant and mutant BTK were inhibited by ibrutinib, the half-maximal effective concentration for BTK with the C481S mutation was significantly higher than for nonmutant BTK (Fig. 1A). In a cellular model, ibrutinib was significantly less effective at blocking BTK autophosphorylation (Fig. 1B) and downstream signaling (as indicated by phosphorylated ERK levels) in cells with mutant BTK than in cells with nonmutant BTK (Fig. 4A in the Supplementary Appendix), whereas dasatinib, a reversible inhibitor of BTK,23 inhibited nonmutant and mutant BTK with similar efficacy. Ibrutinib washout experiments showed that the C481S mutation allows reversible, but not irreversible, inhibition of BTK by ibrutinib, which was confirmed by kinase-binding assays (Fig. 4B and Table 6 in the Supplementary Appendix).
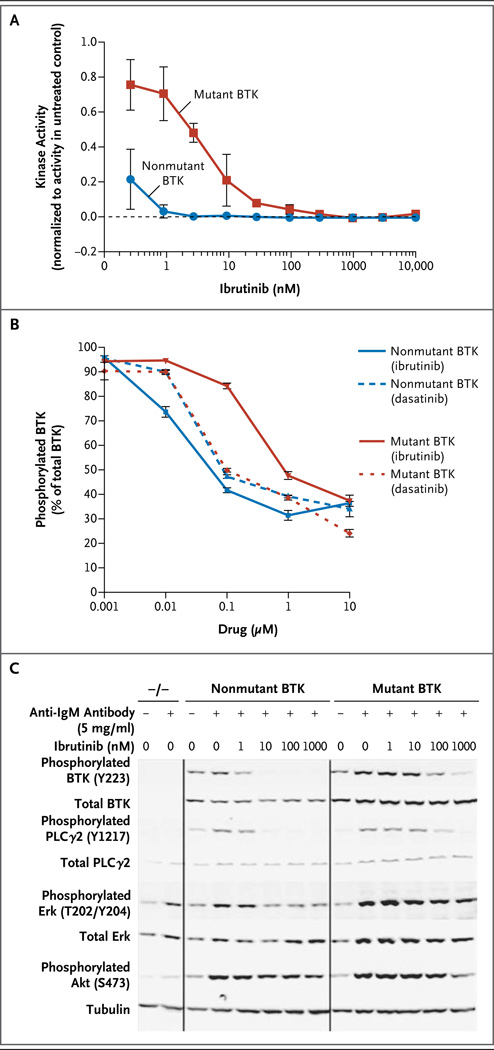
Figure 1. Functional Characterization of Bruton’s Tyrosine Kinase (BTK) with the C481S Mutation.
An assay of recombinant nonmutant BTK versus BTK with the C481S mutation (mutant BTK) showed that the mutant form had enhanced kinase activity that could be inhibited by ibrutinib, though at a much higher half-maximal effective concentration than with nonmutant BTK (Panel A). The data shown reflect two independent experiments with triplicate samples for each treatment. After transfection of mutant or nonmutant BTK into HEK 293T cells, administration of ibrutinib did not inhibit B-cell–receptor signaling in cells with mutant BTK to the same degree as in cells with non-mutant BTK. BTK autophosphorylation at tyrosine 223 (Y223) (Panel B) was significantly more inhibited in cells with nonmutant BTK than in cells with mutant BTK, at 0.01 µM, 0.1 µM, and 1 µM of ibrutinib (P<0.001), and the difference in inhibition between nonmutant BTK and mutant BTK was significantly greater with ibrutinib than with dasatinib (P<0.001). The data shown in Panels A and B are normalized. I bars in both panels indicate standard errors. After transfection of nonmutant or mutant BTK into BTK−/− DT40 cells, B-cell–receptor signaling was inhibited by ibrutinib in cells with nonmutant BTK to a greater degree than in cells with mutant BTK (Panel C). The data shown in Panels B and C reflect at least three independent experiments.
Next, we compared the function of nonmutant BTK and BTK with the C481S mutation by stably transfecting constructs into DT40 (BTK−/−) chicken B cells. After activation of the B-cell receptor, signaling downstream of BTK was inhibited by ibrutinib, as shown by decreased downstream signaling (phosphorylation of PLCγ2, ERK, and AKT) and calcium flux; this inhibition was diminished in mutant BTK as compared with nonmutant BTK (Fig. 1C, and Fig. 5 in the Supplementary Appendix). These data clearly show that the C481S mutation in BTK confers relative resistance to ibrutinib by preventing irreversible binding, providing confirmation that this is a functionally relevant resistance mutation.
