Abstract
OBJECTIVE
To perform an econometric analysis to examine the influence of procedure volume, variation in hospital accounting methodology, and use of various analytic methodologies on cost of robotically assisted hysterectomy for benign gynecologic disease and endometrial cancer.
METHODS
A national sample was used to identify women who underwent laparoscopic or robotically assisted hysterectomy for benign indications or endometrial cancer from 2006 to 2012. Surgeon and hospital volume were classified as the number of procedures performed before the index surgery. Total costs as well as fixed and variable costs were modeled using multivariable quantile regression methodology.
RESULTS
A total of 180,230 women, including 169,324 women who underwent minimally invasive hysterectomy for benign indications and 10,906 patients whose hysterectomy was performed for endometrial cancer, were identified. The unadjusted median cost of robotically assisted hysterectomy for benign indications was $8,152 (interquartile range [IQR] $6,011–10,932) compared with $6,535 (IQR $5,127–8,357) for laparoscopic hysterectomy (P<.001). The cost differential decreased with increasing surgeon and hospital volume. The unadjusted median cost of robotically assisted hysterectomy for endometrial cancer was $9,691 (IQR $7,591–12,428) compared with $8,237 (IQR $6,400–10,807) for laparoscopic hysterectomy (P<.001). The cost differential decreased with increasing hospital volume from $2,471 for the first 5 to 15 cases to $924 for more than 50 cases. Based on surgeon volume, robotically assisted hysterectomy for endometrial cancer was $1,761 more expensive than laparoscopy for those who had performed fewer than five cases; the differential declined to $688 for more than 50 procedures compared with laparoscopic hysterectomy.
CONCLUSION
The cost of robotic gynecologic surgery decreases with increased procedure volume. However, in all of the scenarios modeled, robotically assisted hysterectomy remained substantially more costly than laparoscopic hysterectomy.
Recent population-based studies have shown that robotic-assisted hysterectomy is now frequently performed for benign gynecologic diseases and for oncologic indications.1,2 Despite the rapid uptake of robotic surgery, the comparative effectiveness of robotically assisted hysterectomy remains uncertain.1–11
To date, the majority of previous studies have been unable to demonstrate improved outcomes for robotic-assisted hysterectomy compared with laparoscopic hysterectomy.1–11 Although the morbidity profile of robotic-assisted hysterectomy appears to be reasonable, a major concern for the procedure stems from the high costs associated with the operation.1,4,9–12 Compared with laparoscopic hysterectomy, costs for robotic-assisted hysterectomy are 16% to 34% higher.1,2,9,12 The high cost of robotic surgery is likely driven by a number of factors, including capital costs for the robotic system, maintenance, the cost of disposable instrumentation, and the longer operative times that these procedures often require.12
Although the high cost of robotic surgery represents a major public health concern, proponents of robotic surgery have suggested that the technology can be made more cost-effective. First, previous studies may, in part, reflect the learning curve of a new technology with longer operative times.13–18 Second, many cost studies have reported data across multiple hospitals that capture costs from a variety of cost-reporting methods. Finally, cost data are often not normally distributed and thus are sensitive to the analytic methodology used.19,20 Given these concerns, we performed a detailed economic analysis of the cost of robotic-assisted hysterectomy and examined the influence of procedural volume, hospital accounting systems, and the use of various analytic methodologies on cost for women undergoing robotic-assisted hysterectomy.
MATERIALS AND METHODS
The Perspective database was used for analysis. Perspective captures comprehensive billing data of all hospital admissions from more than 500 acute care facilities from throughout the United States. The database collected data for nearly 5.5 million discharges in 2006, which represents approximately 15% of hospitalizations in the United States.21 The study was deemed exempt by the Columbia University Institutional Review Board.
Women 18 to 90 years of age who underwent a minimally invasive hysterectomy from 2006 to 2012 were analyzed. We initially selected patients who had a code for a laparoscopic hysterectomy (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM] codes 68.31, 68.41, 68.51). Those women who had either an ICD-9-CM procedure code for a robotic-assisted procedure (ICD-9-CM 17.42 or 17.44) or a recorded charge code for robotic instrumentation were classified as having undergone a robotically assisted hysterectomy as previously described.2,22 Women with a gynecologic malignancy other than endometrial cancer were excluded. The cohort was then stratified into the following two groups: those with endometrial cancer (ICD-9-CM 182.x) and those without gynecologic cancer who underwent hysterectomy for benign indications.
Clinical and demographic characteristics including age at the time of the procedure (younger than 50, 50–59, 60–69, and older than 70 years), race (white, black, other), marital status (married, single, unknown), year of diagnosis (2006 to 2012), and insurance status (commercial, Medicare, Medicaid, uninsured, and unknown) were recorded. For women who underwent hysterectomy for benign indications, we noted the following gynecologic conditions: leiomyomas; endometriosis; abnormal bleeding; benign ovarian neoplasms; and pelvic organ prolapse. The performance of concomitant gynecologic procedures, including anterior colporrhaphy, posterior colporrhaphy, salpingo-oophorectomy, incontinence surgery, and lymphadenectomy, were also noted.
Hospital characteristics including location (metropolitan and nonmetropolitan), region of the country (northeast, midwest, west, and south), size (fewer than 400 beds, 400–600 beds, and more than 600 beds), and teaching status (teaching and nonteaching) were recorded for each patient. Risk adjustment for comorbid medical conditions was performed using the Elixhauser comorbidity index. Women were classified based on the number of medical comorbidities as 0, 1, or 2 or more, as previously reported.23
Physician and hospital volume were determined for each patient. Both hospital and surgeon volume were calculated individually for each patient and estimated as the number of procedures performed at a given patient’s hospital or by a given patient’s surgeon before the index procedure. Separate volume-based calculations were performed for robotic-assisted and laparoscopic hysterectomy. Volume was calculated separately for procedures for endometrial cancer and benign indications. Volume was included as a continuous variable in all of the multivariable models.24
To determine the effect of complications on cost, we examined perioperative morbidity. The following perioperative complications were analyzed: intraoperative complications (bladder injury, ureteral injury, intestinal injury, vascular injury, and other operative injury); surgical site complications (wound complications, abscess, hemorrhage, bowel obstruction, ileus); and medical complications (venous thromboembolism, myocardial infarction, cardiopulmonary arrest, acute renal failure, respiratory failure, stroke, bacteremia or sepsis, shock, and pneumonia).2,9 Any morbidity, a composite score of any of these complications, was analyzed.
