Abstract
Objective
To evaluate the association of metabolic risk factors with severity and two-year progression of early degenerative cartilage changes at the knee, measured with T2 relaxation times in middle-aged subjects from the Osteoarthritis Initiative.
Methods
Cartilage segmentation and T2 map generation was performed in 3T knee MR images from 403, 45 – 60 year old subjects without radiographic osteoarthritis (OA). The influence of risk factors on baseline and longitudinal progression of T2 was analyzed using linear regression, adjusting for age, gender and other OA risk factors.
Results
Four metabolic risk factors (i) high abdominal circumference (P<0.001), (ii) hypertension (P=0.040), (iii) high fat consumption (P=0.019) and (iv) self-reported diabetes (P=0.012) were individually associated with higher baseline T2. When the four metabolic risk factors were considered in a multivariate regression model, higher T2 remained significantly associated with abdominal circumference (P<0.001) and diabetes (P=0.031) and there was a trend for high fat consumption (P=0.096). Of individual risk factors, only diabetes remained associated with higher baseline T2 after adjustment for BMI. After adjustment for BMI, baseline T2 increased in dose-reponse fashion with the number of metabolic risk factors present (P=0.032 for linear trend), and subjects with ≥3 metabolic factors (versus <3) had significantly higher baseline T2 (mean difference, 1.2ms; lower 95% confidence interval (CI), 0.3ms; upper 95% CI, 2.1ms; P=0.011). Metabolic risk factors were not significantly associated with increases in T2 during follow-up.
Conclusion
Metabolic risk factors are associated with higher T2, suggesting that increased cartilage degeneration may be caused by modifiable metabolic disorders.
Introduction
Osteoarthritis (OA) is the most common musculoskeletal disorder affecting millions of elderly individuals (1). Apart from relieving debilitating symptoms with analgesia, currently there is no treatment that targets and inhibits the progressive degenerative structural changes. This adds weight to the importance of modifiable factors that may contribute to an increased risk for developing OA (2, 3) and to the ability to detect OA early before irreversible damage to the joint has occurred.
The progressive loss of hyaline articular cartilage in OA (1, 4) can be detected and monitored by magnetic resonance imaging (MRI) (5, 6). Recent studies have demonstrated the potential of MRI for detecting early biochemical shifts in cartilage matrix prior to irreversible morphological damage or clinical symptoms. T2 relaxation time mapping has been used as a biomarker to non-invasively detect early cartilage degeneration quantitatively (7) by virtue of its correlation with the water content and deterioration of the collagen network (8–11). T2 has been shown to be a sensitive indicator of the effects of knee OA risk factors on knee cartilage (11–15) and to predict disease progression.
OA is increasingly understood as a systemic disease, especially in terms of a possible relationship to metabolic disorders linked to obesity (16–19). Several studies have found an increased risk of OA of the knee and other joints associated with both individual, and the accumulation of, metabolic risk factors that are considered part of the metabolic syndrome (17, 18, 20, 21). To our knowledge, no studies have examined the association of metabolic risk factors with MRI measures of cartilage degradation, and T2 mapping specifically, in knees without radiographic OA.
The purpose of this study was to evaluate the association of metabolic risk factors, with baseline knee cartilage T2 and with 2 year changes in these measurements. We hypothesized, that (1) metabolic risk factors would be associated with higher baseline T2 and with greater increases in T2 over two years; and (2) that an increasing number of metabolic risk factors present would be associated with higher T2 and greater progression of T2.
Patients and Methods
Subjects
The Osteoarthritis Initiative (OAI) is an NIH-funded longitudinal, observational multi-center cohort study, focusing primarily on knee OA. The study enrolled 4796 subjects aged 45 to 79 years and at annual follow-up visits obtains clinical assessments and knee joint imaging, including MRI with T2 mapping sequences of the knee (22).
The OAI protocol, amendments, and informed consent documentation were approved by the local institutional review boards. Data used in the preparation of this article were obtained from the OAI public database (http://www.oai.ucsf.edu/). Specific datasets used are baseline clinical dataset 0.2.2, as well as baseline and two year follow up image datasets 0.E.1 and 3.E.1.
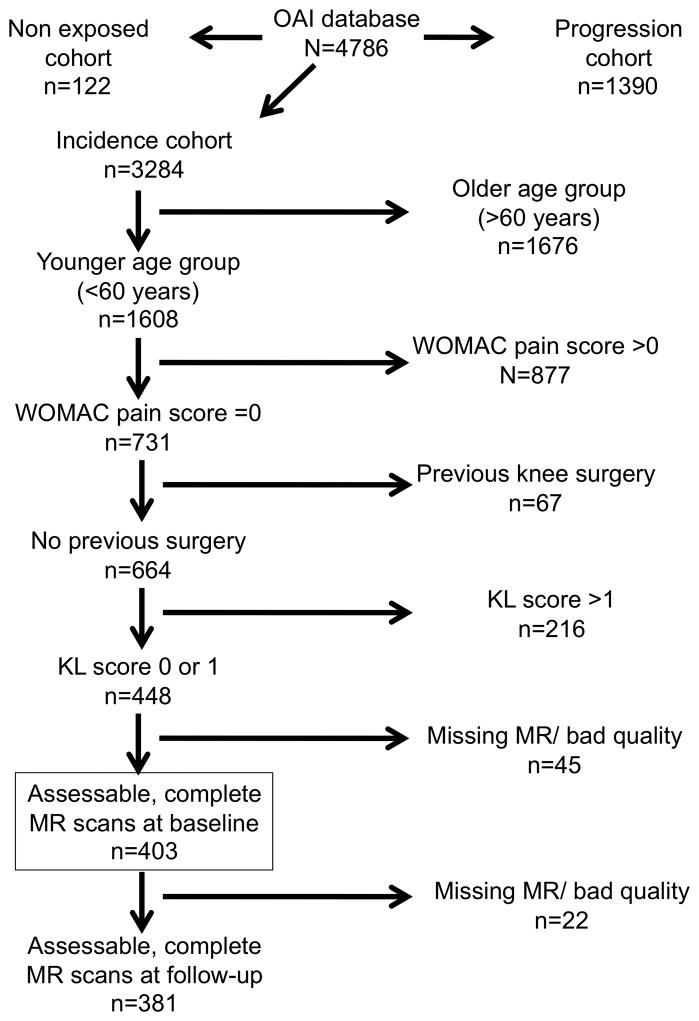
Individuals included in the present study were from the OAI Incidence cohort, which did not have symptomatic radiographic knee OA (defined as KL grade ≥2 and frequent pain in the same knee) at baseline but had one or more risk factors for developing knee OA. In order to focus on early knee degenerative changes in a middle-aged cohort, we selected the younger half of the cohort (ages 45–60 years old) who had baseline Western Ontario and McMaster University (WOMAC) pain scores of 0 (23) and a Kellgren-Lawrence (KL) grade <2 in the study knee. In addition to the OAI exclusion criteria, we excluded those in which the study knee had knee surgery with hardware implantation or poor MR quality and missing MR sequences. Follow-up T2 data were available for 381 of 403 individuals who met all study criteria at baseline (Figure 1).
