Abstract
Importance
Many patients and physicians assume that the safety and effectiveness of newly approved therapeutics is well understood; however, the strength of the clinical trial evidence supporting approval decisions by the Food and Drug Administration (FDA) has not been evaluated.
Objectives
To characterize pivotal efficacy trials, clinical trials that served as the basis of FDA approval, for newly approved novel therapeutics.
Design and Setting
Cross-sectional analysis using publicly available FDA documents for all novel therapeutics approved between 2005 and 2012.
Main Outcome Measures
We classified pivotal efficacy trials according to the following design features: randomization, blinding, comparator and trial endpoint. “Surrogate outcomes” were defined as any endpoint using a biomarker that is expected to predict clinical benefit. We also determined the number of patients, trial duration, and trial completion rates.
Results
Between 2005 and 2012, FDA approved 188 novel therapeutics for 206 indications on the basis of 448 pivotal efficacy trials. Median number of pivotal trials per indication was two (interquartile range: 1–2.5), although 74 (36.8%) indications were approved on the basis of a single pivotal trial. Nearly all trials were randomized (89.3%, 95% Confidence Interval [CI], 86.4%–92.2%), double-blinded (79.5%, 95% CI, 75.7%–83.2%), and used either an active or placebo comparator (87.1%, 95% CI, 83.9%–90.2%). Median number of patients enrolled per indication among all pivotal trials was 760 (interquartile range: 270–1550). At least one pivotal trial with a duration of 6 months or greater supported the approval of 68 (33.8%, 95% CI, 27.2%–40.4%) indications. Pivotal trials using surrogate endpoints as their primary outcome formed the exclusive basis of approval for 91 (45.3%, 95% CI, 38.3%–52.2%) indications, clinical outcomes for 67 (33.3%, 95% CI, 26.8%–39.9%), and clinical scales for 36 (17.9%, 95% CI, 12.6%–23.3%). Trial features differed by therapeutic and indication characteristics, such as therapeutic area, expected length of treatment, orphan status and accelerated approval.
Conclusions and Relevance
The quality of clinical trial evidence used by the FDA as the basis of recent novel therapeutic approvals varied widely across indications.
INTRODUCTION
The approval of a drug by the Food and Drug Administration (FDA) conveys that the product is safe and effective. An Internet-based survey of a national probability sample of 4316 U.S. adults, 2944 respondents (68% response rate), found that 39% report believing that FDA only approves “extremely effective” drugs and 25% only drugs without serious side effects.1 Some physicians make similar assumptions about effectiveness and safety, expecting that patients are likely to benefit from newly approved therapies.2–5
FDA review of new drug applications is guided by the Federal Food, Drug and Cosmetic Act, which requires “adequate and well controlled investigations” to determine efficacy.6 FDA guidance suggests that drug manufacturers submit at least two trials, each providing independent evidence of efficacy – such studies are known as “pivotal” efficacy trials – but also implies flexibility, describing circumstances in which a single efficacy trial might be sufficient to support approval.7 Moreover, for certain applications, FDA provides written guidance on the design of pivotal efficacy trials, including features of trial design, such as sample selection and choice of comparator,8–10 and may provide further guidance in meetings with individual sponsors.11 As an example, for therapeutics evaluated through the accelerated approval pathway, which aims to speed approval of therapeutics that treat life-threatening diseases, FDA permits pivotal efficacy trials to use surrogate endpoints that are “reasonably likely” to predict clinical benefit.12
The clinical research findings available at the time of a drug’s approval have important implications: if made public, these findings represent the only source of information available to patients and their physicians as they decide whether to use a newly approved drug. However, flexible approval standards may lead to some therapeutics being approved by FDA on the basis of numerous rigorously designed clinical trials and others on fewer or less robust studies, leading to differing levels of certainty about the risks and benefits for newly approved drugs. Accordingly, we sought to systematically examine this issue, evaluating the strength of the clinical trial evidence supporting FDA approval decisions for novel therapeutics – pharmacologics and biologics – between 2005 and 2012 by characterizing key features of pivotal efficacy trials, such as trial size, design, duration and endpoints.
METHODS
Data Sources
Drugs@FDA is a publicly accessible database available through the FDA’s website that lists regulatory actions, such as approvals and drug labeling changes, for all currently approved prescription therapeutics. Records for each approved therapeutic are hyperlinked to FDA medical reviews, which are lengthy documents that outline the clinical evidence used to establish the efficacy and safety of the novel therapeutic prior to approval. The Drugs@FDA database was downloaded on January 11, 2012 and on May 1, 2013. Medical reviews were accessed several times between January 2012 and June 2013.
Study Sample
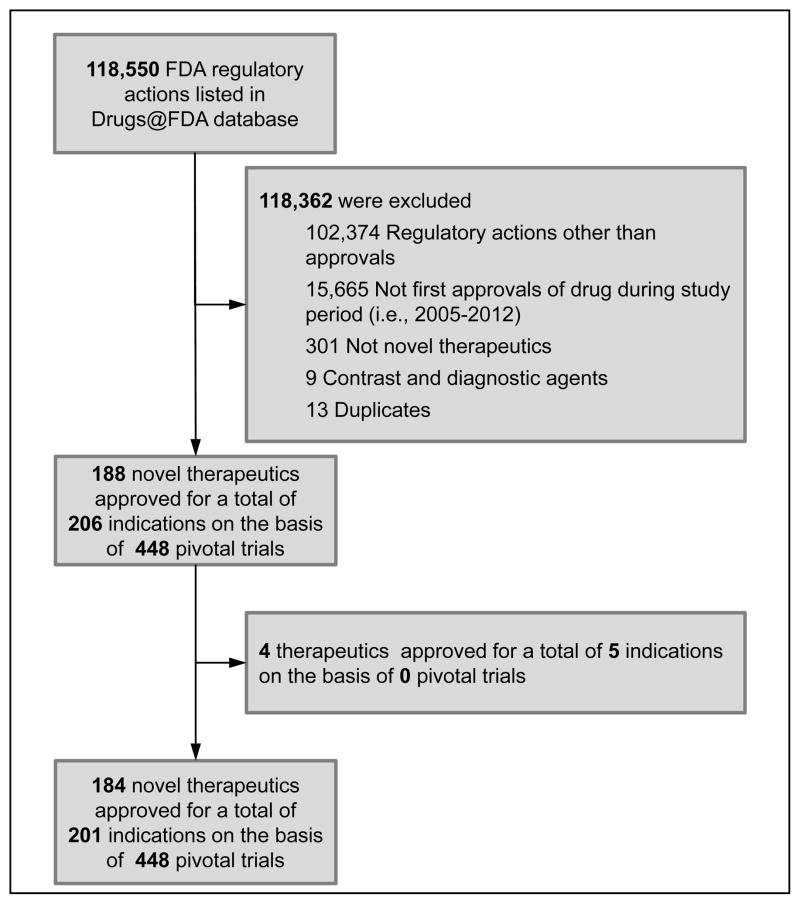
We constructed a sample of novel therapeutics (i.e., new molecular entities or novel biologic drugs) first approved by FDA between January 1, 2005 and December 31, 2012, excluding generic drugs, reformulations and combination therapies of non-novel therapeutics (Figure). We also excluded non-therapeutic agents, such as diagnostic and contrast agents (for details, see Appendix 1), and removed any duplicate records.