We compared BTK phosphorylation on exposure to ibrutinib in cells obtained at baseline and at the time of relapse from one of the patients in whom a BTK C481S mutation developed. At baseline, BTK phosphorylation was completely abrogated by ibrutinib with either washout or continuous exposure to ibrutinib. At the time of relapse, however, ibrutinib inhibited BTK phosphorylation only with continuous exposure, a finding that shows that the drug binds the mutant reversibly in patients (Fig. 6 in the Supplementary Appendix).
IDENTIFIED MUTATIONS IN PLCγ2 AS RESISTANCE MECHANISMS IN VITRO AND IN PATIENTS
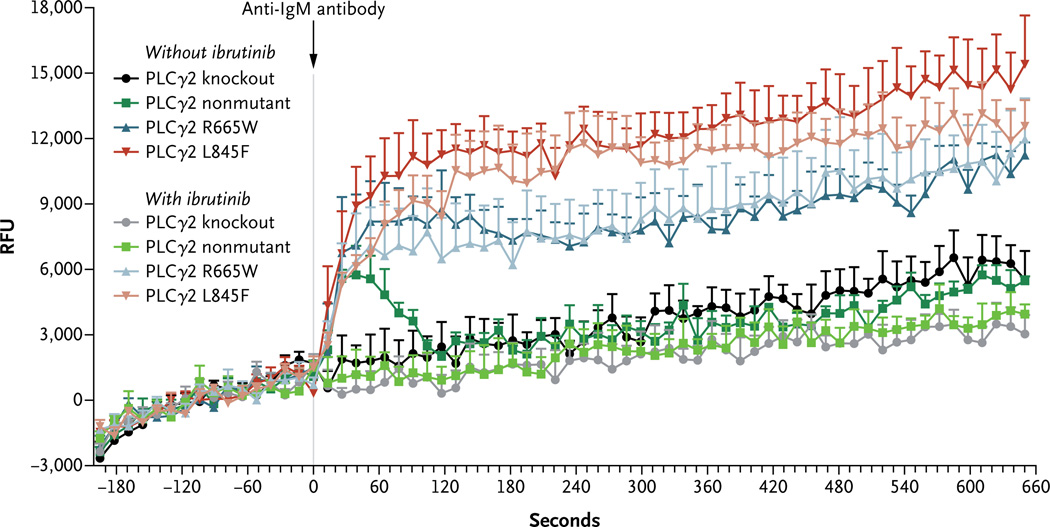
It has been shown that the S707Y mutation in PLCγ2 has a gain-of-function effect owing to disruption of an autoinhibitory SH2 domain.21 We therefore chose to focus on functional characterization of the R665W and L845F mutations. We stably transfected nonmutated PLCγ2, PLCγ2 with the L845F mutation, or PLCγ2 with the R665W mutation into HEK 293T cells and DT40 cells, which lack endogenous PLCγ2 expression (Fig. 7A in the Supplementary Appendix). We examined calcium flux in DT40 cells after stimulation with anti-IgM antibody in the presence of nonmutant or mutant PLCγ2. In contrast to BTK with the C481S mutation, for which calcium flux was very modestly inhibited by ibrutinib, PLCγ2 with either the R665W mutation or the L845F mutation showed enhanced IgM antibody–mediated calcium flux that was not inhibited by ibrutinib (Fig. 2). Thus, these mutations allow for B-cell receptor–mediated signaling that is independent of BTK. Similarly, after stimulation with anti-IgM antibody, cells with either the R665W mutation or the L845F mutation in PLCγ2 showed less inhibition in the presence of ibrutinib than nonmutant cells, as measured by phosphorylation of ERK and AKT (Fig. 7B in the Supplementary Appendix). These data show that the R665W and L845F mutations in PLCγ2 are potentially gain-of-function mutations in the presence of B-cell–receptor stimulation and could be relevant as mutations conferring resistance to ibrutinib in patients.
Figure 2. Functional Characterization of PLCγ2 with the R665W and L845F Mutations.