Figure 1.
Flow-chart of the selected subjects from the OAI.
Metabolic Risk factors
Based on the data available from the OAI, four metabolic risk factors corresponding to the components of the metabolic syndrome (central obesity, hypertension, impaired glucose tolerance and dyslipidemia (24–26)) were assessed for their association with T2 relaxation times. We used abdominal circumference instead of body mass index (BMI) as the measure of central obesity since it has been found to have stronger associations than BMI with visceral adiposity, insulin resistance and cardiovascular disease risk (27, 28), and current consensus definitions (24, 29, 30) use abdominal circumference as one of the risk factors comprising the metabolic syndrome. Abdominal circumference (cm) was measured by a clinic examiner, using a tape measure over bare skin, with the subject standing. Central obesity was defined as a waist circumference ≥102 cm in men and 88 cm in women, using the AHA/NHLBI (ATP III) cutpoints (24), Blood pressure was assessed in a sitting position and high blood pressure defined as systolic >130 mmHg and/or diastolic >85 mmHg using the “International Diabetes Federation consensus” (IDF) definition (25). For impaired glucose tolerance and dyslipidemia we were unable to replicate consensus criteria for metabolic syndrome, since these require blood specimens not available to the authors. Instead, we used factors related to these two components. For a factor related to glucose tolerance, we used self-report of diabetes. Participants who answered yes to the question “Do you have diabetes (high blood sugar)?” were classified as diabetic. For a factor related to dyslipidemia, we relied on dietary fat consumption, since this has been linked to metabolic syndrome in some studies (31, 32) and a recent study has suggested that greater consumption of n-6 polyunsaturatd fatty acids is related to an increased risk of subchondral BMLs in the knee (33). Fat consumption (g/day) was calculated from the Block Brief 2000 Food Frequency Questionnaire administered at the baseline examination (http://www.nutritionquest.com/). Since recommended fat consumption is less than 78 g/day (34), this threshold was used to define high fat consumption. Due to the use of these imperfect proxy measures, we do not classify subjects on the presence of metabolic syndrome.
Imaging
Magnetic resonance (MR) image acquisition
MRI knee examinations were obtained with one of four identical 3T MRI systems (Trio, Siemens, Erlangen, Germany) using identical standard knee coils and protocols, specifically obtained for the OAI. In the right knee, or the left knee if the right had contraindications for MRI, a sagittal two-dimensional multislice multiecho (MSME) spin echo sequence for T2 mapping (TR=2700 ms, seven TEs=10ms, 20ms, 30ms, 40ms, 50ms, 60ms, 70ms, field of view (FOV)=12cm, slice thickness =3mm with 0.5mm gap, in-plane spatial resolution =0.313×0.446mm2, bandwidth =250Hz/pixel) was performed for quantitative T2 relaxation time assessment (22). Further details regarding MRI techniques and protocols have been previously published (13, 35).
T2 relaxation time measurements
The MSME spin echo sequences were transferred to a remote workstation (SPARC; Sun Microsystems, Mountain View, California). Images were analyzed by using software developed at our institution with an interactive display language (IDL; Research Systems, Boulder, Colorado) environment. Segmentation of artifact free cartilage areas of the patella, medial and lateral femoral condyle and medial and lateral tibia in every section was performed by one observer (M.S.K.) and supervised by two radiologists (P.M.J., T.M.L.). Due to pulsation artifacts from the popliteal artery resulting in significant artifacts, the trochlea was excluded. Mean T2 of the baseline and two-year follow-up time point was calculated individually (for each compartment) and globally (mean of all compartments) from the segmented regions of interest, skipping the first echo and using a noise-corrected exponential fitting as previously described (36). To illustrate T2 progression over time, the individual longitudinal increase over time was calculated as an absolute value (T2 follow up-T2 baseline).
Reproducibility of T2 measurements
Averaged over all compartments, inter-observer agreement for T2 measurements in our group was described previously with an inter-reader reproducibility error for mean T2 of 1.57%, respectively 0.53ms (37). Mean intra-reader reproducibility for T2 measurements was 1.17% (38).
Measurement of covariates
Participants were asked about a history of knee injury that resulted in difficulty walking for at least 2 days (yes/no) and about a history of any surgery of the knee (yes/no). Familial predisposition for knee OA was defined as a total knee replacement for OA in a biological parent or sibling (yes/no). Hands were examined by the OAI examiner at the baseline visit according to a protocol available on the OAI website (http://oai.epi-ucsf.org/datarelease/forms.asp). Heberden’s nodes were considered present if bony enlargements were found in ≥3 DIP joints were affected in either hand. Isometric strength measurements were performed using a Good Strength Chair (Metitur, Jyväskylä, Finland; www.oai.ucsf.edu/datarelease/OperationsManuals.asp) for knee flexion and extension. Two submaximal practice trials were completed before force was measured three times for 3 seconds, each separated by 30 seconds; the highest value is used for maximal strength reported (N) (39).
