Abstract
The mechanical microenvironment surrounding cells has a significant impact on cellular function. One prominent example is that the stiffness of the substrate directs stem cell differentiation. However, the underlying mechanisms of how mechanical cues affect stem cell functions are largely elusive. Here, we report that in human mesenchymal stem cells (HMSCs), substrate stiffness can regulate cellular responses to a β-adrenergic receptor (β-AR) agonist, Isoproterenol (ISO). Fluorescence resonance energy transfer based A-Kinase Activity Reporter revealed that HMSCs displayed low activity of ISO-induced protein kinase A (PKA) signal on soft substrate, whereas a significantly higher activity can be observed on hard substrate. Meanwhile, there is an increasing ISO-induced internalization of β2-AR with increasing substrate stiffness. Further experiments revealed that the effects of substrate stiffness on both events were disrupted by interfering the polymerization of microtubules, but not actin filaments. Mechanistic investigation revealed that inhibiting ISO-induced PKA activation abolished β2-AR internalization and vice versa, forming a feedback loop. Thus, our results suggest that the cellular sensing mechanism of its mechanical environment, such as substrate stiffness, affects its response to chemical stimulation of β-AR signaling and PKA activation through the coordination of microtubules, which may contribute to how mechanical cues direct stem cell differentiation.
Keywords: Mesenchymal stem cell, Substrate stiffness, β-adrenergic receptor, FRET biosensor, Molecular Imaging, Protein kinase A
1. Introduction
Adult stem cells, such as human mesenchymal stem cells (HMSCs), have great potential in tissue engineering and regenerative medicine due to their self-renewal ability and multipotency. HMSCs can be directed to differentiate into cell types of mesoderm lineages, for instance, adipocytes, fibroblasts, chondrocytes, osteoblasts, neuronal cells and myocytes [1]. Numerous studies have revealed a variety of factors that play important roles in the functions of HMSCs [2]. Aside from the traditional biochemical factors including soluble factors and adhesive molecules, biophysical properties of the microenvironment, such as substrate stiffness, have been found to directly and effectively control the cell lineage specification of HMSCs [3]. Therefore, understanding how biochemical and biophysical factors are integrated in HMSCs to determine the process of differentiation is crucial for the advancement of regenerative medicine.
G-protein coupled receptors (GPCRs) are a large family of seven-transmembrane receptors that are linked to intracellular G-proteins. They respond to many types of ligands, such as light-sensitive compounds, odors, pheromones, hormones and neurotransmitters [4]. While the predominant biochemical factors studied for stem cells are various growth factors and cytokines, the limited study on the role of GPCRs in stems cells doesn’t match the long-established field of GPCRs signaling. Providing the expression of GPCRs in stem cells as well as existing crosstalk between the signaling pathways of GPCRs and growth factors, it is not surprising that they have been found to regulate stem cell function in vitro and in vivo [4]. As one type of the GPCRs, β-adrenergic receptors (β-ARs) have three subtypes β1, β2 and β3. Recently, it has been reported that bone marrow-derived hematopoietic stem cells express abundant β2- and β3-ARs involved in progenitor mobilization [5]. The functional expression of β2-ARs is also observed in MSCs for a specific role such as protection against oxidative stress [6]. All these receptors are functionally linked to Gs proteins, which in turn are linked to adenylyl cyclase [7,8]. The binding of β-ARs to agonist, such as Isoproterenol (ISO), results in increased levels of the intracellular second messenger cAMP that subsequently activates protein kinase A (PKA) [9]. PKA is a cytosolic threonine/serine kinase with two regulatory and two catalytic subunits. cAMP binds to each of the regulatory subunits releasing and activating the catalytic subunits. The activated subunit can then translocate to the nucleus and phosphorylate the cAMP response element-binding protein (CREB), which is a transcription factor. In this way, PKA regulates many cellular processes through transcriptional control of cAMP response element, such as essential metabolisms of glycogen, sugar and lipids, and DNA replication [10-12]. Additional reports have demonstrated that direct cAMP/PKA pathway activation in HMSCs in vitro results in robust bone formation in vivo [13]. Therefore, β-AR and its downstream PKA signaling pathway can regulate the functions of HMSCs.