After stimulation of DT40 cells with anti-IgM antibody, calcium flux assays showed calcium release in the cells with nonmutant PLCγ2 that can be completely inhibited by ibrutinib. Cells bearing either the R665W mutation or the L845F mutation showed calcium release that is not inhibited by 1 µM ibrutinib (P = 0.62 for R665W and P = 0.43 for L845F). Error bars represent standard errors. RFU denotes relative fluorescence units.
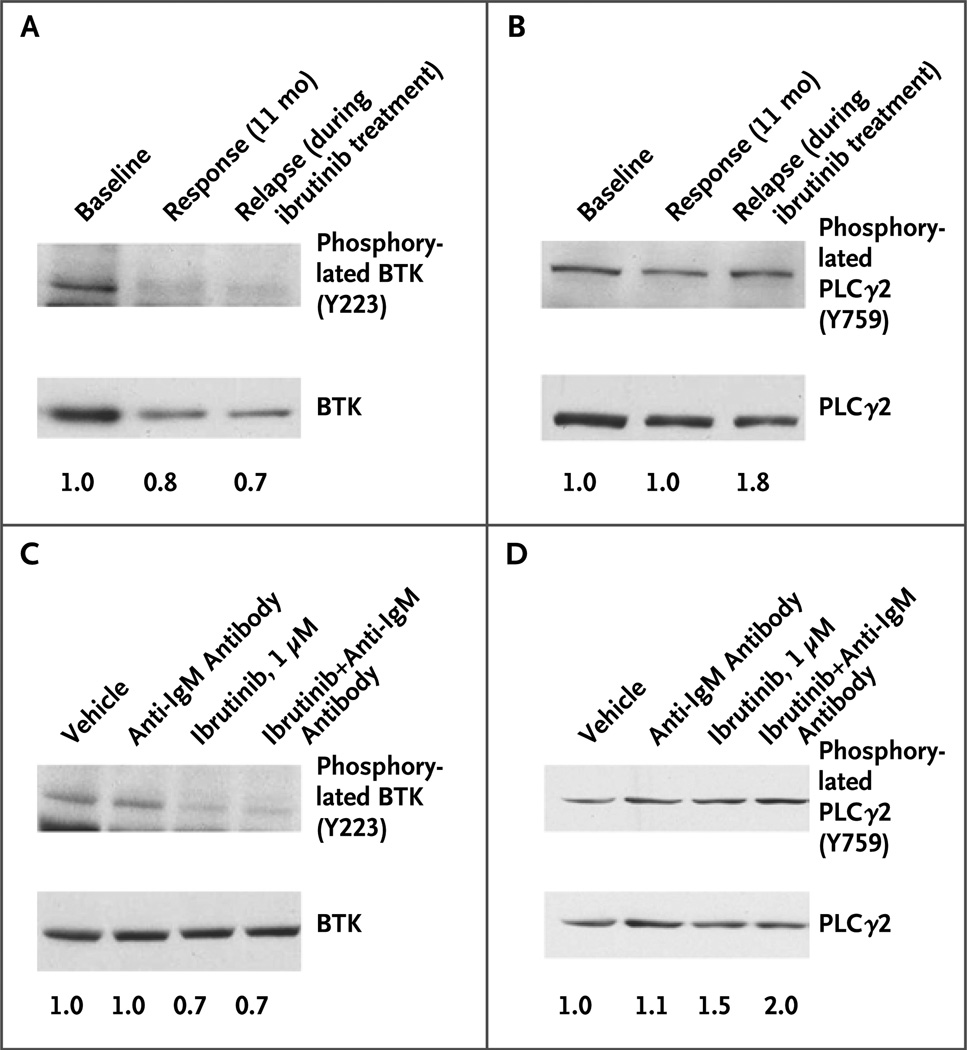
Finally, we examined CLL cells obtained at baseline and at the time of relapse from Patients 5 and 6. In Patient 6, the decrease in BTK phosphorylation at the time of the relapse was similar to the decrease at 11 months of therapy, when the patient was having a response to the drug (Fig. 3A), whereas PLCγ2 showed enhanced phosphorylation at the time of relapse (Fig. 3B). After ibrutinib was discontinued, ibrutinib still inhibited BTK phosphorylation (Fig. 3C) but not PLCγ2 phosphorylation (Fig. 3D). In Patient 5, who had mutations in both BTK and PLCγ2, in vitro ibrutinib did not inhibit either BTK or PLCγ2 phosphorylation (Fig. 8 in the Supplementary Appendix). These data suggest that the gain-of-function phenotype seen in vitro is also relevant in patients.