Statistical analysis
Statistical analysis was performed with JMP software Version 7 (SAS Institute, Cary, NC, USA). For analysis of the association of potential risk factors with baseline T2 and with change in T2, descriptive statistics were obtained applying two-sided t-test and one-way analysis of variance (ANOVA). Multivariate linear regression analyses of risk factors with T2 were adjusted for the effects of other OA risk factors, including age, gender, history of knee injury, history of knee surgery, family history of knee replacement and Heberden’s nodes in hands. The effect of the accumulation of metabolic risk factors was evaluated by including a variable in the regression model for the count (0 to 4) of risk factors present and in a separate analysis a dichotomous variable for the number of risk factors at ≥3 versus <3. Because of the importance of BMI as a well-established risk factor for incident knee OA (2), and to control for possible residual confounding by obesity in multivariate models that include high abdominal circumference, analyses were repeated using continuous measures of BMI. In a sensitivity analysis, we adjusted for isometric knee strength since there is some evidence that fatty infiltration of muscle is a cause of both glycemic dysregulation and muscle weakness that leads to cartilage degradation. On the other hand, muscle weakness may be on the causal pathway from metabolic obesity to cartilage degradation and would not be included as a covariate. Means±standard deviation (SD) or±standard error of the mean (SEM) of T2 and 95% confidence intervals (CI) around adjusted differences in T2 are presented as indicated. Results were considered as significant if *P<0.05.
Results
Subject characteristics
Mean age of the subjects in this study (n=403) was 52.1±3.9 (Mean±SD) years and BMI was 28.5±4.9kg/m². The correlation of abdominal circumference with BMI, considered as continuous variables, was 0.87 (P<0.001). There were no significant differences between men and women for age, BMI, abdominal circumference or blood pressure (Table 1). Mean abdominal circumference in both men and women was above the threshold used for central obesity. Dietary fat consumption was significantly lower in women (52.0±27.0g/day) than in men (62.0±32.3g/day; P=0.002). With respect to metabolic risk factors: (i) high abdominal circumference was present in 298 subjects (73.9%), (ii) hypertension in 113 subjects (28.0%), (iii) self-reported diabetes found in n=9 subjects (2.2%) and (iv) high fat consumption was found in 74 subjects (18.4%). A single one of these four metabolic risk factors was present in 164 subjects (40.7%), two were present in 89 (22.1%), three in 24 subjects (6.0%) and all four in just 2 subjects (0.5%); consequently ≥3 metabolic risk factors were present in 26 subjects.
Table 1.
Baseline characteristics of the study sample. Mean ±standard deviation for female and male subjects is presented.
| Parameter | Mean women (n=199) | Mean men (n=204) |
|---|---|---|
| Age (years) | 52.2 ±4.0 | 52.0 ±3.8 |
| Body mass index (kg /m2) | 28.1 ±5.6 | 28.9 ±4.2 |
| Abdominal circumference (cm) | 101.1 ±15.2 | 102.5 ±12.3 |
| Systolic blood pressure (mmHg) | 116.7 ±13.8 | 120.2 ±13.0 |
| Diastolic blood pressure (mmHg) | 74.6 ±9.3 | 79.1 ±9.2 |
| Fat consumption (g /day) | 52.0 ±27.0 | 62.0 ±32.3 |
| T2 baseline (ms) | 33.6 ±2.4 | 33.6 ±2.2 |
| T2 change (%) | 3.2 ±5.6 | 3.8 ±5.1 |
| T2 change (ms) | 1.0 ±1.9 | 1.2 ±1.7 |
Abdominal circumference, hypertension, fat consumption and diabetes and baseline T2
When each metabolic factor was considered individually and adjusted for other OA risk factors, baseline global T2 was higher in subjects with high abdominal circumference (mean difference 1.3ms; 95% CI:0.8–1.8; P<0.001)), hypertension (0.5ms; 0.0–1.1; P=0.041), diabetes (2.1ms; 0.5–3.7; P=0.010) and fat consumption (0.7ms; 0.1–1.3; P=0.023) compared to those without these risk factors (Table 2). For hypertension, systolic blood pressure had a significant influence (P=0.046) while diastolic blood pressure did not (P=0.753). Examining T2 in the individual knee compartments (data not shown), the most significant influence of abdominal circumference (P<0.001) and hypertension (medial tibia, P=0.036; lateral tibia, P=0.030) was seen for tibial T2 and the most significant influence of fat consumption was seen for the medial femoral condyle (P=0.015). If adjusted for continuous BMI, the association of the individual parameter diabetes remained significant (P=0.046), while fat consumption (P=0.210) and hypertension (P=0.477) were not significant.
Table 2.
Mean differences (lower 95% confidence interval (CI); upper 95% CI) describing the influence of individual metabolic risk factors on baseline global T2 (ms) and T2 progression (ms), with metabolic factors considered one at time adjusted for other OA risk factorsa (A), metabolic factors considered one at time adjusted for BMI and other OA risk factorsa (A1) with all four metabolic factors included in the multivariate regression model (B).