As pharmaceutical drugs targeting GPCRs are among the oldest and most established therapeutics, it is an attractive idea to tap into the existing arsenal of drugs for their potential applications in stem cell engineering both in vitro and in vivo. Ample evidence has indicated that the biophysical factors in the microenvironment of stem cells regulate the cellular response to the soluble factors and pharmaceutical drugs [14]. Therefore, understanding the effect of biophysical factors on GPCRs-mediated stem cell function is crucial to the application of these drugs. To visualize PKA activation with high spatiotemporal resolutions, genetically-encoded FRET-based A-Kinase Activity Reporter (AKAR2) was applied in HMSCs [9,15]. Agonist-induced receptor internalization was also measured by imaging GFP-tagged receptors. In this study, we investigated how substrate stiffness affects such agonist-mediated PKA pathway in HMSCs and explored the underlying mechanism.
2. Materials and Methods
2.1. Bis-acrylamide-PA gel fabrication
Polyacrylamide (PA) gels were prepared as previously described [16,17]. Briefly, 40% w/v acrylamide and 2% w/v bis-acrylamide solutions (Bio-Rad, Hercules, CA) were mixed with 10 mM HEPES buffer. PA gels with different stiffness were obtained by varying the final concentrations of acrylamide solution (3-7.5%) and bis-acrylamide cross-linker (0.03-0.6%). To polymerize the solution, 2.5 μL of 10% w/v ammonium persulfate (Bio-Rad) and 0.25 μl of N,N,N’,N’-Tetramethylethylenediamine (TEMED; Bio-Rad) were added with the solution to yield a final volume of 500 μl. After polymerization, sulfo-SANPAH (N-Sulfosuccinimidyl-6-(4′-azido-2′-nitrophenylamino) hexanoate; Pierce Biotechnology, Rockford, IL), a photo-activating cross-linker, was used to crosslink the extracellular matrix molecules onto the gel surface. The powder of sulfo-SANPAH was dissolved in 10 mM HEPES buffer containing 0.5% DMSO and the sulfo-SANPAH solution (0.5 mg/ml) was added on top of the PA gel. Gel dishes were placed at a distance of ~15 cm from the UV light of the hood for 6 min and rinsed three times with 10 mM HEPES buffer for 10 min. A 200 μl of collagen type I solution (40 ug/ml) (BD Sciences, Bedford, MA) was incubated on top of the sulfo-SANPAH-coated PA gels at 37 °C overnight to deposit collagen for subsequent cell seeding.
2.2. Cell culture and transfection
Human mesenchymal stem cells (HMSCs; Lonza Walkersville, Inc., Walkersville, MD) were cultured with mesenchymal stem cell growth medium (MSCGM, PT-3001, Lonza) containing 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin in a humidified incubator of 95% O2 and 5% CO2 at 37 °C. The DNA plasmids were transfected into the cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) reagent according to the product instructions.
2.3. Gene construction and DNA plasmids
The original construct of FRET-based AKAR2 has been described previously [9]. In order to enhance the FRET response, the FRET acceptor was replaced by an improved version of yellow fluorescent protein for FRET (YPet) [18]. Briefly, the fragment containing ECFP, forkhead-associated domain 1 (FHA1) and PKA substrate sequence (SAGKPGSGEGSTKGLRRATLVDGGTGGS) was fused to YPet, and subcloned into pcDNA3.1 for mammalian cell expression. A green fluorescent protein (GFP)-tagged version of β2-adrenergic receptor (β2-AR), GFP-Gαs and mCherry-tubulin have been well-described in previous reports [19-21].