Figure.
Flow diagram showing sample construction of novel therapeutics approved by the Food and Drug Administration between 2005 and 2012.
Therapeutic and Indication Characteristics
Using information provided within the Drugs@FDA database, we categorized each novel therapeutic by year of approval and as a pharmacologic (i.e., small molecule) or biologic.13 Additionally, therapeutics were classified by orphan status, a designation made by FDA that affords extended market exclusivity for drugs that treat rare diseases (n.b., the Drugs@FDA database only indicates orphan status for biologics approved after 2010). Using FDA approval letters, which are also hyperlinked in the Drugs@FDA database, we identified therapeutics approved through the accelerated approval pathway, and the indication for which all novel therapeutics were initially approved for use. Subsequently, indications were categorized by expected length of treatment: acute, intermediate, or chronic. The expected length of use for acute treatments was less than one month, between one month, and two years for intermediate treatments and greater than two years for chronic treatments (for details, see Appendix 2). Additionally, we used the World Health Organization’s Anatomic Therapeutic Classification system, contextualized for clinical relevance, to categorize each indication into one of eight therapeutic areas.14 Finally, two investigators (NSD and JSR) determined the total number of patients exposed to the novel therapeutic during clinical development, the total safety population (for details, see Appendix 3).
Identification of Pivotal Efficacy Trials
For each novel therapeutic, one investigator (JAA) identified the pivotal efficacy trials used as the basis for approval. Generally, these trials are labeled in FDA medical reviews as “pivotal” and their design and findings are discussed in detail. For approvals where no trial was explicitly labeled “pivotal” in the FDA medical review, we identified trials described as essential to approval or those that were prioritized within the review, using criteria like substantial discussion of study design (i.e., inclusion and exclusion criteria, thorough description of study protocol) and independent analysis of results (i.e., not pooled with other studies). Additionally, any efficacy trial reviewed as part of a resubmitted application was considered pivotal to approval. Two investigators (NSD and JSR) subsequently validated identification of all pivotal efficacy trials through independent review, resolving conflicts by consensus.
Pivotal Efficacy Trial Features
Each pivotal trial was categorized according to its use of randomization and blinding based on the FDA reviewer’s description of the trial. In addition, we also recorded the type of comparator, primary trial endpoint(s), the number of treated patients (overall and intervention group), trial duration and completion rate (Appendix 3). Completion rate was calculated by dividing the number of patients completing the trial by the number of treated patients (overall and intervention group). Primary trial endpoints were classified as “clinical outcomes”, “clinical scales” or “surrogate outcomes” based on an established framework and a recent Institute of Medicine Report (Appendix 3).15,16 Clinical outcomes, such as death or rate of hospitalization, measure patient survival or function. Clinical scales, such as the Crohn’s Disease Activity Index or the visual analogue scale for pain, represent rubrics for the quantification of subjective patient-reported symptoms. Surrogate outcomes, such as hemoglobin A1C or hepatitis C ribonucleic acid levels, represent biomarkers expected to predict clinical benefit. The initial abstraction was performed by one investigator (JAA). Again, two investigators (NSD and JSR) subsequently validated characterization of all pivotal trials through independent review and abstraction, resolving conflicts by consensus.
Statistical analysis
Using descriptive statistics, we characterized the novel therapeutics included in our sample and the indications for which they were initially approved for use. Next, we used descriptive statistics to characterize features across the overall sample of pivotal efficacy trials as well as the features of these trials aggregated at the indication level, the summary of all pivotal efficacy trials used to support the approval of each indication. We then used chi-square, Wilcoxon and Kruskal-Wallis tests as appropriate to examine differences among novel therapeutic and indication characteristics, including therapeutic area, expected length of therapy, drug type, orphan status, and accelerated approval, all of which were pre-planned prior to data collection. Analyses were performed using Microsoft Excel 2010 (Microsoft Corporation; Redmond, WA) and JMP 7.0.1 (SAS Institute; Cary, NC). All statistical tests were two-tailed and used a type I error rate of 0.01 to account for multiple comparisons across 5 therapeutic/indication characteristics.
RESULTS
Between 2005 and 2012, FDA approved 188 novel therapeutics: 154 (81.9%) were pharmacologics, 34 (18.1%) biologics. FDA had granted orphan status to 31 (16.5%) and 22 (11.7%) were approved through the accelerated approval pathway (Table 1). These 188 novel therapeutics were approved for use for 206 indications: 171 (91.0%) for a single indication, 16 (8.5%) for two indications and one (0.5%) for three. Over half of indications required chronic treatment (n=108; 52.4%), 58 (28.2%) intermediate-length treatment, and 40 (19.4%) acute treatment. Three therapeutic areas accounted for nearly half of indications: 41 (19.9%) were used to treat cancer, 29 (14.1%) infectious disease and 23 (11.2%) cardiovascular disease, diabetes mellitus or hyperlipidemia. Median safety population, total number of patients exposed to the novel therapeutic during clinical development, was 1143 (Inter-Quartile Range [IQR]: 503–2600).
Table 1.
Novel therapeutics and associated indications approved by the Food and Drug Administration between 2005 and 2012.
| Novel Therapeutics (n=188) | |
| Approval Year | |
| 2005 | 19 (10.1) |
| 2006 | 22 (11.7) |
| 2007 | 17 (9.0) |
| 2008 | 20 (10.6) |
| 2009 | 25 (13.3) |
| 2010 | 20 (10.6) |
| 2011 | 28 (14.9) |
| 2012 | 37 (19.7) |
| Review Cycles Required for Approval | |
| Single | 134 (71.3) |
| Multiple | 54 (28.7) |
| Novel Therapeutic Type | |
| Pharmacologic | 154 (81.9) |
| Biologic | 34 (18.1) |
| Orphan Status | |
| Orphan designation | 31 (16.5) |
| No orphan designation | 157 (83.5) |
| Accelerated Approval | |
| Accelerated approval | 22 (11.7) |
| Regular approval | 166 (88.3) |
| Associated Indications (n=206) | |
| Therapeutic Area | |
| Cancer | 41 (19.9) |
| Infectious disease | 29 (14.1) |
| Cardiovascular disease, diabetes mellitus and hyperlipidemia | 23 (11.2) |
| Neurologic | 17 (8.3) |
| Dermatologic | 15 (7.3) |
| Autoimmune and musculoskeletal | 13 (6.3) |
| Psychiatric | 10 (4.9) |
| Other | 58 (28.2) |
| Expected Length of Treatment | |
| Acute | 40 (19.4) |
| Intermediate | 58 (28.2) |
| Chronic | 108 (52.4) |
Pivotal Efficacy Trial Features
Pivotal efficacy trials were identified for 201 of 206 indications (Figure); four novel therapeutics were approved (one for two indications) without a pivotal efficacy trial. A total of 448 pivotal trials were identified: 283 (63.2%) were explicitly labeled “pivotal”, 165 (36.8%) were inferred as pivotal based on the criteria described previously. The vast majority of pivotal trials were randomized (n=400; 89.3%, 95% Confidence Interval [CI], 86.4%–92.2%) and double-blinded (n=356; 79.5%, 95% CI, 75.7%–83.2%) (Table 2). Over half of trials used a placebo comparator (n=247; 55.1%, 95% CI, 50.5%–59.8%), 143 (31.9%, 95% CI, 27.6%–36.3%) used an active comparator, and 58 (12.9%, 95% CI, 9.8%–16.1%) had no comparator. The primary endpoint was a surrogate outcome for 219 (48.9%, 95% CI, 44.2%–53.5%) trials, 130 (29.0%, 95% CI, 24.8%–33.2%) used clinical outcomes and 99 (22.1%, 95% CI, 18.2%–26.0%) used clinical scales. Median total and intervention group patient populations were 446 (IQR: 205–678) and 271 (IQR: 133–426), while median trial duration was 14.0 weeks (IQR: 6.0–26.0 weeks); 113 (25.2%, 95% CI, 21.2%–29.3%) lasted 6 months or longer (Table 3). The median completion rate was 86.6% (IQR: 77.9%–93.1%).