| BASELINE T2 Metabolic Factor | A: Individual risk factors | A1: Individual Risk Factors additionally adjusted for BMI | B: All four metabolic factors in the same model |
|---|---|---|---|
| Abdominal circumference | 1.3 (0.8; 1.8) | 0.4 (−0.2; 1.0) | 1.2 (0.7; 1.7) |
| P-value | P<0.001 | P=0.164 | P<0.001 |
| Blood pressure | 0.5 (0.0;1.0) | 0.2 (−0.3; 0.7) | 0.2 (−0.3; 0.7) |
| P-value | P=0.041 | P=0.477 | P=0.362 |
| Diabetes | 2.1 (0.5;3.8) | 1.6 (0.0; 3.1) | 1.8 (0.2; 2.4) |
| P-value | P=0.010 | P=0.046 | P=0.026 |
| Fat consumption | 0.7 (0.1;1.3) | 0.4 (−0.2; 0.9) | 0.5 (−0.1; 1.0) |
| P-value | P=0.023 | P=0.210 | P=0.096 |
| T2 PROGRESSION Metabolic Factor | A: Individual risk factors | A1: Individual Risk Factors additionally adjusted for BMI | B: All four metabolic factors in the same model |
|---|---|---|---|
| Abdominal circumference | 0.3 (−0.1; 0.7) | 0.6 (−1.0; 2.3) | 0.2 (−0.2; 0.7) |
| P-value | P=0.155 | P=0.439 | P=0.307 |
| Blood pressure | 0.2 (−0.2; 0.7) | 0.5 (−0.9; 1.8) | 0.1 (−0.3; 0.6) |
| P-value | P=0.367 | P=0.507 | P=0.0.541 |
| Diabetes | 1.3 (−0.1; 2.6) | 3.0 (−1.0; 7.0) | 1.2 (−0.1; 2.6) |
| P-value | P=0.060 | P=0.145 | P=0.080 |
| Fat consumption | 0.2 (−0.4; 0.7) | 0.3 (−1.2; 1.9) | 0.1 (−0.4; 0.6) |
| P-value | P=0.566 | P=0.671 | P=0.732 |
All analyses include other OA risk factors as covariates: age, gender, Herbeden’s nodes at the hands; family history of joint replacement; previous knee surgery; previous knee injury
In a multivariate regression model including all four metabolic factors as well as other OA risk factors, abdominal circumference (P<0.001) and diabetes (P=0.026) were significantly associated with global T2 and fat consumption had a non-significant trend (P=0.096; Table 2).
Number of metabolic risk factors present and baseline T2
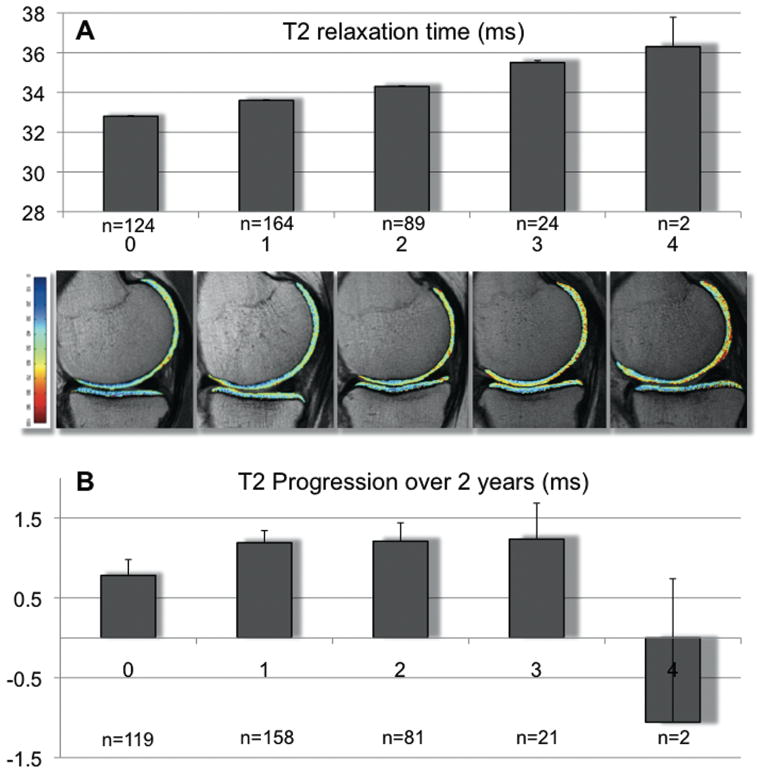
Since all of the individual metabolic factors are putative measures of the same underlying concept, they are expected to be interrelated and to explain some of the variation in the other factors. Therefore, we evaluated whether the accumulation of individual risk factors was associated with baseline T2, also adjusting for BMI. Baseline T2 was significantly higher in individuals with a higher number of individual metabolic risk factors present (Figure 2A), with P<0.001 for number of metabolic risk factors adjusted for the other baseline risk factors; and P=0.032 when additionally adjusted for continuous BMI. Mean (±SEM) adjusted (also for continuous BMI) global baseline T2 increased stepwise from 32.8±0.2ms for 0 risk factors to 36.3±2.0ms when all 4 risk factors were present. This increase was seen for all individual knee compartments, but it was only significant for the medial and lateral tibial compartment (P<0.001).
Figure 2.
A: Adjusted mean baseline global T2 relaxation times (ms; ±SEM) for subgroups with increasing numbers of metabolic risk factors (0–4), adjusted for other OA risk factors and a continuous measure of BMI. T2 increased stepwise with increasing number of metabolic risk factors from 32.8±0.2ms for none to 36.3±2.0ms for four risk factors. For the number of metabolic risk factors present, (0–4), P<0.001 without adjustment for BMI and P=0.032 with adjustment for BMI. Other OA risk factors are age, gender, Herbeden’s nodes, family history of joint replacement, previous knee surgery and previous knee injury. Underneath representative cartilage T2 color maps overlaid on the first-echo images of the MSME sequence of each group. Blue color indicates low, red color high cartilage T2. Subjects without any metabolic risk factor showed lower T2 than subjects with metabolic risk factors in an increasing manner.
B: Progression of T2 relaxation times (%; ±SEM) over two years for subgroups with increasing numbers of metabolic risk factors. Analyses as described for A. For the number of metabolic risk factors present, (0–4), P<0.071 without adjustment for BMI and P=0.191 with adjustment for BMI.
Individuals with who had ≥3 metabolic risk factors had significantly higher basline T2 (35.5±0.5ms) compared to individuals with ≤2 metabolic risk factors (33.5±0.1ms; P<0.001; Table 3). If additionally adjusted for continuous BMI, the P-value for differences in T2 was P=0.011 (mean difference, 1.2ms; lower 95%CI, 0.3ms; upper 95%CI, 2.1ms). The most significant differences were found for the medial and lateral tibia (P<0.001). The lateral femoral condyle showed a non-significant trend (P=0.075).
Table 3.