The primary outcome of the analysis was cost. Cost represents the monetary value to perform a service, whereas charges are based on what a hospital bills for the service. We directly analyzed cost. Perspective captures cost data through an itemized log of all items and services billed to a patient during the acute hospitalization. Cost data represent the cost of the entire index hospitalization. Within the database, hospitals report cost either through direct internal accounting systems or through Medicare cost-to-charge ratios.21,25 The type of accounting performed by each hospital was recorded and separate sensitivity analyses based on the type of accounting system were performed as described. All costs were adjusted for inflation using the Consumer Price Index and reported in 2012 U.S. dollars.26 Converted cost data were inspected and patients with spurious costs (less than $500) were removed from the cost analyses.2 We examined total costs and performed separate analyses for fixed and variable costs. Fixed costs are those costs attributable to capital equipment and maintenance, whereas variable costs are attributable to the operation of the hospital irrespective of fixed costs.27
Women who underwent hysterectomy for benign disease and those who underwent hysterectomy for endometrial cancer were analyzed separately. Frequency distributions between categorical variables for those who underwent laparoscopic hysterectomy and those who underwent robotically assisted hysterectomy were compared using χ2 tests, and median values of continuous variables were compared using Wilcoxon rank-sum tests. Cost data for each of the groups are reported as medians with interquartile ranges. Distributions of cost based on previous procedural volume are displayed graphically with previous surgical volume broken down into deciles.
Multivariable adjustments of cost were performed using quantile (median) regression methodology.19 Quantile regression directly estimates the adjusted median costs and 95% confidence intervals (CIs) were derived based on bootstrap resampling methods. A series of cost models were developed. We first developed an unadjusted model based only on the route of hysterectomy. A similar model was constructed that only included hospitals that reported cost based on direct internal accounting systems. A fully adjusted model that reports cost after adjustment for all of the clinical, demographic, physician, and hospital characteristics was then described. Similarly, a fully adjusted model excluding patients with any perioperative complication was shown. Finally, a series of stratified models were developed that included only patients based on the volume of the attending surgeon (fewer than 5 procedures, 5–15 procedures, 16–30 procedures, and more than 50 procedures).
A series of sensitivity analyses of the adjusted and unadjusted cost models were performed after log-transformation of the data. All analyses were performed with SAS 9.2. All statistical tests were two-sided. P<.05 was considered statistically significant.
RESULTS
A total of 180,230 women were identified. The cohort included 169,324 women who underwent minimally invasive hysterectomy for benign indications and 10,906 patients whose hysterectomy was performed for endometrial cancer. Robotically assisted hysterectomy accounted for 30.4% of the hysterectomies for benign disorders and 59.6% of hysterectomies for endometrial cancer. The clinical and demographic characteristics of the cohort are displayed in Table 1.
Table 1.
Clinical and Demographic Characteristics for Women Who Underwent Minimally Invasive Hysterectomy
| Characteristic | Benign Indications
|
Endometrial Cancer
|
||||
|---|---|---|---|---|---|---|
| Laparoscopic | Robotically Assisted | P | Laparoscopic | Robotically Assisted | P | |
| Total | 117,832 (69.6) | 51,492 (30.4) | 4,404 (40.4) | 6,502 (59.6) | ||
| Age (y) | <.001 | <.001 | ||||
| Younger than 50 | 90,346 (76.7) | 37,088 (72.0) | 536 (12.2) | 683 (10.5) | ||
| 50–59 | 19,042 (16.2) | 9,626 (18.7) | 1,345 (30.5) | 1,826 (28.1) | ||
| 60–69 | 6,120 (5.2) | 3,371 (6.6) | 1,413 (32.1) | 2,348 (36.1) | ||
| 70 or older | 2,324 (2.0) | 1,407 (2.7) | 1,110 (25.2) | 1,645 (25.3) | ||
| Race | <.001 | <.001 | ||||
| White | 86,233 (73.2) | 36,319 (70.5) | 3,039 (69.0) | 4,886 (75.2) | ||
| Black | 11,420 (9.7) | 6,405 (12.4) | 332 (7.5) | 390 (6.0) | ||
| Other | 20,179 (17.1) | 8,768 (17.0) | 1,033 (23.5) | 1,226 (18.9) | ||
| Year of diagnosis | <.001 | <.001 | ||||
| 2006 | 13,412 (11.4) | 146 (0.3) | 475 (10.8) | 12 (0.2) | ||
| 2007 | 15,251 (12.9) | 507 (1.0) | 739 (16.8) | 95 (1.5) | ||
| 2008 | 15,239 (12.9) | 1,445 (2.8) | 754 (17.1) | 297 (4.6) | ||