Table 2.
Design of pivotal efficacy trials providing the basis for approval of novel therapeutics by the Food and Drug Administration between 2005 and 2012, stratified by therapeutic and indication characteristics.
| Therapeutic/Indication Characteristic | Pivotal Trials, No. | Randomized, No. % (95% CI) | Double-Blinded, No. % (95% CI) | Comparator, No. % (95% CI) | Endpoint, No. % (95%CI) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Active | Placebo | None | Surrogate Outcome | Clinical Outcome | Clinical Scale | ||||
| All | 448 | 400 89.3% (86.4%–92.2%) |
356 79.5% (75.7%–83.2%) |
143 31.9% (27.6%–36.3%) |
247 55.1% (50.5%–59.8%) |
58 12.9% (9.8%–16.1%) |
219 48.9% (44.2%–53.5%) |
130 29.0% (24.8%–33.2%) |
99 22.1% (18.2%–26.0%)s |
| Therapeutic Area | |||||||||
| Cancer | 55 | 26 47.3% (33.7%–60.9%) |
15 27.3% (15.1%–39.4%) |
10 18.2% (7.7%–28.7%) |
16 29.1% (16.7%–41.5%) |
29 52.7% (39.1%–66.3%) |
46 83.6% (73.5%–93.7%) |
9 16.4% (6.3%–26.5%) |
0 0.0% (0.0%–0.0%) |
| Infectious Disease | 57 | 53 93.0% (86.1%–99.8%) |
45 78.9% (68.0%–89.9%) |
39 68.4% (56.0%–80.9%) |
13 22.8% (11.6%–34.0%) |
5 8.8% (1.2%–16.3%) |
33 57.9% (44.7%–71.1%) |
24 42.1% (28.9%–55.3%) |
0 0.0% (0.0%–0.0%) |
| CV/DM/Lipids | 73 | 72 98.6% (95.9%–100.0%) |
68 93.2% (87.2%–99.1%) |
26 35.6% (24.4%–46.9%) |
45 61.6% (50.2%–73.1%) |
2 2.7% (0.0%–6.6%) |
62 84.9% (76.5%–93.3%) |
11 15.1% (6.7%–23.5%) |
0 0.0% (0.0%–0.0%) |
| Neurology | 38 | 38 100.0% (100.0%–100.0%) |
38 100.0% (100.0%–100.0%) |
6 15.8% (3.6%–27.9%) |
30 78.9% (65.4%–92.5%) |
2 5.3% (0.0%–12.7%) |
0 0.0% (0.0%–0.0%) |
25 65.8% (50.0%–81.6%) |
13 34.2% (18.4%–50.0%) |
| Dermatology | 29 | 27 93.1% (83.3%–100.0%) |
22 75.9% (59.3%–92.4%) |
5 17.2% 2.6%–31.9%) |
20 69.0% (51.1%–86.9%) |
4 13.8% (0.4%–27.1%) |
5 17.2% (2.6%–31.9%) |
15 51.7% (32.4%–71.1%) |
9 31.0% (13.1%–48.9%) |
| Autoimmune/Musculoskeletal | 36 | 36 100.0% (100%–100%) |
34 94.4% (86.6%–100%) |
11 30.6% (14.7%–46.3%) |
25 69.4% (53.6%–85.3%) |
0 0.0% (0%–0%) |
6 16.7% (3.9%–29.5%) |
2 5.6% (0%–13.4%) |
28 77.8% (63.5%–92.0%) |
| Psychiatry | 43 | 43 100.0% (100.0%–100.0%) |
43 100.0% (100.0%–100.0%) |
19 44.2% (28.7%–60.0%) |
24 55.8% (40.3%–71.3%) |
0 0.0% (0.0%–0.0%) |
5 11.6% (1.6%–21.6%) |
7 16.3% (4.8%–27.8%) |
31 72.1% (58.1%–86.1%) |
| Other | 117 | 105 89.7% (84.2%–95.3%) |
91 77.8% (70.1%–85.4%) |
27 23.1% (15.3%–30.8%) |
74 63.2% (54.4%–72.1%) |
16 13.7% (7.4%–20.0%) |
62 53.0% (43.8%–62.2%) |
37 31.6% (23.1%–40.2%) |
18 15.4% (8.7%–22.0%) |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||||
| Expected Length of Treatment | |||||||||
| Acute | 78 | 72 92.3% (86.3%–98.4%) |
63 80.8% (71.8%–89.7%) |
32 41.0% (29.9%–52.1%) |
35 44.9% (33.6%–56.2%) |
11 14.1% (6.2%–22.0%) |
31 39.7% (28.6%–50.8%) |
41 52.6% (41.2%–63.9%) |
6 7.7% (1.6%–13.7%) |
| Intermediate | 99 | 68 68.7% (59.4%–78.0%) |
54 54.5% (44.6%–64.5%) |
30 30.3% (21.1%–39.5%) |
38 38.4% (28.6%–48.1%) |
31 31.3% (22.0%–40.6%) |
53 53.5% (43.5%–63.5%) |
22 22.2% (13.9%–30.6%) |
24 24.2% (15.7%–32.8%) |
| Chronic | 271 | 260 95.9% (93.6%–98.3%) |
239 88.2% (84.3%–92.1%) |
81 29.9% (24.4%–35.4%) |
174 64.2% (58.5%–70.0%) |
16 5.9% (3.1%–8.7%) |
135 49.8% (43.8%–55.8%) |
67 24.7% (19.6%–29.9%) |
69 25.5% (20.2%–30.7%) |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||||
| Novel Therapeutic Type | |||||||||
| Pharmacologic | 384 | 340 88.5% (85.3%–91.7%) |
307 79.9% (75.9%–84.0%) |
127 33.1% (28.3%–37.8%) |
204 53.1% (48.1%–58.1%) |
53 13.8% (10.3%–17.3%) |
196 51.0% (46.0%–56.1%) |
121 31.5% (26.8%–36.2%) |
67 17.4% (13.6%–21.2%) |
| Biologic | 64 | 60 93.8% (87.7%–99.8%) |
49 76.6% (65.9%–87.2%) |
16 25.0% (14.1%–35.9%) |
43 67.2% (55.4%–79.0%) |
5 7.8% (1.1%–14.6%) |
23 35.9% (23.6%–48.0%) |
9 14.1% (5.3%–22.8%) |
32 50.0% (37.4%–62.6%) |
| P value | 0.28 | 0.51 | 0.10 | < 0.001 | |||||
| Orphan Status | |||||||||
| Yes | 56 | 30 53.6% (40.0%–67.0%) |
21 37.5% (24.4%–50.6%) |
12 21.4% (10.3%–32.5%) |
16 28.6% (16.4%–40.8%) |
28 50.0% (36.5%–63.5%) |
41 73.2% (61.2%–85.2%) |
10 17.9% (7.5%–28.2%) |
5 8.9% (1.2%–16.6%) |
| No | 392 | 370 94.4% (92.1%–96.7%) |
335 85.5% (82.0%–89.0%) |
131 33.4% (28.7%–38.1%) |
231 58.9% (54.0%–63.8%) |
30 7.7% (5.0%–10.3%) |
178 45.4% (40.5%–50.4%) |
120 30.6% (26.0%–35.2%) |
94 24.0% (19.7%–28.2%) |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||||
| Accelerated Approval | |||||||||
| Yes | 40 | 18 45.0% (28.9%–61.1%) |
12 30.0% (15.2%–44.8%) |
6 15.0% (3.4%–26.6%) |
12 30.0% (15.2%–44.8%) |
22 55.0% (38.9%–71.1%) |
38 95.0% (87.9%–100.0%) |
2 5.0% (0.0%–12.1%) |
0 0.0% (0.0%–0.0%) |
| No | 408 | 382 93.6% (91.2%–96.0%) |
344 84.3% (80.8%–87.9%) |
137 33.6% (29.0%–38.2%) |
235 57.6% (52.8%–62.4%) |
36 8.8% (6.1%–11.6%) |
181 44.4% (39.5%–49.2%) |
128 31.4% (26.9%–35.9%) |
99 24.3% (20.1%–28.4%) |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||||
Notes: CI=Confidence Interval; CV/DM/Lipids=Cardiovascular disease, diabetes mellitus, and hyperlipidemia.