Adjusted mean differences of baseline global T2 (ms) ±SEM and adjusted mean differences of T2 progression (ms) ±SEM for subjects with ≤2 metabolic factors (n=377) compared with subjects with ≥3 metabolic factors (n=26). P-values are from multivariate regression models adjusted for A: other OA risk factorsa, B: additionally adjusted for continuous BMI.
| BASELINE T2 | T2 PROGRESSION | |||
|---|---|---|---|---|
| Compartment | A | B | A | B |
| Global | 2.0 (1.0; 2.9) <0.001 |
1.2 (0.3, 2.1) P=0.011 |
0.1 (−0.7; 0.9) P=0.828 |
0.1 (−0.8; 0.9) P=0.871 |
| PAT | 1.0 (−0.6; 2.6) P=0.228 |
0.5 (−1.2; 2.2) P=0.570 |
1.3 (−0.3; 2.9) P=0.115 |
1.3 (−0.3 3.0) P=0.112 |
| MFC | 1.4 (0.3; 2.4) P=0.014 |
1.3 (0.1; 2.4) P=0.029 |
0.0 (−1.0; 1.1) P=925 |
0.3 (−0.8; 1.4) P=0.578 |
| LFC | 0.9 (−0.1; 1.9) P=0.076 |
0.9 (−0.2, 1.9) P=0.105 |
0.6 (−0.4; 1.6) P=0.256 |
0.5 (−0.5; 1.6) P=0.308 |
| MT | 3.8 (2.5; 5.1) P<0.001 |
2.4 (1.1; 3.6) P<0.001 |
1.4 (0.1; 2.8) P=0.040 |
0.7 (−0.7; 2.0) P=0.354 |
| LT | 2.3 (0.9; 3.7) P=0.001 |
0.6 (−0.7; 1.8) P=0.387 |
0.1 (−1.0; 1.2) P=0.843 |
0.1 (−1.0; 1.3) P=0.813 |
All analyses include other OA risk factors as covariates: Herbeden’s nodes at the hands; family history of joint replacement; previous knee surgery; previous knee injury; age and gender. PAT, patella; MFC, medial femoral condyle; LFC, lateral femoral condyle; MT, medial tibia; LT, lateral tibia.
In a sensitivity analysis, we additionally adjusted the analysis of ≥3 versus ≥2 risk factors for knee flexion and extension isometric strength. This had essentially no effect on our results. For example, subjects with ≥3 risk factors had higher baseline global T2 (+1.8(0.8;2.9)ms, P<0.001) and higher medial femoral condyle T2 (+1.5(0.3;2.7)ms, P=0.012) after this adjustment.
Progression analysis
Mean (±SD) longitudinal change of global T2 in all subjects over time was 3.5±5.3% (1.1±1.8ms). None of the individual metabolic factors was significantly associated with change in global T2 (P=0.130 to P=0.977; data not shown). There was a statistical trend for the association of global T2 progression with the number of metabolic risk factors present (P<0.071 without and P=0.191 with adjustment for BMI; Figure 2b). While individuals with ≥3 metabolic risk factors had slightly greater increases in global and compartment-specific mean T2 than subjects with ≥2 metabolic risk factors (Table 3), especially in the medial tibia, none of these differences was significant after adjustment for BMI.
Discussion
The present study demonstrated a significant association of metabolic risk factors with higher baseline T2 relaxation times. The individual factors (high abdominal circumference, hypertension, high fat consumption and diabetes) were associated with significantly higher baseline T2, although of the individual factors only the association with diabetes remained significant after adjustment for BMI. However, the number of risk factors present in an individual was associated with higher baseline T2 values independently of BMI. These results suggest that subjects with an accumulation of, metabolic risk factors have more severe cartilage degradation. Since all of these risk factors are modifiable, our results suggest potential avenues to prevent or delay knee cartilage degradation and possibly the development of knee OA.
Cartilage T2 relaxation mapping is a non-invasive biomarker to detect early cartilage matrix degeneration, mainly collagen disarrangement and increases in water content (16). A correlation with the severity of OA has consistently been shown (6, 40–43). High T2 was associated with increased severity of cartilage defects and was able to predict cartilage loss (11, 15, 41, 44). The difference in adjusted global T2 between those with ≤2 risk factors and those with ≥3 risk factors was 2.0ms(1.0;2.9), and 1.2ms(0.3,2.1) after further adjustment for BMI as a continuous measure. In previous longitudinal analyses we found that differences of 1.0SD in baseline T2 were significantly associated with a 40% to 70% increase in the risk of compartment-specific cartilage loss and worsening bone marrow lesions in the knee (15, 41). In a cross-sectional analysis, knees with WOMAC pain scores ≥5 had T2 values that were about 2.0ms higher than knees with WOMAC pain scores of 0 (41).
In contrast, in both the present and previous studies there is less evidence supporting the relevance of progression of T2 changes over time. In knees from the OAI normal control cohort we observed a significant increase in T2 values over 2 years, which was moderately correlated with increases in cartilage damage over the same period (12, 14). But we also recently reported that while obese individuals had significantly greater baseline T2 than non-obese individuals, we did not find greater increases in T2 over 36 months (41). The results of the present study mirror these previous findings, with stronger and more consistent cross-sectional associations of the obesity-related risk factors studied with baseline T2 than associations with T2 change over 24 months. There are several possible reasons for the less robust associations seen with T2 change over time: (i) The follow-up time of 24 months was relatively short and the changes observed relatively small compared to the variability in T2 progression. (ii) Differences in baseline T2 may be larger and reflect cumulative damage over time. T2 progression may therefore be a less sensitive outcome for measuring association. (iii) There is also more recent evidence suggesting that T2 progression occurs more slowly when significant cartilage degradation is already present and T2 is elevated (44–46). The upper 95% confidence bounds for effects on T2 progression are around 0.7ms, close to what may be clinically relevant differences. Thus we cannot rule out clinically important differences in T2 progression that are, nevertheless, not statistically significant.