Figure 3. Characterization of R665W PLCγ2 Mutation in a Patient.
One patient who was found to have an R665W mutation in PLCγ2 had samples available for immunoblot analysis at baseline, during the period of response to ibrutinib, and at the time of relapse, before ibrutinib was discontinued. At baseline, BTK was phosphorylated, and this phosphorylation was inhibited by ibrutinib both during the period of response to the drug and at the time of relapse (Panel A). However, PLCγ2 showed evidence of sustained activation at the time of relapse through phosphorylation at tyrosine 759 (Y759) (Panel B), a finding that suggests that the R665W mutation is a gain-of-function mutation. At the time of relapse, after the drug had been discontinued, fresh cells were treated with vehicle, plate-immobilized anti- IgM antibody, 1 µM ibrutinib, or 1 µM ibrutinib plus anti-IgM antibody. BTK phosphorylation was inhibited by ibrutinib (Panel C), but PLCγ2 phosphorylation was not (Panel D); these findings show that the R665W mutation is not sensitive to ibrutinib in vitro. Densitometry values, shown below the lanes, reflect the ratio of phosphorylated protein to total protein in Panels B, C, and D; in Panel A, the values reflect the ratio of phosphorylated protein to loading control because total BTK protein expression can change over time during ibrutinib therapy.
ABSENCE OF BTK OR PLCγ2 MUTATIONS IN PATIENTS WITH PROLONGED LYMPHOCYTOSIS DURING IBRUTINIB THERAPY
In patients treated with ibrutinib, a characteristic lymphocytosis develops as CLL cells are mobilized from lymph nodes and the spleen. Although lymphocytosis typically resolves within 8 months, a subgroup of patients have lymphocytosis that lasts longer than 12 months in the presence of a continued response to ibrutinib.24 To determine whether such patients have new mutations in BTK or PLCγ2 and may therefore be at risk for relapse, we sequenced these two genes using Ion Torrent technology in nine patients who had lymphocytosis for at least 12 months after the initiation of ibrutinib therapy. The sequencing depth for BTK at C481 and for PLCγ2 at R665 and S707 was more than 700 reads, and the sequencing depth for PLCγ2 at L845 was more than 100 reads. No patient had evidence of any mutation in BTK or PLCγ2 (data not shown). These observations suggest that persistent lymphocytosis is not associated with known resistance mutations.
DISCUSSION
Acquired resistance to ibrutinib is due at least in part to recurrent mutations in BTK and PLCγ2. Our functional studies suggest that the C481S mutation in BTK confers resistance to ibrutinib by preventing irreversible drug binding. The S707Y, R665W, and L845F mutations in PLCγ2 are all potentially gain-of-function mutations that allow B-cell receptor–mediated activation that is independent of BTK. Calcium-flux assays showed enhanced and sustained activation after anti-IgM antibody stimulation, which is probably very relevant in patients in whom CLL cells are exposed to chronic antigen stimulation. None of these mutations were detected before drug exposure. The clinical significance of the very low variant reads on Ion Torrent sequencing for the R665W mutation in PLCγ2 at baseline is unknown, and more patients will need to be evaluated to determine whether this signal is identifying patients at risk for relapse. We also found that patients with persistent lymphocytosis during ibrutinib treatment did not have evidence of these resistance mutations on deep sequencing. We have previously documented by immunoblot analysis24 that BTK and PLCγ2 are inhibited in these patients, and these data suggest that patients with prolonged lymphocytosis during ibrutinib therapy do not have detectable small clones of resistant cells that would put them at high risk for relapse. However, we recognize that our inability to identify small numbers of mutant clones in the presence of large numbers of nonmutant clones may mean that such variants were present but were not identified.