2.4. Imaging acquisition, data analysis, and microscopy
Cells were starved with 0.5% FBS for 36 - 48 hr before imaging experiments. During the imaging process, the cells were maintained in a CO2-independent medium (Invitrogen, CA) at 37°C. As indicated, cells were treated with Isoproterenol (ISO, 10 μM; Sigma) or Forskolin (FSK, 10 μM; Calbiochem). For inhibition studies of PKA activity, H89 (20 μM; Sigma) was added 1 hr before treatment of ISO. To disrupt the polymerization of actin filaments and microtubules, the experiments were performed by pretreating the cells for 1 hr before imaging with Cytochalasin D (CytoD, 1 μM, Sigma) or Nocodazole (NOC, 5 μM, Sigma), respectively. To disrupt lift rafts, methyl-beta-cyclodextrin (MβCD, 20 μM; Sigma) was pretreated with the cells for 1 hr before imaging. The concentration of inhibitors used and their effects were verified in previous studies [22-24]. Images were acquired on a Zeiss Axiovert 200M microscope (Carl Zeiss) equipped with a cooled charge-coupled device (CCD) camera (Cascade 512B, Photometrics) and a 440DF20 excitation filter, a 455DRLP dichroic mirror, and two emission filters controlled by a filter changer (480DF30 for CFP and 535DF25 for YFP). Time-lapse images were acquired every 1 min and the FRET/ECFP emission ratio images were computed and analyzed in MetaFluor 6.2 software (Universal Imaging, West Chester, PA). The number of β2-AR-GFP particle was quantified using Image J software.
2.5. Statistical analysis
All statistical data were expressed as the mean ± standard error of the mean (SEM). Statistical evaluation was completed using Excel software to perform a Student’s t-test to determine the statistical differences between groups. A significant difference was determined by the P-value (< 0.05).
3. Results
3.1. Substrate stiffness tunes the level of β-AR agonist-induced PKA activation
As mechanical factors play crucial roles in the production of intracellular second messengers [25,26] and affect cellular physiology and stem cell differentiation [3,27], we have investigated whether substrate stiffness can regulate PKA activity in HMSCs. To visualize the dynamics of PKA activation in live cells, we utilized a FRET-based AKAR2 reporter previously described [9]. In order to enhance the dynamic range of the FRET reporter, the original FRET acceptor was replaced by yellow fluorescent protein for FRET (YPet) while the FRET donor ECFP was kept the same (Fig. S1) [18]. The cells expressing ECFP-AKAR2-YPet reporter were cultured on PA gel substrates with different stiffness, and then stimulated with a β-AR agonist, ISO, a reagent structurally similar to adrenaline and known as a medication used for the treatment of heart diseases and asthma [28,29]. This drug can activate both β1- and β2-ARs at the membrane, and eventually stimulate the downstream PKA via cAMP. Thus, PKA activity was monitored by real-time live cell imaging and its activation level was assessed by the FRET/ECFP emission ratio (Fig. S1).