| 2009 | 17,132 (14.5) | 4,644 (9.0) | 611 (13.9) | 678 (10.4) | ||
| 2010 | 19,245 (16.3) | 9,129 (17.7) | 657 (14.9) | 1,251 (19.2) | ||
| 2011 | 19,522 (16.6) | 15,924 (30.9) | 601 (13.7) | 1,888 (29.0) | ||
| 2012 | 18,031 (15.3) | 19,697 (38.3) | 567 (12.9) | 2,281 (35.1) | ||
| Insurance | <.001 | .10 | ||||
| Commercial | 89,414 (75.9) | 39,550 (76.8) | 2,304 (52.3) | 3,340 (51.4) | ||
| Medicare | 7,822 (6.6) | 4,390 (8.5) | 2,304 (38.7) | 2,639 (40.6) | ||
| Medicaid | 11,897 (10.1) | 4,548 (8.8) | 158 (3.6) | 223 (3.4) | ||
| Uninsured | 3,825 (3.3) | 1,279 (2.5) | 143 (3.3) | 167 (2.6) | ||
| Unknown | 4,874 (4.1) | 1,725 (3.5) | 97 (2.2) | 133 (2.1) | ||
| Marital status | <.001 | <.001 | ||||
| Married | 72,221 (61.3) | 30,337 (58.9) | 2,126 (48.3) | 3,236 (49.8) | ||
| Single | 33,348 (28.3) | 15,873 (30.8) | 1,640 (37.2) | 2,518 (38.7) | ||
| Unknown | 12,263 (10.4) | 5,282 (10.3) | 638 (14.5) | 748 (11.5) | ||
| Area of residence | <.001 | <.001 | ||||
| Metropolitan | 102,532 (87.0) | 45,785 (88.9) | 4,207 (95.5) | 6,302 (96.9) | ||
| Nonmetropolitan | 15,300 (13.0) | 5,707 (11.1) | 197 (4.5) | 200 (3.1) | ||
| Region | <.001 | <.001 | ||||
| Eastern | 9,308 (7.9) | 4,693 (9.1) | 876 (19.9) | 1,227 (18.9) | ||
| Midwest | 24,277 (20.6) | 11,784 (22.9) | 966 (21.9) | 1,313 (20.2) | ||
| South | 60,850 (51.6) | 27,928 (54.2) | 1,802 (40.9) | 2,654 (40.8) | ||
| West | 23,397 (19.9) | 7,087 (13.8) | 760 (17.3) | 1,308 (20.1) | ||
| Comorbidity | <.001 | .12 | ||||
| 0 | 48,460 (41.1) | 19,679 (38.2) | ||||
| 1 | 32,666 (27.7) | 14,444 (28.1) | 768 (17.4) | 1,039 (16.0) | ||
| 2 | 17,434 (67.9) | 8,254 (16.0) | 1,053 (23.9) | 1,555 (23.9) | ||
| 3 or more | 19,272 (16.4) | 9,115 (17.7) | 2,583 (58.7) | 3,908 (60.1) | ||
| Hospital type | <.001 | <.001 | ||||
| Nonteaching | 81,850 (69.5) | 32,998 (64.1) | 2,074 (47.1) | 2,674 (41.1) | ||
| Teaching | 35,982 (30.5) | 18,494 (35.9) | 2,330 (52.9) | 3,828 (58.9) | ||
| Hospital size (beds) | <.001 | <.001 | ||||
| Fewer than 400 | 70,809 (60.1) | 25,509 (49.5) | 1,805 (41.0) | 2,029 (31.2) | ||
| 400–600 | 28,020 (23.8) | 16,668 (32.4) | 1,633 (37.1) | 2,610 (40.1) | ||
| More than 600 | 19,003 (16.1) | 9,315 (18.1) | 966 (21.9) | 1,863 (28.7) | ||
| Hospital volume | ||||||
| Lowest | 19,561 (25.1) | 12,888 (25.0) | 1,137 (25.8) | 1,624 (25.0) | ||
| Second | 29,377 (24.9) | 12,877 (25.0) | 1,055 (24.0) | 1,625 (25.0) | ||
| Third | 29,410 (25.0) | 12,839 (24.9) | 1,103 (25.1) | 1,610 (24.8) | ||
| Highest | 29,484 (25.0) | 12,888 (25.0) | 1,109 (25.2) | 1,643 (25.3) | ||
| Surgeon volume | ||||||
| Lowest | 29,961 (25.4) | 12,142 (23.6) | 1,177 (26.7) | 1,471 (22.6) | ||
| Second | 28,289 (24.0) | 13,161 (25.6) | 954 (21.7) | 1,697 (26.1) | ||
| Third | 30,409 (25.8) | 13,249 (25.7) | 1,189 (27.0) | 1,739 (26.8) | ||
| Highest | 29,173 (24.8) | 12,940 (25.1) | 1,084 (24.6) | 1,595 (24.5) | ||
| Procedure | ||||||
| Anterior repair | 7,217 (6.1) | 1,187 (2.3) | <.001 | 52 (1.2) | 19 (0.3) | <.001 |
| Posterior repair | 6,690 (5.7) | 1,200 (2.3) | <.001 | 40 (0.9) | 17 (0.3) | <.001 |
| Anti-incontinence | 11,884 (10.1) | 3,139 (6.1) | <.001 | 69 (1.6) | 68 (1.1) | .02 |
| Oophorectomy | 63,058 (53.5) | 26,693 (51.8) | <.001 | 4,218 (95.8) | 6,316 (97.1) | .001 |
| Lymphadenectomy | 2,446 (55.5) | 4,489 (69.0) | <.001 | |||
| Indication | ||||||
| Leiomyoma | 54,662 (46.4) | 27,390 (53.2) | <.001 | |||
| Endometriosis | 39,106 (33.2) | 16,125 (31.3) | <.001 | |||
| Abnormal bleeding | 70,149 (59.5) | 30,128 (58.5) | <.001 | |||
| Benign neoplasm | 32,131 (27.3) | 15,032 (29.2) | <.001 | |||
| Pelvic organ prolapse | 20,940 (17.8) | 4,682 (9.1) | <.001 | |||
| Cost data | <.001 | <.001 | ||||
| Direct costing | 85,679 (72.7) | 40,530 (78.7) | 3,173 (72.1) | 5,217 (80.2) | ||
| Cost-to-charge ratio | 32,153 (27.3) | 10,962 (21.3) | 1,231 (28.0) | 1,285 (19.8) | ||
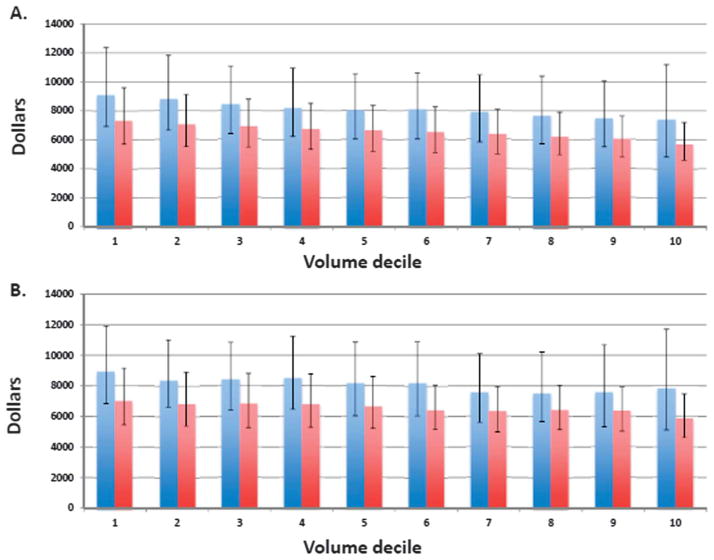
The unadjusted median cost of robotically assisted hysterectomy for benign indications was $8,152 (interquartile range [IQR] $6,011–10,932) compared with $6,535 (IQR $5,127–8,357) for laparoscopic hysterectomy (Table 2). Median fixed costs were $3,591 (IQR $2,347–5,345) for robotic-assisted hysterectomy compared with $2,965 (IQR $2,137–4,051) for laparoscopic hysterectomy, whereas variable costs were $4,384 (IQR $3,170–5,883) and $3,440 (IQR $2,633–4,513) for the two procedures, respectively. The cost of both robotic-assisted and laparoscopic hysterectomy for benign indications decreased with increasing procedural volume. Figure 1 displays the median total cost of robotically assisted and laparoscopic hysterectomy for benign indications based on previous surgeon (Fig. 1A) and hospital (Fig. 1B) volume.