Table 3.
Exposure to novel therapeutics approved by the Food and Drug Administration between 2005 and 2012 during pivotal efficacy trials that provided the basis for approval, stratified by therapeutic and indication characteristics.
| Therapeutic/Indication Characteristic | Pivotal Trials, No. | Median Patients, No. (IQR) | Duration* | Median Overall Completion Rate (IQR) | ||
|---|---|---|---|---|---|---|
| Overall | Intervention Group | Median Weeks (IQR) | ≥ 6 Months, No. % (95% CI) | |||
| All Trials (n=448) | 448 | 446 (205–678) | 271 (133–426) | 14.0 (6.0–26.0) | 113 25.2% (21.2%–29.3%) |
86.6 (77.9–93.1) |
| Therapeutic Area | ||||||
| Cancer | 55 | 266 (84–610) | 154 (84–359) | 18.5 (8.9–29.2) | 17 30.9% (18.3%–43.5%) |
82.5 (75.0–91.3) |
| Infectious Disease | 57 | 585 (319–697) | 305 (234–366) | 5.0 (2.5–24.0) | 10 17.5% (7.4%–27.7%) |
91.6 (87.1–96.0) |
| CV/DM/Lipids | 73 | 651 (406–926) | 441 (271–710) | 24.0 (10.0–26.0) | 23 31.5% (20.6%–42.4%) |
88.0 (76.8–92.4) |
| Neurology | 38 | 358 (234–613) | 253 (127–333) | 16.0 (12.0–21.0) | 7 18.4% (5.5%–31.3%) |
83.9 (76.7–88.5) |
| Dermatology | 29 | 233 (121–491) | 126 (64–204) | 4.3 (2.0–13.0) | 3 10.3% (0.0%–22.1%) |
94.4 (90.5–98.0) |
| Autoimmune/Musculoskeletal | 36 | 525 (362–749) | 345 (230–502) | 24.0 (24.0–28.0) | 12 33.3% (17.2%–49.5%) |
82.8 (76.9–92.2) |
| Psychiatry | 43 | 432 (275–590) | 231 (128–343) | 6.0 (6.0–8.0) | 0 0% (0.0%–0.0%) |
70.1 (54.8–83.6) |
| Other | 117 | 416 (122–695) | 238 (83–451) | 24.0 (10.5–39.0) | 41 35.0% (26.3%–43.8%) |
87.0 (79.4–93.1) |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Expected Length of Treatment | ||||||
| Acute | 78 | 312 (154–599) | 212 (97–318) | 2.5 (2.0–4.7) | 1 1.3% (0.0%–3.8%) |
93.1 (82.4–98.1) |
| Intermediate | 99 | 296 (108–625) | 196 (86–365) | 12.0 (4.6–24.0) | 22 22.2% (13.9%–30.6%) |
82.4 (67.5–91.3) |
| Chronic | 271 | 499 (312–745) | 311 (168–499) | 24.0 (12.0–26.0) | 90 33.2% (27.6%–38.9%) |
86.0 (79.2–92.1) |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Novel Therapeutic Type | ||||||
| Pharmacologic | 384 | 461 (222–693) | 275 (138–426) | 12.0 (6.0–24.0) | 86 22.4% (18.2%–26.6%) |
85.8 (77.5–92.9) |
| Biologic | 64 | 344 (108–658) | 217 (70–410) | 24.0 (18.0–49.6) | 27 42.2% (29.7%–54.6%) |
90.8 (81.3–94.5) |
| P value | 0.07 | 0.05 | < 0.001 | 0.002 | 0.03 | |
| Orphan Status | ||||||
| Yes | 56 | 150 (71–288) | 98 (53–184) | 13.5 (4.0–37.5) | 16 28.6% (16.4%–40.8%) |
88.7 (81.1–94.7) |
| No | 392 | 480 (276–717) | 294 (157–454) | 14.0 (6.0–26.0) | 97 24.7% (20.5%–29.0%) |
86.3 (77.7–93.1) |
| P value | < 0.001 | < 0.001 | 0.70 | 0.52 | 0.18 | |
| Accelerated Approval | ||||||
| Yes | 40 | 157 (89–342) | 142 (78–260) | 23.7 (9.4–24.0) | 9 22.5% (9.0%–36.0%) |
87.4 (76.0–94.8) |
| No | 408 | 470 (240–711) | 289 (142–446) | 12.0 (6.0–26.0) | 104 25.5% (21.2%–29.7%) |
86.6 (78.0–93.1) |
| P value | < 0.001 | < 0.001 | 0.13 | 0.85 | 0.61 | |
Notes: IQR=Interquartile Range; CI=Confidence Interval; CV/DM/Lipids=Cardiovascular disease, diabetes mellitus, and hyperlipidemia.
Excludes “single administration” trials, in which the investigational drug was given only once.
Trial Features by Therapeutic & Indication Characteristics
Features of pivotal efficacy trials differed by therapeutic and indication characteristics. Trials of therapeutics used for cancer were least likely to be randomized (47.3%, 95% CI, 33.7%–60.9% vs. 95.2%, 95% CI, 93.0%–97.3%; p<0.001) and double-blinded (27.3%, 95% CI, 15.1%–39.4% vs. 86.8%, 95% CI, 83.4%–90.1%; p<0.001) (Table 2). An active comparator was used more frequently in trials of therapeutics approved for infectious disease indications when compared with therapeutics for other indications (68.4%, 95% CI, 56.0%–80.9% vs. 26.6%, 95% CI, 22.2%–31.0%; p<0.001). Clinical outcomes and scales were infrequently used in trials of therapeutics approved for cancer (n=9; 16.4%, 95% CI, 6.3%–26.5%) and cardiovascular disease, diabetes mellitus, and hyperlipidemia (n=11; 15.1%, 95% CI, 6.7%–23.5%). Surrogate endpoints were used in nearly all trials among therapeutics approved through the accelerated approval pathway (n=38; 95.0%, 95% CI, 87.9%–100%), in contrast to fewer than half (n=181; 44.4%, 95% CI, 39.5%–49.2%) of trials among non-accelerated approval therapeutics.