There is growing evidence that OA is a multi-systemic disease with interrelated risk factors and metabolic disorders. OA has been linked to obesity, but also to other cardiovascular risk factors, like dyslipidemia, hypertension, and insulin resistance that characterize the “Metabolic Syndrome” (18, 47). This is in agreement with our findings, since T2 continuously increased with the number of metabolic risk factors present. Large longitudinal studies have confirmed that overweight precedes the development of knee OA (2). OA is also moderately associated with obesity in non-weight-bearing joints such as hand joints (48). We found a correlation of central obesity with increased T2, indicating more advanced cartilage matrix degeneration. Abdominal circumference has been found to be higher correlated with cardiovascular disease than BMI. There is limited evidence on whether this remains true for OA (49). We primarily concentrated on the parameter abdominal circumference, since it is implied in “Metabolic Syndrome” definitions. Replacing abdominal circumference with BMI showed similar results. Considering the high correlation of 0.87 of these two parameters, it is challenging to assess whether one parameter has a higher impact. Although other studies like the Japanese Road study (18) did not adjust for BMI, we additionally presented results for BMI, to account for its clinical relevance. Interestingly, the group with ≥3 metabolic risk factors showed significantly higher T2 despite adjustment for BMI. This supports the hypothesis, that an accumulation of risk factors increases the risk for OA.
Self reported diabetes had a significant effect on early degenerative changes but not on their progression. These may be chance findings given the small numbers with self-reported diabetes, or be due to our relatively short follow-up interval. An equally plausible explanation is that once diabetes is diagnosed and presumably glycemia is controlled, diabetes correlated risk for OA is reduced; as seen for cardiovascular complications, diabetic retinopathy or chronic kidney disease (50, 51). Past studies have revealed mixed results with respect to any association of hypertension with knee OA. An association of hypertension and knee OA, independent of body weight, was reported in one study (52), but other groups have not been able to verify this (53). Our study also revealed an influence of hypertension on T2 relaxation times, which, however, was attenuated by adjustment for other metabolic factors. Greater absolute fat intake was associated with increased baseline T2. This is consistent with other studies showing that fatty acid intake influences adipose tissue expression of leptin, which may play a role in osteoarthritis by promoting nitric oxide synthesis in chondrocytes (54). Increased saturated fatty acid consumption may increase the risk of developing bone marrow lesions and dietary modification of fatty acid intake may be one strategy in the prevention of knee OA (33, 55, 56).
In a model that includes all four metabolic risk factors, the effect of each risk factor is attenuated and only abdominal circumference and diabetes remain significantly associated with T2. Since all four factors are putative measures of a single concept, they are expected to be interrelated and to explain some of the variation in the other factors. High abdominal circumference and BMI are both measures of obesity and are highly interrelated; and neither is significantly associated with T2 when both are included in the same model (data not shown).
There are several limitations of our study. First, this was an observational study. Clinical trials are needed to determine if modifying risk factors can protect against the development of cartilage degradation and OA. Second, although the metabolic risk factors for high T2 correspond to the consensus components of the “Metabolic Syndrome”, these latter require the use blood samples to determine impaired glucose tolerance and dyslipidemia. We used imperfect proxies for these factors, relying on self-report of diabetes and dietary fat consumption. Although there is evidence supporting the association of these proxy measures with metabolic abnormalities, using them does not allow us to classify individuals in our study for the presence of “Metabolic Syndrome”. Rather, our focus is on the association of the individual metabolic factors for which we have data, and the accumulation of these factors in an individual subject, with our outcome of T2.
In conclusion, we found that metabolic risk factors (i) high abdominal circumference, (ii) hypertension, (iii) high fat consumption and (iv) diabetes were associated with increased baseline T2. The more of these factors were present, the higher the T2 was found, suggesting an abnormal biochemical composition of cartilage in these individuals. These results underline the importance of public health initiatives targeting the growing prevalence of these risk factors in the modern Western society.
Significance and Innovations.
The study demonstrated a significant association of metabolic risk factors with higher cartilage T2 relaxation times, suggesting an abnormal biochemical composition of cartilage in these individuals, in a large cohort.
The individual metabolic risk factors ((i) large abdominal circumference, (ii) hypertension, (iii) high fat consumption and (iv) diabetes) as well as the number of these risk factors that were present in a subject were associated with significantly higher baseline T2, suggesting more severe cartilage degradation. The difference between the group with ≥3 metabolic factors and the group with <3 metabolic factors (P<0.001) remained significant after adjustment for BMI (P=0.011).
Since all of these risk factors are modifiable, our results suggest potential avenues to prevent or delay knee cartilage degradation and possibly the development of knee OA.
Acknowledgments
Funding sources:
This study was funded by NIH U01 AR059507 and P50 AR060752 as well as through the OAI, which is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners. This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation.
Footnotes
The study was performed at:
Musculoskeletal and Quantitative Imaging Group (MQIR), Department of Radiology and Biomedical imaging, University of California, San Francisco, 185 Berry Street, Suite 350, San Francisco, CA, 94107, USA
Conflict of Interest:
There is no conflict of interest for any of the authors.
Contributor Information
Pia M. Jungmann, Email: pia.jungmann@tum.de.
Mareen S. Kraus, Email: Mareen_Kraus@hotmail.com.
Hamza Alizai, Email: Alizai@uthscsa.edu.
Lorenzo Nardo, Email: Lorenzo.Nardo@ucsf.edu.
Thomas Baum, Email: Thomas.Baum@tum.de.
Michael C. Nevitt, Email: mnevitt@psg.ucsf.edu.
Chuck E. McCulloch, Email: cmcculloch@epi.ucsf.edu.
Gabby B. Joseph, Email: Gabby.Joseph@ucsf.edu.
John A. Lynch, Email: jlynch@psg.ucsf.edu.
Thomas M. Link, Email: Thomas.Link@ucsf.edu.