Table 2.
Unadjusted Cost Estimates for Minimally Invasive Hysterectomy for Benign Indications Stratified by Route of Surgery and Hospital and Physician Volume
| Case volume | Laparoscopic ($) | Robotically Assisted ($) | P | Cost Differential (Robotically Assisted vs Laparoscopic) ($) |
|---|---|---|---|---|
| Hospital volume | ||||
| Any volume | ||||
| Total cost | 6,535 (5,127–8,357) | 8,152 (6,011–10,932) | <.001 | 1,617 |
| Variable cost | 2,965 (2,137–4,051) | 3,591 (2,347–5,345) | <.001 | 626 |
| Fixed cost | 3,440 (2,633–4,513) | 4,384 (3,170–5,883) | <.001 | 944 |
| Fewer than 5 cases | ||||
| Total cost | 7,262 (5,579–9,638) | 9,315 (6,956–12,495) | <.001 | 2,053 |
| Variable cost | 3,865 (2,819–5,257) | 4,859 (3,704–6,759) | <.001 | 994 |
| Fixed cost | 3,288 (2,335–4,671) | 4,260 (2,788–5,989) | <.001 | 972 |
| 5–15 cases | ||||
| Total cost | 7,024 (5,475–9,149) | 9,031 (6,934–12,044) | <.001 | 2,007 |
| Variable cost | 3,766 (2,767–5,087) | 4,788 (3,644–6,487) | <.001 | 1,022 |
| Fixed cost | 3,223 (2,290–4,386) | 4,035 (2,754, –5,794) | <.001 | 812 |
| 16–30 cases | ||||
| Total cost | 6,862 (5,357–8,983) | 8,591 (6,649–11,371) | <.001 | 1,729 |
| Variable cost | 3,643 (2,760–4,893) | 4,723 (3,537–6,255) | <.001 | 1,080 |
| Fixed cost | 3,120 (2,208–4,252) | 3,722 (2,597–5,309) | <.001 | 602 |
| 31–50 cases | ||||
| Total cost | 6,888 (5,404–8,985) | 8,545 (6,678–11,147) | <.001 | 1,657 |
| Variable cost | 3,639 (2,763–4,893) | 4,661 (3,440–6,183) | <.001 | 1,022 |
| Fixed cost | 3,137 (2,234–4,278) | 3,806 (2,640–5,298) | <.001 | 669 |
| More than 50 cases | ||||
| Total cost | 6,466 (5,076–8,225) | 8,026 (5,838–10,789) | <.001 | 1,560 |
| Variable cost | 3,399 (2,610–4,431) | 4,299 (3,095–5,791) | <.001 | 900 |
| Fixed cost | 2,929 (3,119–3,997) | 3,525 (2,274–5,312) | <.001 | 596 |
| Physician volume | ||||
| Any volume | ||||
| Total cost | 6,535 (5,127–8,357) | 8,152 (6,011–10,932) | <.001 | 1,617 |
| Variable cost | 2,965 (2,137–4,051) | 3,591 (2,347–5,345) | <.001 | 626 |
| Fixed cost | 3,440 (2,633–4,513) | 4,384 (3,170–5,883) | <.001 | 944 |
| Fewer than 5 cases | ||||
| Total cost | 7,113 (5,574–9,168) | 8,818 (6,660–11,837) | <.001 | 1,705 |
| Variable cost | 3,678 (2,805–4,882) | 4,718 (3,485–6,327) | <.001 | 1,040 |
| Fixed cost | 3,284 (2,372–4,480) | 3,994 (2,659–5,739) | <.001 | 710 |
| 5–15 cases | ||||
| Total cost | 6,674 (5,252–8,419) | 8,141 (6,153–10,732) | <.001 | 1,467 |
| Variable cost | 3,488 (2,609–4,384) | 4,429 (3,240–4,811) | <.001 | 941 |
| Fixed cost | 2,865 (2,076–3,939) | 3,592 (2,384–5,212) | <.001 | 727 |
| 16–30 cases | ||||
| Total cost | 6,400 (5,029–8,144) | 7,959 (5,921–10,584) | <.001 | 1,559 |
| Variable cost | 3,388 (2,609–4,384) | 4,314 (3,070–5,773) | <.001 | 926 |
| Fixed cost | 2,865 (2,076–3,939) | 3,473 (2,280–5,114) | <.001 | 608 |
| 31–50 cases | ||||
| Total cost | 6,234 (4,952–7,856) | 7,624 (5,636–10,243) | <.001 | 1,390 |
| Variable cost | 3,353 (2,591–4,311) | 4,095 (2,983–5,558) | <.001 | 742 |
| Fixed cost | 2,772 (2,009–3,720) | 3,319 (2,136–4,913) | <.001 | 547 |
| More than 50 cases | ||||
| Total cost | 5,854 (4,668–7,399) | 7,473 (5,075–10,645) | <.001 | 1,619 |
| Variable cost | 3,135 (2,409–4,097) | 4,025 (2,881–5,574) | <.001 | 890 |
| Fixed cost | 2,620 (1,905–3,567) | 3,267 (2,053–5,364) | <.001 | 647 |
Data are median (interquartile range) unless otherwise specified.