Resistance to tyrosine kinase inhibitors through mutations that alter drug binding are seen with other targets and other cancers, including BCR-ABL in chronic myeloid leukemia,11 EGFR25,26 and ALK27 in lung cancer, KIT in gastrointestinal stromal tumors,28 and FLT3 in acute myeloid leukemia.29,30 Unlike the mutations in this group, however, the C481S mutation in BTK is not a second mutation in a previously mutated gene, but rather a primary mutation in a gene that is not recurrently mutated in CLL, and it is mutated at the amino acid required for drug binding. This mutation is not known to cause the X-linked agammaglobulinemia phenotype31 but instead results in retained catalytic activity. Our identification of a previously identified activating PLCγ2 mutation21 and our functional characterization of the two additional mutations showed that the activation of B-cell–receptor signaling distal to BTK can also confer resistance. This pattern of a mutation localized to the irreversible binding site of ibrutinib and the immediate downstream kinase is not commonly seen with reversible kinase inhibitors and may be a result of continuous (rather than intermittent) pressure on the drug target. As other potent irreversible kinase inhibitors are developed for key signaling pathways in cancer, it will be of interest to determine whether this pattern of resistance is generalized. Studies of the stability of the mutated RNA and proteins are ongoing.
Although acquired resistance to ibrutinib has developed in only a small proportion of patients, these data suggest that patients with increased genomic instability, including those with del(17p13.1), del(11q22.3), or a complex karyotype, may be at risk for relapse. On the basis of available data, patients with del(17p13.1) or a complex karyotype seem to be the most rational choice for combination therapies designed to avoid the development of resistance.
Even though this series of patients had a uniform presence of mutations in BTK, PLCγ2, or both, it is clear that other mechanisms of resistance are also present in a subgroup of patients. In a recent preliminary study involving three patients with ibrutinib resistance, sequencing revealed the S707Y mutation in PLCγ2 that we identified in Patient 5 in our study but did not reveal BTK mutations.32 In patients without B-cell–receptor pathway mutations, resistance may be mediated through mutations in other coding genes providing alternative survival signals that are not inhibited by ibrutinib or through noncoding RNA, epigenetic activation or silencing, or selective gene amplification. In addition, other mutations may act in combination with the BTK or PLCγ2 mutations to drive resistance, such as the additional driver mutations outlined in Table 1 in the Supplementary Appendix. Work is ongoing to identify alternative mechanisms of resistance and also to determine the role, if any, of other identified mutations.
Although ibrutinib inhibits other similar kinases that contain C481, including ITK and BLK,7,22 mutations have not been observed in these other targets, findings that suggest that BTK is its critical target. Knowledge of downstream mediators of resistance may lead to the development of rational combinations to prevent or treat resistant disease.
Supplementary Material
Acknowledgments
Supported by the National Cancer Institute (P50 CA140158, R01 CA177292, and K23 CA178183), the Four Winds Foundation, the D. Warren Brown Foundation, Mr. and Mrs. Michael Thomas, Mr. and Mrs. Al Lipkin, the Harry T. Mangurian Jr. Foundation, the Leukemia and Lymphoma Society, Pharmacyclics, the Conquer Cancer Foundation, an American Society of Hematology Scholar Award, Else Kr.ner-Fresenius-Stiftung (2010_Kolleg24, Project 2012_A146), Helmholtz Virtual Institute (VH-VI-404, TP2), and the German Research Foundation (SFB 1074 Project B2).
We thank Elisabeth Van Der Helm, Stella Chang, Padmaja Magadala, Michelle Francesco, Lisa Smith, and Kelly Smucker for providing technical assistance.