The median intervention group patient population was smaller among therapeutics with orphan status when compared with those without (98 [IQR: 53–184] vs. 294 [IQR: 157–454]; p<0.001), and in trials of therapeutics approved via the accelerated approval pathway when compared to non-accelerated approval therapeutics (142 [IQR: 78–260] vs. 289 [IQR: 142–446]; p<0.001) (Table 3). Trial duration varied according to expected length of treatment (p<0.001) and was shorter for pharmacologics when compared with biologics (12.0 [IQR: 6.0–24.0] vs. 24.0 [IQR: 18.0–49.6] weeks; p<0.001).
Aggregated Pivotal Efficacy Trial Features Supporting Approved Indications
Among 201 indications, median number of trials per indication was 2 (IQR: 1–2.5; Table 4); 74 (36.8%) indications were approved on the basis of a single trial, 77 (38.3%) on two, and 50 (24.9%) on three or more. Among the aggregated pivotal efficacy trials supporting these indications, median total and intervention group patient populations were 760 (IQR: 270–1550) and 445 (IQR: 169–936). Although at least one trial using clinical outcomes or clinical scales supported the approval of 73 (36.3%, 95% CI, 29.6%–43.0%) and 39 (19.4%, 95% CI, 13.9%–24.9%) indications, respectively (Table 5), trials using clinical outcomes or clinical scales formed the exclusive basis of approval for slightly fewer: 67 (33.3%, 95% CI, 26.8%–39.9%) and 36 (17.9%, 95% CI, 12.6%–23.3%) indications. In addition, trials using surrogate endpoints as their primary outcome formed the exclusive basis of approval for 91 (45.3%, 95% CI, 38.3%–52.2%) indications. At least one trial of at least 6 months in duration supported the approval of 68 (33.8%, 95% CI, 27.2%–40.4%) indications
Table 4.
Number of aggregated pivotal efficacy trials and total number of patients providing the basis for approval of indications for novel therapeutics by the Food and Drug Administration between 2005 and 2012, stratified by therapeutic and indication characteristics.
| Therapeutic/Indication Characteristic | Indications, No. | Median Pivotal Efficacy Trials, No. (IQR) | Median Patients in Aggregated Pivotal Efficacy Trials, No. (IQR) | Median Total Safety Population,* No. (IQR) | |
|---|---|---|---|---|---|
| Overall | Intervention Group | ||||
| All Indications | 201 | 2 (1.0–2.5) | 760 (270–1550) | 445 (169–936) | 1143 (503–2600) |
| Therapeutic Area | |||||
| Cancer | 41 | 1 (1.0–1.0) | 397 (180–634) | 277 (159–414) | 511 (295–1100) |
| Infectious Disease | 27 | 2 (2.0–2.0) | 1171 (763–1408) | 605 (462–817) | 1408 (840–1979) |
| CV/DM/Lipids | 23 | 3 (1.0–5.0) | 3645 (1446–5942) | 2291 (832–3947) | 3422 (1579–6570) |
| Neurology | 17 | 2 (2.0–3.0) | 1088 (448–1394) | 661 (279–877) | 2315 (1729–3145) |
| Dermatology | 15 | 2 (1.0–2.0) | 374 (233–1005) | 187 (127–376) | 1193 (1048–2228) |
| Autoimmune/Musculoskeletal | 13 | 2 (2.0–3.0) | 1209 (289–2893) | 804 (223–1906) | 1955 (379–3233) |
| Psychiatry | 10 | 4 (2.0–5.5) | 1492 (947–3000) | 878 (417–1812) | 3290 (1596–4099) |
| Other | 55 | 2 (1.0–2.0) | 418 (105–1608) | 238 (78–968) | 700 (296–1781) |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Expected Length of Treatment | |||||
| Acute | 36 | 2 (2.0–2.0) | 586 (305–1194) | 349 (155–613) | 889 (471–1560) |
| Intermediate | 57 | 1 (1.0–2.0) | 435 (192–787) | 290 (159–507) | 645 (365–1319) |
| Chronic | 108 | 2 (1.0–3.0) | 1203 (361–2062) | 694 (234–1407) | 1857 (698–3262) |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Novel Therapeutic Type | |||||
| Pharmacologic | 164 | 2 (1.0–3.0) | 825 (322–1607) | 503 (209–956) | 1206 (554–2806) |
| Biologic | 37 | 1 (1.0–2.0) | 374 (105–1213) | 229 (70–683) | 890 (288–1839) |
| P value | 0.01 | 0.009 | 0.003 | 0.05 | |
| Orphan Status | |||||
| Yes | 29 | 1 (1.0–2.0) | 238 (162–576) | 190 (107–361) | 483 (290–651) |
| No | 172 | 2 (1.0–3.0) | 961 (339–1659) | 516 (214–1021) | 1371 (663–2909) |
| P value | 0.05 | < 0.001 | < 0.001 | < 0.001 | |
| Accelerated Approval | |||||
| Yes | 23 | 2 (1.0–2.0) | 266 (160–586) | 207 (154–462) | 497 (299–856) |
| No | 178 | 2 (1.0–3.0) | 862 (323–1648) | 503 (180–986) | 1323 (593–2761) |
| P value | 0.11 | < 0.001 | 0.008 | < 0.001 | |
Notes: IQR=Interquartile Range; CV/DM/Lipids=Cardiovascular disease, diabetes mellitus, and hyperlipidemia.
For therapeutics approved for multiple indications, the safety population is the pooled number of patients exposed to the drug.
Table 5.
Proportion of novel therapeutic indications approved by the Food and Drug Administration between 2005 and 2012 on the basis of at least one trial that met the criteria below, stratified by therapeutic and indication characteristics.