Fig. 1.
Cost based on previous surgical volume for robotically assisted hysterectomy for benign indications. Previous surgical volume is stratified by deciles and data are presented as medians with interquartile ranges. Blue bars represent robotically assisted hysterectomy; red bars represent laparoscopic hysterectomy. A. Cost based on previous surgeon volume. B. Cost based on previous hospital volume. Figure is truncated at 200 cases.
Wright. Economics of Robotically Assisted Hysterectomy. Obstet Gynecol 2014.
Table 2 displays the unadjusted median costs for hysterectomy for benign by procedural volume. At hospitals that had performed fewer than five previous cases, robotically assisted hysterectomy was $2,053 more expensive. The cost differential decreased with increasing hospital volume as follows: 5 to 15 cases (+$2,007), 16 to 30 cases (+$1,729), 31 to 50 cases (+$1,657), and more than 50 cases (+$1,560). Among surgeons, robotically assisted hysterectomy was $1,705 more expensive than laparoscopic hysterectomy for those who had performed fewer than five cases, whereas costs declined to +$1,467 for 5 to 15 cases, +$1,559 for 16 to 30 cases, +$1,390 for 31 to 50 cases, and +$1,619 for more than 50 procedures.
The unadjusted median cost of robotic-assisted hysterectomy for endometrial cancer was $9,691 (IQR $7,591–12,428) compared with $8,237 (IQR $6,400–10,807) for laparoscopic hysterectomy (Table 3). Median fixed costs were $4,543 (IQR $3,201–6,164) for robotic-assisted hysterectomy for endometrial cancer compared with $3,790 (IQR $2,832–5,406) for laparoscopic hysterectomy, whereas variable costs were $5,065 (IQR $3,994–6,536) and $4,215 ($3,098–5,644) for the two procedures, respectively. The cost of both robotically assisted and laparoscopic hysterectomy for endometrial cancer decreased with increasing procedural volume. Figure 2 displays the median total cost of robotically assisted and laparoscopic hysterectomy for endometrial cancer based on previous surgeon (Figure 2A) and hospital (Figure 2B) volume.
Table 3.
Unadjusted Cost Estimates for Minimally Invasive Hysterectomy for Endometrial Cancer Stratified by Route of Surgery and Hospital and Physician Volume
| Case volume | Laparoscopic ($) | Robotically Assisted ($) | P | Cost Differential (Robotically Assisted vs Laparoscopic) ($) |
|---|---|---|---|---|
| Hospital volume | ||||
| Any volume | ||||
| Total cost | 8,237 (6,400–10,807) | 9,691 (7,591–12,428) | <.001 | 1,454 |
| Variable cost | 3,790 (2,832–5,406) | 4,543 (3,201–6,164) | <.001 | 753 |
| Fixed cost | 4,215 (3,098–5,644) | 5,065 (3,994–6,536) | <.001 | 850 |
| Fewer than 5 cases | ||||
| Total cost | 7,998 (6,230–10,476) | 10,469 (8,181–13,362) | <.001 | 2,471 |
| Variable cost | 4,096 (3,021–5,510) | 5,453 (3,959–7,240) | <.001 | 1,357 |
| Fixed cost | 3,653 (2,610–5,106) | 4,791 (3,384–6,677) | <.001 | 1,138 |
| 5–15 cases | ||||
| Total cost | 8,204 (6,423–11,261) | 10,188 (8,039–13,273) | <.001 | 1,984 |
| Variable cost | 4,286 (3,123–5,729) | 5,379 (4,223–6,876) | <.001 | 1,093 |
| Fixed cost | 3,807 (2,740–5,711) | 4,708 (3,276–6,408) | <.001 | 901 |
| 16–30 cases | ||||
| Total cost | 8,122 (6,450–11,199) | 9,875 (7,720–12,646) | <.001 | 1,753 |
| Variable cost | 4,199 (3,167–5,754) | 5,283 (4,191–6,717) | <.001 | 1,084 |
| Fixed cost | 3,644 (2,839–5,511) | 4,473 (3,194–6,173) | <.001 | 829 |
| 31–50 cases | ||||
| Total cost | 8,288 (6,533–11,110) | 9,691 (7,986–12,271) | <.001 | 1,403 |
| Variable cost | 4,243 (3,006–5,829) | 5,212 (4,302–6,619) | <.001 | 972 |
| Fixed cost | 3,990 (2,960–5,605) | 4,538 (3,328–6,011) | <.001 | 548 |
| More than 50 cases | ||||
| Total cost | 8,332 (6,426–10,606) | 9,256 (7,257–11,914) | <.001 | 924 |
| Variable cost | 4,270 (3,150–5,559) | 4,701 (3,804–5,985) | <.001 | 431 |
| Fixed cost | 4,270 (3,150–5,559) | 4,502 (3,101–5,973) | <.001 | 232 |
| Physician volume | ||||
| Any volume | ||||
| Total cost | 8,237 (6,400– 10,807) | 9,691 (7,591–12,428) | <.001 | 1,454 |
| Variable cost | 3,790 (2,832–5,406) | 4,543 (3,201–6,164) | <.001 | 753 |
| Fixed cost | 4,215 (3,098–5,644) | 5,065 (3,994–6,536) | <.001 | 850 |
| Fewer than 5 cases | ||||
| Total cost | 8,491 (6,572–11,009) | 10,252 (8,008–13,231) | <.001 | 1,761 |
| Variable cost | 4,306 (3,181–5,681) | 5,362 (4,092–7,006) | <.001 | 1,056 |
| Fixed cost | 3,921 (2,874–5,499) | 4,784 (3,339–6,510) | <.001 | 863 |
| 5–15 cases | ||||
| Total cost | 8,265 (6,370–11,211) | 9,531 (7,609–12,054) | <.001 | 1,266 |
| Variable cost | 4,294 (3,054–5,812) | 5,103 (4,118–6,365) | <.001 | 809 |
| Fixed cost | 3,866 (2,765–5,652) | 4,471 (3,097–5,987) | <.001 | 605 |
| 16–30 cases | ||||
| Total cost | 7,812 (6,170–10,412) | 9,377 (7,432–12,039) | <.001 | 1,565 |
| Variable cost | 3,931 (2,938–5,698) | 4,902 (3,933–6,215) | <.001 | 971 |
| Fixed cost | 3,710 (2,929–5,147) | 4,398 (3,266–5,920) | <.001 | 688 |
| 31–50 cases | ||||
| Total cost | 8,213 (6,252–10,531) | 9,664 (7,539–12,195) | <.001 | 1,451 |
| Variable cost | 4,096 (2,798–5,532) | 4,974 (3,870–6,268) | <.001 | 878 |
| Fixed cost | 3,573 (2,906–5,543) | 4,697 (3,445–6,052) | <.001 | 1,124 |
| More than 50 cases | ||||
| Total cost | 7,267 (5,924–8,611) | 7,955 (6,574–10,457) | <.001 | 688 |
| Variable cost | 3,898 (2,996–4,651) | 4,210 (3,487–5,039) | <.001 | 312 |
| Fixed cost | 3,128 (2,522–4,005) | 3,602 (2,977–5,654) | <.001 | 474 |
Data are median (interquartile range) unless otherwise specified.