References
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–46. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 3.Reijman M, Pols HA, Bergink AP, Hazes JM, Belo JN, Lievense AM, et al. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: the Rotterdam Study. Ann Rheum Dis. 2007;66(2):158–62. doi: 10.1136/ard.2006.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995;38(8):1134–41. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- 5.Burstein D, Gray M. New MRI techniques for imaging cartilage. J Bone Joint Surg Am. 2003;85-A(Suppl 2):70–7. doi: 10.2106/00004623-200300002-00009. [DOI] [PubMed] [Google Scholar]
- 6.Burstein D, Gray M, Mosher T, Dardzinski B. Measures of molecular composition and structure in osteoarthritis. Radiol Clin North Am. 2009;47(4):675–86. doi: 10.1016/j.rcl.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Link TM, Steinbach LS, Ghosh S, Ries M, Lu Y, Lane N, et al. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology. 2003;226(2):373–81. doi: 10.1148/radiol.2262012190. [DOI] [PubMed] [Google Scholar]
- 8.Ding C, Cicuttini F, Scott F, Cooley H, Jones G. Knee structural alteration and BMI: a cross-sectional study. Obes Res. 2005;13(2):350–61. doi: 10.1038/oby.2005.47. [DOI] [PubMed] [Google Scholar]
- 9.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232(2):592–8. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckstein F, Cicuttini F, Raynauld JP, Waterton JC, Peterfy C. Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): morphological assessment. Osteoarthritis Cartilage. 2006;14 (Suppl A):A46–75. doi: 10.1016/j.joca.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 11.Joseph GB, Baum T, Alizai H, Carballido-Gamio J, Nardo L, Virayavanich W, et al. Baseline mean and heterogeneity of MR cartilage T2 are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3 years--data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2012;20(7):727–35. doi: 10.1016/j.joca.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baum T, Stehling C, Joseph GB, Carballido-Gamio J, Schwaiger BJ, Muller-Hocker C, et al. Changes in knee cartilage T2 values over 24 months in subjects with and without risk factors for knee osteoarthritis and their association with focal knee lesions at baseline: Data from the osteoarthritis initiative. J Magn Reson Imaging. 2011 doi: 10.1002/jmri.22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hovis KK, Stehling C, Souza RB, Haughom BD, Baum T, Nevitt M, et al. Physical activity is associated with MR-based knee cartilage T2 measurements in asymptomatic subjects with and without osteoarthritis risk factors. Arthritis Rheum. 2011 doi: 10.1002/art.30419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan J, Pialat JB, Joseph T, Kuo D, Joseph GB, Nevitt MC, et al. Knee cartilage T2 characteristics and evolution in relation to morphologic abnormalities detected at 3-T MR imaging: a longitudinal study of the normal control cohort from the Osteoarthritis Initiative. Radiology. 2011;261(2):507–15. doi: 10.1148/radiol.11102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasad AP, Nardo L, Schooler J, Joseph GB, Link TM. T(1)rho and T(2) relaxation times predict progression of knee osteoarthritis. Osteoarthritis Cartilage. 2013;21(1):69–76. doi: 10.1016/j.joca.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz JD, Agrawal S, Velasquez M. Getting to the heart of the matter: osteoarthritis takes its place as part of the metabolic syndrome. Curr Opin Rheumatol. 2010;22(5):512–9. doi: 10.1097/BOR.0b013e32833bfb4b. [DOI] [PubMed] [Google Scholar]
- 17.Sowers M, Karvonen-Gutierrez CA, Palmieri-Smith R, Jacobson JA, Jiang Y, Ashton-Miller JA. Knee osteoarthritis in obese women with cardiometabolic clustering. Arthritis Rheum. 2009;61(10):1328–36. doi: 10.1002/art.24739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshimura N, Muraki S, Oka H, Kawaguchi H, Nakamura K, Akune T. Association of Knee Osteoarthritis with the Accumulation of Metabolic Risk Factors Such as Overweight, Hypertension, Dyslipidemia, and Impaired Glucose Tolerance in Japanese Men and Women: The ROAD Study. J Rheumatol. 2011;38(5):921–30. doi: 10.3899/jrheum.100569. [DOI] [PubMed] [Google Scholar]
- 19.Zhuo Q, Yang W, Chen J, Wang Y. Metabolic syndrome meets osteoarthritis. Nat Rev Rheumatol. 2012;8(12):729–37. doi: 10.1038/nrrheum.2012.135. [DOI] [PubMed] [Google Scholar]
- 20.Engstrom G, Gerhardsson de Verdier M, Rollof J, Nilsson PM, Lohmander LS. C-reactive protein, metabolic syndrome and incidence of severe hip and knee osteoarthritis. A population-based cohort study. Osteoarthritis Cartilage. 2009;17(2):168–73. doi: 10.1016/j.joca.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Puenpatom RA, Victor TW. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: an analysis of NHANES III data. Postgrad Med. 2009;121(6):9–20. doi: 10.3810/pgm.2009.11.2073. [DOI] [PubMed] [Google Scholar]
- 22.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433–41. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 24.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 25.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366(9491):1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 26.Freiberg MS, Cabral HJ, Heeren TC, Vasan RS, Curtis Ellison R. Alcohol consumption and the prevalence of the Metabolic Syndrome in the US: a cross-sectional analysis of data from the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27(12):2954–9. doi: 10.2337/diacare.27.12.2954. [DOI] [PubMed] [Google Scholar]
- 27.Pouliot MC, Despres JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73(7):460–8. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 28.van Dijk SJ, Feskens EJ, Bos MB, de Groot LC, de Vries JH, Muller M, et al. Consumption of a high monounsaturated fat diet reduces oxidative phosphorylation gene expression in peripheral blood mononuclear cells of abdominally overweight men and women. J Nutr. 2012;142(7):1219–25. doi: 10.3945/jn.111.155283. [DOI] [PubMed] [Google Scholar]
- 29.Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 30.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 31.Freire RD, Cardoso MA, Gimeno SG, Ferreira SR. Dietary fat is associated with metabolic syndrome in Japanese Brazilians. Diabetes Care. 2005;28(7):1779–85. doi: 10.2337/diacare.28.7.1779. [DOI] [PubMed] [Google Scholar]