| Therapeutic/Indication Characteristic | Indications, No. | ≥ 2 Pivotal
Trials No. % (95% CI)* |
Trial Duration† No. % (95% CI) |
Comparator No. % (95% CI) |
Trial Endpoint No. % (95% CI) |
|||
|---|---|---|---|---|---|---|---|---|
| ≥ 6 months | ≥ 12 months | Active | Placebo | Clinical Outcome | Clinical Scale | |||
| All Indications | 201 | 127 63.2% (56.5%–69.9%) |
68 33.8% (27.2%–40.4%) |
17 8.5% (4.6%–12.3%) |
79 39.3% (32.5%–46.1%) |
119 59.2% (52.4%–66.0%) |
73 36.3% (29.6%–43.0%) |
39 19.4% (13.9%–24.9%) |
| Therapeutic Area | ||||||||
| Cancer | 41 | 8 19.5% (6.8%–32.1%) |
16 39.0% 23.4%–54.6%) |
2 4.9% (0.0%–11.8%) |
10 24.4% (10.7%–38.1%) |
15 36.6% (21.2%–52.0%) |
9 22.0% (8.7%–35.2%) |
0 0.0% (0.0%–0.0%) |
| Infectious Disease | 27 | 21 77.8% (61.0%–94.5%) |
5 18.5% (2.9%–34.1%) |
1 3.7% (0.0%–11.3%) |
21 77.8% (61.1%–94.5%) |
7 25.9% (8.3%–43.6%) |
13 48.1% (28.0%–68.3%) |
0 0.0% (0.0%–0.0%) |
| CV/DM/Lipids | 23 | 16 69.6% (49.2%–90.0%) |
12 52.2% (30.0%–74.3%) |
4 17.4% (0.0%–34.2%) |
13 56.5% (34.6%–78.4%) |
16 69.6% (49.2%–89.9%) |
8 34.8% (13.7%–55.8%) |
0 0.0% (0.0%–0.0%) |
| Neurology | 17 | 15 88.2% (71.1%–100.0%) |
4 23.5% (1.0%–46.0%) |
2 11.8% (0.0%–28.8%) |
5 29.4% (5.3%–53.6%) |
15 88.2% (71.1%–100.0%) |
11 64.7% (39.4%–90.0%) |
7 41.2% (15.1%–67.2%) |
| Dermatology | 15 | 11 73.3% (48.0%–98.6%) |
2 13.3% (0.0%–32.8%) |
0 0% (0.0%–0.0%) |
3 20.0% (0.0%–42.9%) |
11 73.3% (48.0%–98.7%) |
8 53.3% (24.7%–81.9%) |
5 33.3% (6.3%–60.3%) |
| Autoimmune/Musculoskeletal | 13 | 11 84.6% (61.9%–100.0%) |
6 46.2% 14.8%–77.5%) |
1 7.7% (0.0%–24.5%) |
6 46.2% (14.8%–77.5%) |
11 84.6% (61.9%–100.0%) |
1 7.7% (0.0%–24.5%) |
10 76.9% (50.4%–100.0%) |
| Psychiatry | 10 | 10 100.0% (100.0%–100.0%) |
0 0% (0.0%–0.0%) |
0 0% (0.0%–0.0%) |
8 80.0% (49.8–100.0%) |
7 70.0% (35.4%–100.0%) |
2 20.0% (0.0%–50.2%) |
8 80.0% (49.8%–100.0%) |
| Other | 55 | 35 63.6% (50.5%–76.8%) |
23 41.8% (28.4%–55.3%) |
7 12.7% (3.6%–21.8%) |
13 23.6% (12.0%–35.2%) |
37 67.3% (54.5%–80.0%) |
21 38.2% (24.9%–51.4%) |
9 16.4% (6.3%–26.5%) |
| P value | < 0.001 | 0.01 | 0.36 | < 0.001 | < 0.001 | 0.008 | < 0.001 | |
| Expected Length of Treatment | ||||||||
| Acute | 36 | 28 77.8% (63.5%–92.0%) |
1 2.8% (0.0%–8.4%) |
0 0% (0.0%–0.0%) |
20 55.6% (38.5%–72.6%) |
17 47.2% (30.0%–64.4%) |
22 61.1% (44.4%–77.8%) |
3 8.3% (0.0%–17.8%) |
| Intermediate | 57 | 21 36.8% (23.9%–49.8%) |
19 33.3% (20.7%–46.0%) |
4 7.0% (0.0%–13.8%) |
17 29.8% (17.6%–42.1%) |
25 43.9% (30.6%–57.1%) |
14 24.6% (13.0%–36.1%) |
10 17.5% (7.4%–27.7%) |
| Chronic | 108 | 78 72.2% (63.6%–80.8%) |
48 44.4% (34.9%–54.0%) |
13 12.0% (5.8%–18.3%) |
42 38.9% (29.5%–48.2%) |
77 71.3% (62.6%–80.0%) |
37 34.3% (25.2%–43.4%) |
26 24.1% (15.9%–32.3%) |
| P value | < 0.001 | < 0.001 | 0.07 | 0.05 | 0.001 | 0.001 | 0.11 | |
| Novel Therapeutic Type | ||||||||
| Pharmacologic | 164 | 110 67.1% (59.8%–74.3%) |
52 31.7% (24.5%–38.9%) |
15 9.1% (4.7%–13.6%) |
71 43.3% (35.6%–51.0%) |
92 56.1% (48.4%–63.8%) |
66 40.2% (32.7%–47.8%) |
23 14.0% (8.7%–19.4%) |
| Biologic | 37 | 17 45.9% (29.1%–62.8%) |
16 43.2% (26.5%–60.0%) |
2 5.4% (0.0%–13.0%) |
8 21.6% (7.7%–35.5%) |
27 73.0% (58.0%–88.0%) |
7 18.9% (5.7%–32.2%) |
16 43.2% (26.5%–60.0%) |
| P value | 0.02 | 0.18 | 0.46 | 0.01 | 0.06 | 0.01 | < 0.001 | |
| Orphan Status | ||||||||
| Yes | 29 | 13 44.8% (25.6%–64.0%) |
12 41.4% (22.3%–60.4%) |
5 17.2% (2.6%–31.9%) |
8 27.6% (10.3%–44.9%) |
11 37.9% (19.1%–56.7%) |
8 27.6% (10.3%–44.9%) |
2 6.9% (0.0%–16.7%) |
| No | 172 | 114 66.3% (59.1%–73.4%) |
56 32.6% (25.5%–39.6%) |
12 7.0% (3.1%–10.8%) |
71 41.3% (33.8%–48.7%) |
108 62.8% (55.5%–70.9%) |
65 37.8% (30.5%–45.1%) |
37 21.5% (15.3%–27.7%) |
| P value | 0.03 | 0.35 | 0.07 | 0.16 | 0.01 | 0.29 | 0.07 | |
| Accelerated Approval | ||||||||
| Yes | 23 | 13 56.5% (34.6%–78.4%) |
8 34.8% (13.7%–55.8%) |
2 8.7% (0.0%–21.2%) |
4 17.4% (0.0%–34.2%) |
7 30.4% (10.0%–50.8%) |
1 4.3% (0.0%–13.4%) |
0 0.0% (0.0%–0.0%) |
| No | 178 | 114 64.0% (56.9%–71.2%) |
60 33.7% (26.7%–40.7%) |
15 8.4% (4.3%–12.5%) |
75 42.1% (34.8%–49.5%) |
112 62.9% (55.8%–70.0%) |
72 40.4% (33.2%–47.7%) |
39 21.9% (15.8%–28.0%) |
| P value | 0.48 | 0.92 | 0.97 | 0.02 | 0.003 | 0.001 | 0.001 | |
Notes: CI=Confidence Interval; CV/DM/Lipids=Cardiovascular disease, diabetes mellitus, and hyperlipidemia.
Two or more pivotal trials served as the basis of FDA approval
Excludes “single administration” trials, in which the investigational drug was given only once.
Aggregated Trial Features Supporting Approved Indications by Therapeutic & Indication Characteristics
The features of the aggregated pivotal efficacy trials supporting approved indications differed by therapeutic and indication characteristics. Most therapeutics approved for cancer indications were on the basis of a single trial, while the approval of therapeutics for cardiovascular disease, diabetes mellitus, and hyperlipidemia and psychiatric indications often relied upon at least three trials (Tables 4 and 5). Median numbers of overall and intervention group patients were larger among aggregated trials supporting indications within these therapeutic areas.