Fig. 2.
Cost based on previous surgical volume for robotically assisted hysterectomy for endometrial cancer. Previous surgical volume is stratified by deciles and data are presented as medians with interquartile ranges. Blue bars represent robotically assisted hysterectomy; red bars represent laparoscopic hysterectomy. A. Cost based on previous surgeon volume. B. Cost based on previous hospital volume.
Wright. Economics of Robotically Assisted Hysterectomy. Obstet Gynecol 2014.
At hospitals that had performed fewer than five previous cases for endometrial cancer, robotically assisted hysterectomy was $2,471 more expensive than laparoscopic hysterectomy. The cost differential decreased with increasing hospital volume: 5 to 15 cases (+1,984), 16 to 30 cases (+$1,753), 31 to 50 cases (+$1,403), and more than 50 cases (+$924). Among surgeons, robotically assisted hysterectomy for endometrial cancer was $1,761 more expensive for those who had performed fewer than five cases, whereas costs declined to +$1,266 for 5 to 15 cases, +$1,565 for 16 to 30 cases, +$1,451 for 31 to 50 cases, and +$688 for more than 50 procedures.
In a series of adjusted models, these results were largely unchanged (Table 4). In a fully adjusted model, compared with laparoscopic hysterectomy, robotically assisted hysterectomy for benign indications was $1,225 (95% CI $1,177–1,272) more expensive and $1,328 (95% CI $1,164–1,491) more costly for endometrial cancer. When the analysis was limited to hospitals that used direct costing methods, the cost differential associated with robotic procedures was greater; robotically assisted benign hysterectomy was $1,995 (95% CI $1,948–2,041) more than laparoscopic hysterectomy. Removing patients who experienced complications had minimal effects on the estimates. Like the unadjusted models, cost declined with volume. Results were similar for fixed and variable costs and after log transformation of the data (not shown).
Table 4.
Cost Models of Minimally Invasive Hysterectomy
| Analysis | Benign Indications | Endometrial Cancer |
|---|---|---|
| Unadjusted | ||
| Total cost | 1,617 (1,581–1,653) | 1,454 (1,279–1,629) |
| Variable cost | 943 (918–969) | 850 (752–948) |
| Fixed cost | 626 (602–649) | 753 (672–835) |
| Unadjusted direct costing hospitals only | ||
| Total cost | 1,995 (1,948–2,041) | 1,875 (1,670–2,081) |
| Variable cost | 1,132 (1,107–1,158) | 1,017 (907–1,128) |
| Fixed cost | 801 (774–828) | 922 (799–1,046) |
| Fully adjusted* | ||
| Total cost | 1,225 (1,177–1,272) | 1,328 (1,164–1,491) |
| Variable cost | 922 (895–948) | 936 (847–1,024) |
| Fixed cost | 374 (349–399) | 453 (361–544) |
| Fully adjusted, complications removed | ||
| Total cost | 1,222 (1,171–1,272) | 1,375 (1,219–1,530) |
| Variable cost | 920 (896–944) | 956 (867–1,045) |
| Fixed cost | 372 (347–396) | 432 (347–517) |
| Fully adjusted, physician volume fewer than 5 cases | ||
| Total cost | 1,443 (1,362–1,524) | 1,495 (1,284–1,705) |
| Variable cost | 974 (927–1,021) | 985 (850–1,120) |
| Fixed cost | 566 (412–619) | 560 (418–701) |
| Fully adjusted, physician volume 16–30 cases | ||
| Total cost | 1,084 (976–1,192) | 1,687 (1,317–2,056) |
| Variable cost | 920 (855–986) | 1,421 (1,203–1,640) |
| Fixed cost | 303 (234–372) | 332 (82–582) |
| Fully adjusted, physician volume more than 50 cases | ||
| Total cost | 1,290 (1,170–1,409) | 832 (393–1,270)† |
| Variable cost | 892 (837–947) | 311 (73–549)† |
| Fixed cost | 334 (271–396) | 585 (313–858)† |
Data are U.S. dollars (95% confidence interval).
Cost differential of robotically assisted compared with laparoscopic hysterectomy.
Fully adjusted model incorporates all clinical, demographic, physician, and hospital characteristics.
Provider area excluded from models because of absence of patients in one cell.
DISCUSSION
Our data suggests that the cost of robotic gynecologic surgery decreases with increasing procedural volume. The reduction in costs was most pronounced for women undergoing robotically assisted hysterectomy for endometrial cancer and was more modest when the surgery was performed for benign indications. Despite the reductions in cost associated with increased procedural volume, in all of the scenarios modeled, robotic-assisted hysterectomy remained substantially more costly than laparoscopic hysterectomy.