- 32.Lottenberg AM, da Afonso MS, Lavrador MS, Machado RM, Nakandakare ER. The role of dietary fatty acids in the pathology of metabolic syndrome. J Nutr Biochem. 2012;23(9):1027–40. doi: 10.1016/j.jnutbio.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Davies-Tuck ML, Wluka AE, Forbes A, English DR, Giles GG, et al. Dietary fatty acid intake affects the risk of developing bone marrow lesions in healthy middle-aged adults without clinical knee osteoarthritis: a prospective cohort study. Arthritis Res Ther. 2009;11(3):R63. doi: 10.1186/ar2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma S, Murphy SP, Wilkens LR, Shen L, Hankin JH, Henderson B, et al. Adherence to the Food Guide Pyramid recommendations among Japanese Americans, Native Hawaiians, and whites: results from the Multiethnic Cohort Study. J Am Diet Assoc. 2003;103(9):1195–8. doi: 10.1016/s0002-8223(03)00981-7. [DOI] [PubMed] [Google Scholar]
- 35.Carballido-Gamio J, Joseph GB, Lynch JA, Link TM, Majumdar S. Longitudinal analysis of MRI T2 knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative: a texture approach. Magn Reson Med. 2011;65(4):1184–94. doi: 10.1002/mrm.22693. [DOI] [PubMed] [Google Scholar]
- 36.Souza RB, Stehling C, Wyman BT, Hellio Le Graverand MP, Li X, Link TM, et al. The effects of acute loading on T1rho and T2 relaxation times of tibiofemoral articular cartilage. Osteoarthritis Cartilage. 2010;18(12):1557–63. doi: 10.1016/j.joca.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Stehling C, Baum T, Mueller-Hoecker C, Liebl H, Carballido-Gamio J, Joseph GB, et al. A novel fast knee cartilage segmentation technique for T(2) measurements at MR imaging - data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2011;19(8):984–9. doi: 10.1016/j.joca.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan J, Stehling C, Muller-Hocker C, Schwaiger BJ, Lynch J, McCulloch CE, et al. Vastus lateralis/vastus medialis cross-sectional area ratio impacts presence and degree of knee joint abnormalities and cartilage T2 determined with 3T MRI - an analysis from the incidence cohort of the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2011;19(1):65–73. doi: 10.1016/j.joca.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berger MJ, Kean CO, Goela A, Doherty TJ. Disease severity and knee extensor force in knee osteoarthritis: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2012;64(5):729–34. doi: 10.1002/acr.21608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baum T, Joseph GB, Arulanandan A, Nardo L, Virayavanich W, Carballido-Gamio J, et al. Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2012;64(2):248–55. doi: 10.1002/acr.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baum T, Joseph GB, Nardo L, Virayavanich W, Arulanandan A, Alizai H, et al. Correlation of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with body mass index: thirty-six-month followup data from a longitudinal, observational multicenter study. Arthritis Care Res (Hoboken) 2013;65(1):23–33. doi: 10.1002/acr.21741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X, Pai A, Blumenkrantz G, Carballido-Gamio J, Link T, Ma B, et al. Spatial distribution and relationship of T1rho and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson Med. 2009;61(6):1310–8. doi: 10.1002/mrm.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8(4):355–68. doi: 10.1055/s-2004-861764. [DOI] [PubMed] [Google Scholar]
- 44.Jungmann P, Kraus M, Nardo L, Liebl H, Alizai G, Joseph G, et al. T2 relaxation time measurements are limited in monitoring progression, once advanced cartilage defects at the knee occur - Longitudinal data from the Osteoarthritis Initiative. J Magn Reson Imaging. 2013 doi: 10.1002/jmri.24137. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crema MD, Roemer FW, Marra MD, Burstein D, Gold GE, Eckstein F, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 2011;31(1):37–61. doi: 10.1148/rg.311105084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koff MF, Amrami KK, Kaufman KR. Clinical evaluation of T2 values of patellar cartilage in patients with osteoarthritis. Osteoarthritis Cartilage. 2007;15(2):198–204. doi: 10.1016/j.joca.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection. Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 48.Carman WJ, Sowers M, Hawthorne VM, Weissfeld LA. Obesity as a risk factor for osteoarthritis of the hand and wrist: a prospective study. Am J Epidemiol. 1994;139(2):119–29. doi: 10.1093/oxfordjournals.aje.a116974. [DOI] [PubMed] [Google Scholar]
- 49.Ghroubi S, Elleuch H, Guermazi M, Kaffel N, Feki H, Abid M, et al. Abdominal obesity and knee ostheoarthritis. Ann Readapt Med Phys. 2007;50(8):661–6. doi: 10.1016/j.annrmp.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Laakso M. Hyperglycemia as a risk factor for cardiovascular disease in type 2 diabetes. Prim Care. 1999;26(4):829–39. doi: 10.1016/s0095-4543(05)70133-0. [DOI] [PubMed] [Google Scholar]
- 51.Mattila TK, de Boer A. Influence of intensive versus conventional glucose control on microvascular and macrovascular complications in type 1 and 2 diabetes mellitus. Drugs. 2010;70(17):2229–45. doi: 10.2165/11585220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 52.Hart DJ, Doyle DV, Spector TD. Association between metabolic factors and knee osteoarthritis in women: the Chingford Study. J Rheumatol. 1995;22(6):1118–23. [PubMed] [Google Scholar]
- 53.Davis MA, Ettinger WH, Neuhaus JM. The role of metabolic factors and blood pressure in the association of obesity with osteoarthritis of the knee. J Rheumatol. 1988;15(12):1827–32. [PubMed] [Google Scholar]
- 54.Hynes GR, Jones PJ. Leptin and its role in lipid metabolism. Curr Opin Lipidol. 2001;12(3):321–7. doi: 10.1097/00041433-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 55.Knoops KT, de Groot LC, Kromhout D, Perrin AE, Moreiras-Varela O, Menotti A, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2004;292(12):1433–9. doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- 56.Salto LM, Cordero-MacIntyre Z, Beeson L, Schulz E, Firek A, De Leon M. En Balance participants decrease dietary fat and cholesterol intake as part of a culturally sensitive Hispanic diabetes education program. Diabetes Educ. 2011;37(2):239–53. doi: 10.1177/0145721710394874. [DOI] [PMC free article] [PubMed] [Google Scholar]