There was no difference in the proportion of indications approved through the accelerated approval pathway on the basis of multiple trials when compared with non-accelerated approval indications (56.5%, 95% CI, 34.6%–78.4% vs. 64.0%, 95% CI, 56.9%–71.2%; p=0.48). More therapeutics indicated for chronic treatment were supported by at least one trial of 6 months duration when compared with therapeutics indicated for acute or intermediate-length treatment (44.4%, 95% CI, 34.9%–54.0% vs. 2.8%, 95% CI, 0%–8.4% and 33.3%, 95% CI, 20.7%–46.0%, respectively; p<0.001).
DISCUSSION
Our characterization of pivotal efficacy trials, trials that serve as the basis of FDA approval, for all novel therapeutics approved between 2005 and 2012 demonstrates that the quality of clinical trial evidence used by the FDA to make approval decisions varied widely across indications. While the vast majority of indications were supported by at least one randomized, double-blinded trial, there was wide variation in trials’ choice of comparators and endpoints, duration, size, and completion rate. In addition, just over one third of indications were approved on the basis of a single pivotal efficacy trial.
The variation in the quality of clinical trial evidence used by FDA to assess novel therapeutic efficacy highlights the agency’s flexible standards for approval. Such regulatory flexibility allows for a customized approach to approval, including the ability to rapidly approve potentially effective therapies for life-threatening diseases, such as certain cancers, or those diseases for which there is no existing effective treatment, such as orphan diseases. These approvals can be made without requiring costly and time-consuming randomized, double-blinded, controlled trials, despite their being regarded as the gold standard for evaluation.17–19 Indeed, FDA has provided guidance on approaches to accelerate clinical development of novel therapeutics8–10,20 and has cited its willingness to rapidly approve new drugs in recent year-end reviews of drug approvals.21,22 Substantial variation has been described among pivotal efficacy trials supporting the approval of cancer drugs,23 and this flexibility may well be warranted given the limited number of effective therapies and poor prognosis associated with cancer.
Understanding the strength of clinical trial evidence of newly approved therapeutics has important implications for patients and physicians. When medications become available on the market, decisions must be made about their use, likely informed by how well safety and effectiveness are understood. Comparative effectiveness information, which is not required as part of FDA approval and involves comparison of an intervention to an active control, was available for less than half of indications, consistent with prior research,24 but leaving uncertainty about the benefits and safety of these medications when compared with other available therapeutics. Similarly, while patient-important clinical outcomes and scales were used in many pivotal trials, trials using surrogate endpoints as their primary outcome formed the exclusive basis of approval for nearly half of the approved indications in our study. This reliance on surrogate outcomes leaves patients and physicians to extrapolate clinical benefits from trials, again raising questions about the certainty of the medications’ benefits in practice.25 Finally, we found that the majority of trials evaluating therapeutics indicated for chronic treatment lasted less than one year, raising questions about the certainty of these medications’ long-term efficacy and safety.
Because comprehensive safety evaluations are difficult to undertake as part of randomized controlled trials, particularly smaller trials, these findings clarify the importance of adopting a “life-cycle” approach, both for drug safety and for improved understanding of drug effectiveness. In 2006, the Institute of Medicine’s Report on the Future of Drug Safety recommended that the FDA monitor and evaluate the benefits and risks of drug therapies not only prior to their approval but throughout their entire market life.26 This so-called life-cycle approach suggests that new information on therapeutics’ benefits and safety be continually collected. It also requires adequate and robust post-market surveillance systems that allow reassessments of drug efficacy and safety after market introduction.
However, communicating this updated information to patients and physicians is critical. A recent Institute of Medicine committee report similarly recommended that the FDA implement a benefit and risk assessment and management plan that would summarize the FDA’s evaluation of a drug’s benefit-risk profile in a single document and be continuously updated during the entire market life of the product.27,28 Alternatively, or as part of this effort, FDA could provide a summative statement, or even a grade, for each approval to signal the quality of clinical trial evidence used to determine safety and efficacy, allowing therapeutics approved on the basis of more robust evidence to be distinguished from those approved on less robust evidence. As the FDA has publicly declared its intention to encourage investigators to use innovative trial designs that are as effective as standard designs but less burdensome and time-consuming and to identify qualifying biomarkers that accurately predict outcomes to make clinical trials more efficient,29 it must also ensure that patients and physicians understand how to interpret these trials and the likelihood of experiencing benefit or harm when deciding to use these newly approved therapeutics.
Our study has several limitations. Although pivotal efficacy trials represent the primary source of information about novel therapeutic efficacy at approval, other trials that provide supplementary efficacy data are discussed transiently in FDA medical reviews, but were not systematically assessed. While FDA is more likely to first approve novel therapeutics for use,30 the agency may also rely on information from use of the drugs in other countries when evaluating these therapeutics for approval, which we did not assess. Additionally, efficacy represents just one component of FDA review, which also covers safety, pharmacology, chemistry and manufacturing. Finally, our study was limited to the approval of new molecular entities and novel biologics; since reformulated and generic drugs can be approved on the basis of bioequivalence studies, it is likely that our study captured the majority of pivotal efficacy trials used for novel therapeutic evaluations. Moreover, our findings are consistent with a prior study of high-risk cardiovascular devices, which found many to be approved on the basis of trials that lack adequate strength and may be prone to bias.31
In conclusion, the quality of clinical trial evidence used by the FDA as the basis of novel therapeutic approvals between 2005 and 2012 varied widely across indications. This variation has important implications for patients and physicians as they make decisions about the use of newly approved therapeutics and has the potential to inform both the current FDA regulatory approval standards and post-market surveillance initiatives.
Supplementary Material
Acknowledgments
Funding/support and role of the sponsor: Support for this project was provided in part by The Pew Charitable Trusts. The sponsor played no role in the design of the study, analysis or interpretation of findings, or drafting the manuscript and did not review or approve the manuscript prior to submission. The authors assume full responsibility for the accuracy and completeness of the ideas presented. Drs. Krumholz and Ross receive support from Medtronic, Inc. to develop methods of clinical trial data sharing, from the Centers of Medicare and Medicaid Services (CMS) to develop and maintain performance measures that are used for public reporting, and from the Food and Drug Administration (FDA) to develop methods for post-market surveillance of medical devices. Dr. Krumholz is supported by a National Heart Lung Blood Institute Cardiovascular Outcomes Center Award (1U01HL105270-02). Dr. Ross is supported by the National Institute on Aging (K08 AG032886) and by the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program.
Footnotes
Data access and responsibility: Mr. Downing and Dr. Ross had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of interest: Dr. Krumholz reports that he chairs a scientific advisory board for United Healthcare. Dr. Ross reports that he is a member of a scientific advisory board for FAIR Health, Inc.
Author contributions: Mr. Downing and Dr. Ross were responsible for the conception and design of this work, drafted the manuscript and conducted the statistical analysis. Mr. Downing and Drs. Aminawung and Ross were responsible for acquisition of data. Dr. Ross obtained funding and provided supervision. All authors participated in the analysis and interpretation of the data and critically revised the manuscript for important intellectual content.