The introduction of new surgical technologies is often associated with a learning curve.13,15–17 An institutional analysis of 325 patients who underwent robotic-assisted hysterectomy noted that although increasing surgical experience was associated with shorter operative times and decreased length of stay, surgical experience had no effect on complications. The investigators noted that operative time decreased from 3.5 hours during the first 6 months of robot use to 2.7 hours per procedure.13 We noted that cost decreased with the number of previous procedures performed for both hospitals and surgeons.
The association between surgical volume and outcomes has been well-described over the course of the past two decades.28,29 For high-risk oncologic and cardiovascular procedures, increased surgeon and hospital procedural volume are associated with decreased morbidity and mortality.28,29 For lower-morbidity procedures, including most gynecologic operations, the association between increased volume and decreased complications is more modest.30–33 However, for many gynecologic procedures, increased procedural volume is associated with lower resource use and decreased costs.30–33 The current study suggests similar trends for robotically assisted hysterectomy.
The relationship between increased volume and decreased cost appeared to be greater when robotically assisted hysterectomy was performed for endometrial cancer than when the operation was used for benign indications. Further, for hysterectomy for benign indications, the relative magnitudes of cost reductions for robotically assisted and laparoscopic hysterectomy associated with increasing surgeon volume were similar. We noted that surgeon volume had a more meaningful influence on cost than hospital volume. Given that hysterectomy is performed much more frequently for benign indications than for cancer, the limited reduction in the cost differential for benign indications with higher volume has important public health implications.34
Our study addresses a number of methodologic concerns in the analysis of the cost. First, both hospital volume and surgeon procedural volume were measured as the number of operations performed before the index case and as continuous variables.24 This approach was meant to account for the learning curve for the operation. Second, separate analyses were performed for hospitals that used direct internal accounting systems compared with those that estimate cost based on cost-to-charge ratios. When analyzing only those hospitals that directly report actual costs, we noted that the cost differential of robotic-assisted hysterectomy was greater than that in the analysis of all hospitals. We used a number of statistical methodologies to account for right skewed data such as cost.19,20
We recognize a number of important limitations. We cannot exclude the possibility that some procedures were misclassified. However, the classification system we used to identify robotic procedures has been used in previous work and was previously validated.2 Our classification of physician volume only captured a surgeon’s patients within a given hospital, and we were unable to link physicians across hospitals. There is likely variation across hospitals in fixed costs for the robotic platform and instrumentation based on different negotiated prices. We were unable to capture some factors, including tumor characteristics, weight, and surgical history, that undoubtedly influenced outcomes. Finally, our data present cost from a hospital perspective. Previous work has suggested that increased use of robotic-assisted hysterectomy is associated with a decreased rate of abdominal hysterectomy that likely provides important cost reductions from a societal standpoint.
Our study suggests that costs are reduced with both increased surgeon experience and hospital experience, although the reduction in cost is affected to a greater degree by surgeon rather than hospital volume. Although the cost reduction is multifactorial, the cost savings are likely from a combination of shorter operative times and reduced length of stay. Our findings are also notable in that no matter how the two procedures were modeled, in similar circumstances laparoscopic hysterectomy always remained less costly than robotically assisted hysterectomy. Even for very high-volume surgeons and centers, robotically assisted hysterectomy remained more costly. Based on these data, it appears unlikely that robotic-assisted hysterectomy can achieve cost parity with laparoscopic hysterectomy based on surgical experience alone and that reductions in the cost of robotic instrumentation will be required for the procedure to become cost-effective.2,35 Strategies to reduce the cost of robotic instrumentation as well as initiatives to promote access to high-quality laparoscopic surgery are warranted.
Acknowledgments
Dr. Wright (NCI R01CA169121-01A1) and Dr. Hershman (NCI R01CA134964) are recipients of grants and Dr. Tergas is the recipient of a fellowship (NCI R25 CA094061-11) from the National Cancer Institute.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.Rosero EB, Kho KA, Joshi GP, Giesecke M, Schaffer JI. Comparison of robotic and laparoscopic hysterectomy for benign gynecologic disease. Obstet Gynecol. 2013;122:778–86. doi: 10.1097/AOG.0b013e3182a4ee4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright JD, Ananth CV, Lewin SN, Burke WM, Lu YS, Neugut AI, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309:689–98. doi: 10.1001/jama.2013.186. [DOI] [PubMed] [Google Scholar]
- 3.Paraiso MF, Ridgeway B, Park AJ, Jelovsek JE, Barber MD, Falcone T, et al. A randomized trial comparing conventional and robotically assisted total laparoscopic hysterectomy. Am J Obstet Gynecol. 2013;208:368, e1–7. doi: 10.1016/j.ajog.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Wright KN, Jonsdottir GM, Jorgensen S, Shah N, Einarsson JI. Costs and outcomes of abdominal, vaginal, laparoscopic and robotic hysterectomies. JSLS. 2012;16:519–24. doi: 10.4293/108680812X13462882736736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desille-Gbaguidi H, Hebert T, Paternotte-Villemagne J, Gaborit C, Rush E, Body G. Overall care cost comparison between robotic and laparoscopic surgery for endometrial and cervical cancer. Eur J Obstet Gynecol Reprod Biol. 2013;171:348–52. doi: 10.1016/j.ejogrb.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Sarlos D, Kots L, Stevanovic N, von Felten S, Schar G. Robotic compared with conventional laparoscopic hysterectomy: a randomized controlled trial. Obstet Gynecol. 2012;120:604–11. doi: 10.1097/AOG.0b013e318265b61a. [DOI] [PubMed] [Google Scholar]