Footnotes
Presented in part at the Annual Meeting of the American Society of Clinical Oncology, Chicago, May 31–June 4, 2013; and the 15th International Workshop on Chronic Lymphocytic Leukemia, Cologne, Germany, September 9–11, 2013.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Dal Porto JM, Gauld SB, Merrell KT, Mills D, Pugh-Bernard AE, Cambier J. B cell antigen receptor signaling 101. Mol Immunol. 2004;41:599–613. doi: 10.1016/j.molimm.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Deglesne PA, Chevallier N, Letestu R, et al. Survival response to B-cell receptor ligation is restricted to progressive chronic lymphocytic leukemia cells irrespective of Zap70 expression. Cancer Res. 2006;66:7158–7166. doi: 10.1158/0008-5472.CAN-06-0085. [DOI] [PubMed] [Google Scholar]
- 3.Bernal A, Pastore RD, Asgary Z, et al. Survival of leukemic B cells promoted by engagement of the antigen receptor. Blood. 2001;98:3050–3057. doi: 10.1182/blood.v98.10.3050. [DOI] [PubMed] [Google Scholar]
- 4.Puente XS, Pinyol M, Quesada V, et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475:101–105. doi: 10.1038/nature10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Lawrence MS, Wan Y, et al. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N Engl J Med. 2011;365:2497–2506. doi: 10.1056/NEJMoa1109016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–6296. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–1189. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [Erratum, N Engl J Med 2014; 370:786.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 11.Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 12.Cortes J, Jabbour E, Kantarjian H, et al. Dynamics of BCR-ABL kinase domain mutations in chronic myeloid leukemia after sequential treatment with multiple tyrosine kinase inhibitors. Blood. 2007;110:4005–4011. doi: 10.1182/blood-2007-03-080838. [DOI] [PubMed] [Google Scholar]
- 13.Quintás-Cardama A, Kantarjian HM, Cortes JE. Mechanisms of primary and secondary resistance to imatinib in chronic myeloid leukemia. Cancer Control. 2009;16:122–131. doi: 10.1177/107327480901600204. [DOI] [PubMed] [Google Scholar]
- 14.Stoklosa T, Poplawski T, Koptyra M, et al. BCR/ABL inhibits mismatch repair to protect from apoptosis and induce point mutations. Cancer Res. 2008;68:2576–2580. doi: 10.1158/0008-5472.CAN-07-6858. [DOI] [PubMed] [Google Scholar]
- 15.Landau DA, Carter SL, Stojanov P, et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell. 2013;152:714–726. doi: 10.1016/j.cell.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furman RR, Cheng S, Lu P, et al. Ibrutinib resistance in chronic lymphocytic leukemia. N Engl J Med. 2014;370:2352–2354. doi: 10.1056/NEJMc1402716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKenna AHM, Hanna M, Banks E, et al. The Genome Analysis Toolkit: a Map-Reduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cibulskis KLM, Lawrence MS, Carter SL, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31:213–219. doi: 10.1038/nbt.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cingolani PPA, Platts A, Wang L, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118, iso-2, iso-3. Fly (Austin) 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Q, Lee GS, Brady J, et al. A hypermorphic missense mutation in PLCG2, encoding phospholipase Cγ2, causes a dominantly inherited autoinflammatory disease with immunodeficiency. Am J Hum Genet. 2012;91:713–720. doi: 10.1016/j.ajhg.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539–2549. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hantschel O, Rix U, Schmidt U, et al. The Btk tyrosine kinase is a major target of the Bcr-Abl inhibitor dasatinib. Proc Natl Acad Sci U S A. 2007;104:13283–13288. doi: 10.1073/pnas.0702654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woyach JA, Smucker K, Smith LL, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood. 2014;123:1810–1817. doi: 10.1182/blood-2013-09-527853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non–small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 26.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Awad MM, Katayama R, McTigue M, et al. Acquired resistance to crizotinib from a mutation in CD74–ROS1. N Engl J Med. 2013;368:2395–2401. doi: 10.1056/NEJMoa1215530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonescu CR, Besmer P, Guo T, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res. 2005;11:4182–4190. doi: 10.1158/1078-0432.CCR-04-2245. [DOI] [PubMed] [Google Scholar]
- 29.Smith CC, Wang Q, Chin CS, et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012;485:260–263. doi: 10.1038/nature11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heidel F, Solem FK, Breitenbuecher F, et al. Clinical resistance to the kinase inhibitor PKC412 in acute myeloid leukemia by mutation of Asn-676 in the FLT3 tyrosine kinase domain. Blood. 2006;107:293–300. doi: 10.1182/blood-2005-06-2469. [DOI] [PubMed] [Google Scholar]
- 31.Väliaho J, Smith CI, Vihinen M. BTK-base: the mutation database for X-linked agammaglobulinemia. Hum Mutat. 2006;27:1209–1217. doi: 10.1002/humu.20410. [DOI] [PubMed] [Google Scholar]
- 32.Burger JA, Landau D, Hoellenriegel J, et al. Clonal evolution in patients with chronic lymphocytic leukemia (CLL) developing resistance to BTK inhibition. Blood. 2013;122:866. doi: 10.1038/ncomms11589. abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.