References
- 1.Schwartz LM, Woloshin S. Communicating uncertainties about prescription drugs to the public: a national randomized trial. Arch Intern Med. 2011;171(16):1463–1468. doi: 10.1001/archinternmed.2011.396. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Zhang H, Yu CH, Li JY, Jiang Y. The attitudes of oncology physicians and nurses toward phase I, II, and III cancer clinical trials. Contemp Clin Trials. 2011;32(5):649–653. doi: 10.1016/j.cct.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Chen DT, Wynia MK, Moloney RM, Alexander GC. US physician knowledge of the FDA-approved indications and evidence base for commonly prescribed drugs: results of a national survey. Pharmacoepidem Dr S. 2009;18(11):1094–1100. doi: 10.1002/pds.1825. [DOI] [PubMed] [Google Scholar]
- 4.Healy D. Pharmageddon. Berkeley and Los Angeles, California: University of California Press; 2012. [Google Scholar]
- 5.Anderson GM, Juurlink D, Detsky AS. Newly approved does not always mean new and improved. JAMA. 2008;299(13):1598–1600. doi: 10.1001/jama.299.13.1598. [DOI] [PubMed] [Google Scholar]
- 6.21 U.S.C., United States Code. Edition; Title 21 - Food and Drugs; Chapter 9 - Federal Food, Drug, and Cosmetic Act; Subchapter V - Drugs and Devices; Part A - Drugs and Devices. [Accessed October 7, 2013.];Section 355 - New drugs; subsection (d) grounds for refusing application; approval of application; “substantial evidence” defined. 2010 Available at: http://www.gpo.gov/fdsys/pkg/USCODE-2010-title21/html/USCODE-2010-title21-chap9-subchapV-partA-sec355.htm.
- 7.U.S. Food and Drug Administration. [Accessed October 7, 2013];Guidance for Industry: Providing Clinical Evidence of Effectiveness for Human Drugs and Biological Products. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm078749.pdf.
- 8.U.S. Food and Drug Administration. [Accessed October 7, 2013];Guidance for Industry: Non-Inferiority Clinical Trials. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM202140.pdf.
- 9.U.S. Food and Drug Administration. [Accessed October 7, 2013];Guidance for Industry: Enrichment Strategies for Clinical Trials to Support Approval of Human Drugs and Biological Products (Draft Guidance) Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM332181.pdf.
- 10.U.S. Food and Drug Administration. [Accessed October 7, 2013];Guidance for Industry: Adaptive Design Clinical Trials for Drugs and Biologics (Draft Guidance) Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM201790.pdf.
- 11.Booz Allen Hamilton Inc. [Accessed October 7, 2013];Independent Evaluation of FDA’s First Cycle Review Performance - Final Report. Available at: http://www.fda.gov/ForIndustry/UserFees/PrescriptionDrugUserFee/ucm127117.htm.
- 12.21 U.S.C., United States Code. Edition; Title 21 - Food and Drugs; Chapter 1 - Food and Drug Administration; Subchapter D - Drugs for Human Use; Part 314 - Applications for FDA Approval to Market a New Drug. [Accessed October 7, 2013.];2012 Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=314&showFR=1&subpartNode=21:5.0.1.1.4.8.
- 13.U.S. Food and Drug Administration. [Accessed December 28, 2011];Drugs@FDA: FDA Approved Drug Products. Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/
- 14.WHO Collaborating Center for Drug Statistics Methodology. [Accessed October 7, 2013];ATC/DDD Index. 2013 Available at: http://www.whocc.no/atc_ddd_index/
- 15.Clement FM, Harris A, Li JJ, Yong K, Lee KM, Manns BJ. Using Effectiveness and Cost-effectiveness to Make Drug Coverage Decisions A Comparison of Britain, Australia, and Canada. JAMA. 2009;302(13):1437–1443. doi: 10.1001/jama.2009.1409. [DOI] [PubMed] [Google Scholar]
- 16.Institute of Medicine. Evaluation of biomarkers and surrogate endpoints in chronic disease. Washington, DC: National Academy Press; 2010. [PubMed] [Google Scholar]
- 17.Concato J, Shah N, Horwitz RI. Randomized, Controlled Trials, Observational Studies, and the Hierarchy of Research Designs. New England Journal of Medicine. 2000;342(25):1887–1892. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith GCS, Pell JP. Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials. BMJ. 2003;327(7429):1459–1461. doi: 10.1136/bmj.327.7429.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller FG, Joffe S. Equipoise and the Dilemma of Randomized Clinical Trials. New England Journal of Medicine. 2011;364(5):476–480. doi: 10.1056/NEJMsb1011301. [DOI] [PubMed] [Google Scholar]
- 20.Mitka M. FDA and PhRMA seek better ways to assess drug safety, efficacy in clinical trials. JAMA: the journal of the American Medical Association. 2012;307(24):2576–2577. doi: 10.1001/jama.2012.6684. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Food and Drug Administration. [Accessed October 7, 2013];FY 2011 Innovative Drug Approval. Available at: http://www.fda.gov/AboutFDA/ReportsManualsForms/Reports/ucm330502.htm.
- 22.U.S. Food and Drug Administration. [Accessed October 7, 2013];Drugs@FDA: FDA Approved Drug Products. Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/
- 23.Kesselheim AS, Myers JA, Avorn J. Characteristics of clinical trials to support approval of orphan vs nonorphan drugs for cancer. JAMA: the journal of the American Medical Association. 2011;305(22):2320–2326. doi: 10.1001/jama.2011.769. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg NH, Schneeweiss S, Kowal MK, Gagne JJ. Availability of comparative efficacy data at the time of drug approval in the united states. JAMA: the journal of the American Medical Association. 2011;305(17):1786–1789. doi: 10.1001/jama.2011.539. [DOI] [PubMed] [Google Scholar]
- 25.Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Annals of internal medicine. 1996;125(7):605–613. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- 26.Institute of Medicine. The Future of Drug Safety: Promoting and Protecting the Health of the Public. Washington, DC: National Academy Press; 2006. [Google Scholar]
- 27.Institute of Medicine. Ethical and Scientific Issues in Studying the Safety of Approved Drugs. Washington, DC: National Academy Press; 2012. [PubMed] [Google Scholar]
- 28.Psaty BM, Meslin EM, Breckenridge A. A lifecycle approach to the evaluation of FDA approval methods and regulatory actions: opportunities provided by a new IOM report. JAMA: the journal of the American Medical Association. 2012;307(23):2491–2492. doi: 10.1001/jama.2012.5545. [DOI] [PubMed] [Google Scholar]
- 29.Hamburg MA. Shattuck lecture. Innovation, regulation, and the FDA. N Engl J Med. 2010;363(23):2228–2232. doi: 10.1056/NEJMsa1007467. [DOI] [PubMed] [Google Scholar]
- 30.Downing NS, Aminawung JA, Shah ND, Braunstein JB, Krumholz HK, Ross JS. Regulatory Review of Novel Therapeutics - Comparison of Three Regulatory Agencies. New England Journal of Medicine. 2012;366:2284–2293. doi: 10.1056/NEJMsa1200223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhruva SS, Bero LA, Redberg RF. Strength of study evidence examined by the FDA in premarket approval of cardiovascular devices. JAMA: the journal of the American Medical Association. 2009;302(24):2679–2685. doi: 10.1001/jama.2009.1899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.