- 7.Sarlos D, Kots L, Stevanovic N, Schaer G. Robotic hysterectomy versus conventional laparoscopic hysterectomy: outcome and cost analyses of a matched case-control study. Eur J Obstet Gynecol Reprod Biol. 2010;150:92–6. doi: 10.1016/j.ejogrb.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Paraiso MF, Jelovsek JE, Frick A, Chen CC, Barber MD. Laparoscopic compared with robotic sacrocolpopexy for vaginal prolapse: a randomized controlled trial. Obstet Gynecol. 2011;118:1005–13. doi: 10.1097/AOG.0b013e318231537c. [DOI] [PubMed] [Google Scholar]
- 9.Wright JD, Burke WM, Wilde ET, Lewin SN, Charles AS, Kim JH, et al. Comparative effectiveness of robotic versus laparoscopic hysterectomy for endometrial cancer. J Clin Oncol. 2012;30:783–91. doi: 10.1200/JCO.2011.36.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H, Lu D, Wang L, Shi G, Song H, Clarke J. Robotic surgery for benign gynaecological disease. The Cochrane Database of Systematic Reviews. 2012;(2):Art. No.: CD008978. doi: 10.1002/14651858.CD008978.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Sarlos D, Kots LA. Robotic versus laparoscopic hysterectomy: a review of recent comparative studies. Curr Opin Obstet Gynecol. 2011;23:283–8. doi: 10.1097/GCO.0b013e328348a26e. [DOI] [PubMed] [Google Scholar]
- 12.Barbash GI, Glied SA. New technology and health care costs–the case of robot-assisted surgery. N Engl J Med. 2010;363:701–4. doi: 10.1056/NEJMp1006602. [DOI] [PubMed] [Google Scholar]
- 13.Woelk JL, Casiano ER, Weaver AL, Gostout BS, Trabuco EC, Gebhart JB. The learning curve of robotic hysterectomy. Obstet Gynecol. 2013;121:87–95. doi: 10.1097/aog.0b013e31827a029e. [DOI] [PubMed] [Google Scholar]
- 14.Geller EJ, Lin FC, Matthews CA. Analysis of robotic performance times to improve operative efficiency. J Minim Invasive Gynecol. 2013;20:43–8. doi: 10.1016/j.jmig.2012.08.774. [DOI] [PubMed] [Google Scholar]
- 15.Lim PC, Kang E, Park do H. A comparative detail analysis of the learning curve and surgical outcome for robotic hysterectomy with lymphadenectomy versus laparoscopic hysterectomy with lymphadenectomy in treatment of endometrial cancer: a case-matched controlled study of the first one hundred twenty two patients. Gynecol Oncol. 2011;120:413–8. doi: 10.1016/j.ygyno.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 16.Seamon LG, Fowler JM, Richardson DL, Carlson MJ, Valmadre S, Phillips GS, et al. A detailed analysis of the learning curve: robotic hysterectomy and pelvic-aortic lymphadenectomy for endometrial cancer. Gynecol Oncol. 2009;114:162–7. doi: 10.1016/j.ygyno.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Lenihan JP, Jr, Kovanda C, Seshadri-Kreaden U. What is the learning curve for robotic assisted gynecologic surgery? J Minim Invasive Gynecol. 2008;15:589–94. doi: 10.1016/j.jmig.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 18.Seamon LG, Cohn DE, Richardson DL, Valmadre S, Carlson MJ, Phillips GS, et al. Robotic hysterectomy and pelvic-aortic lymphadenectomy for endometrial cancer. Obstet Gynecol. 2008;112:1207–13. doi: 10.1097/AOG.0b013e31818e4416. [DOI] [PubMed] [Google Scholar]
- 19.Koenker R. Quantile regression. New York (NY): Cambridge University Press; 2005. [Google Scholar]
- 20.Chen J, Vargas-Bustamante A, Mortensen K, Thomas SB. Using quantile regression to examine health care expenditures during the great recession. Health Serv Res. 2013 doi: 10.1111/1475-6773.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2010;303:2359–67. doi: 10.1001/jama.2010.796. [DOI] [PubMed] [Google Scholar]
- 22.Pasic RP, Rizzo JA, Fang H, Ross S, Moore M, Gunnarsson C. Comparing robot-assisted with conventional laparoscopic hysterectomy: impact on cost and clinical outcomes. J Minim Invasive Gynecol. 2010;17:730–8. doi: 10.1016/j.jmig.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 23.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–33. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 24.Livingston EH, Cao J. Procedure volume as a predictor of surgical outcomes. JAMA. 2010;304:95–7. doi: 10.1001/jama.2010.905. [DOI] [PubMed] [Google Scholar]
- 25.Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035–42. doi: 10.1001/jama.2010.672. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Department of Labor. Bureau of labor statistics consumer price index. 2011 Available at: ftp://ftp.bls.gov/pub/special.requests/cpi/cpiai.txt. Retrieved September 1, 2011.
- 27.Kollef MH, Hamilton CW, Ernst FR. Economic impact of ventilator-associated pneumonia in a large matched cohort. Infect Control Hosp Epidemiol. 2012;33:250–6. doi: 10.1086/664049. [DOI] [PubMed] [Google Scholar]
- 28.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 29.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–27. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 30.Rogo-Gupta LJ, Lewin SN, Kim JH, Burke WM, Sun X, Herzog TJ, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116:1341–7. doi: 10.1097/AOG.0b013e3181fca8c5. [DOI] [PubMed] [Google Scholar]
- 31.Wallenstein MR, Ananth CV, Kim JH, Burke WM, Hershman DL, Lewin SN, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709–16. doi: 10.1097/AOG.0b013e318248f7a8. [DOI] [PubMed] [Google Scholar]
- 32.Wright JD, Hershman DL, Burke WM, Lu YS, Neugut AI, Lewin SN, et al. Influence of surgical volume on outcome for laparoscopic hysterectomy for endometrial cancer. Ann Surg Oncol. 2012;19:948–58. doi: 10.1245/s10434-011-2090-8. [DOI] [PubMed] [Google Scholar]
- 33.Wright JD, Lewin SN, Deutsch I, Burke WM, Sun X, Herzog TJ. Effect of surgical volume on morbidity and mortality of abdominal hysterectomy for endometrial cancer. Obstet Gynecol. 2011;117:1051–9. doi: 10.1097/AOG.0b013e31821647a0. [DOI] [PubMed] [Google Scholar]
- 34.Wright JD, Herzog TJ, Tsui J, Ananth CV, Lewin SN, Lu YS, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122:233–41. doi: 10.1097/AOG.0b013e318299a6cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnett JC, Judd JP, Wu JM, Scales CD, Jr, Myers ER, Havrilesky LJ. Cost comparison among robotic, laparoscopic, and open hysterectomy for endometrial cancer. Obstet Gynecol. 2010;116:685–93. doi: 10.1097/AOG.0b013e3181ee6e4d. [DOI] [PubMed] [Google Scholar]