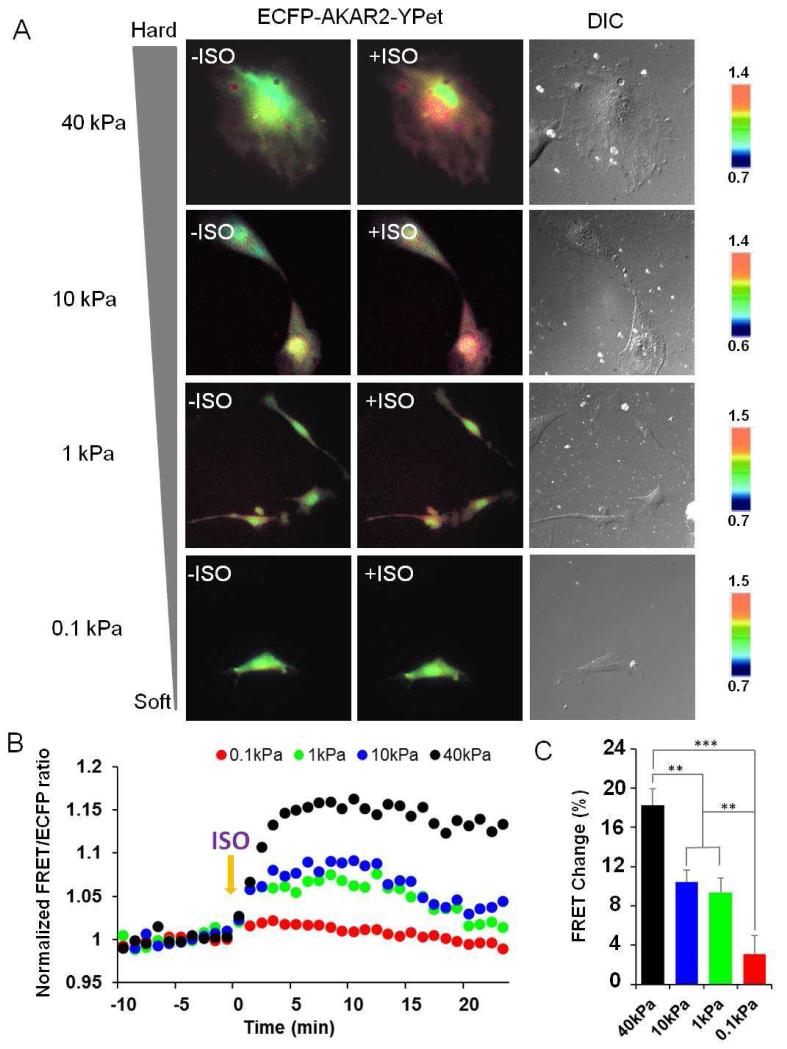
As shown in Figure 1A, ISO stimulation induced increasing FRET response in HMSCs cultured on gels with increasing stiffness, indicating increasing levels of PKA activation. As shown in Figure 1B-C on the quantification of the responses, the PKA activation reached its maximal level around five minutes after the ISO addition. The statistical analysis of multiple cells confirmed this pattern. The cells cultured on hard gel (40 kPa) showed the largest increase in FRET ratio (~18%, n=10) upon ISO treatment, whereas relatively small changes in FRET ratio occurred in cells seeded on substrates with soft (3%, n=6, ***P<0.001) or intermediate stiffness (9-10%, n=7, **P<0.01), suggesting that the mechanical properties of the substrate can tune the β-AR agonist-induced PKA signaling.
Figure 1. Distinct responses of PKA activation upon ISO stimulation in HMSCs cultured on substrates with different stiffness.
(A) The cells expressing ECFP-AKAR2-YPet reporter show the FRET ratio changes before and after treatment with a β-AR agonist, ISO, on substrates with different stiffness. Blue and red colors represent low and high FRET ratios, respectively. (B, C) Time courses and bar graphs indicate the average of normalized FRET/ECFP emission ratio changes of the reporter from multiple cells in response to ISO (n=6-10, **P<0.01 and ***P<0.001).
On the contrary, the pattern of Forskolin (FSK)-induced PKA activation was different from that of ISO-induced PKA signaling (Fig. S2). There is a similar level of increase in PKA activity upon FSK treatment regardless of the stiffness magnitudes of substrates where cells are seeded (Fig. S2). FSK is a cell-permeable agonist of intracellular enzyme, adenylyl cyclase, which synthesizes cAMP from ATP. This increase of cAMP level bypassed the activation of membrane receptors such as β-AR. Therefore, these results suggest that substrate stiffness can tune agonist-induced β-AR mediated PKA activation.
3.2. Substrate stiffness regulates the degree of agonist-induced β2-AR internalization
After an agonist binds to β-AR, the receptor can be phosphorylated by PKA directly or indirectly through activation of G-protein coupled receptor kinases (GRKs) [8,30]. Increased phosphorylation of β-AR is known to be involved in the process of the internalization that subsequently leads to sequestration and desensitization of the receptors [7]. Therefore, we hypothesized that changes in the activity of PKA regulated by substrate stiffness could affect the β-AR internalization.
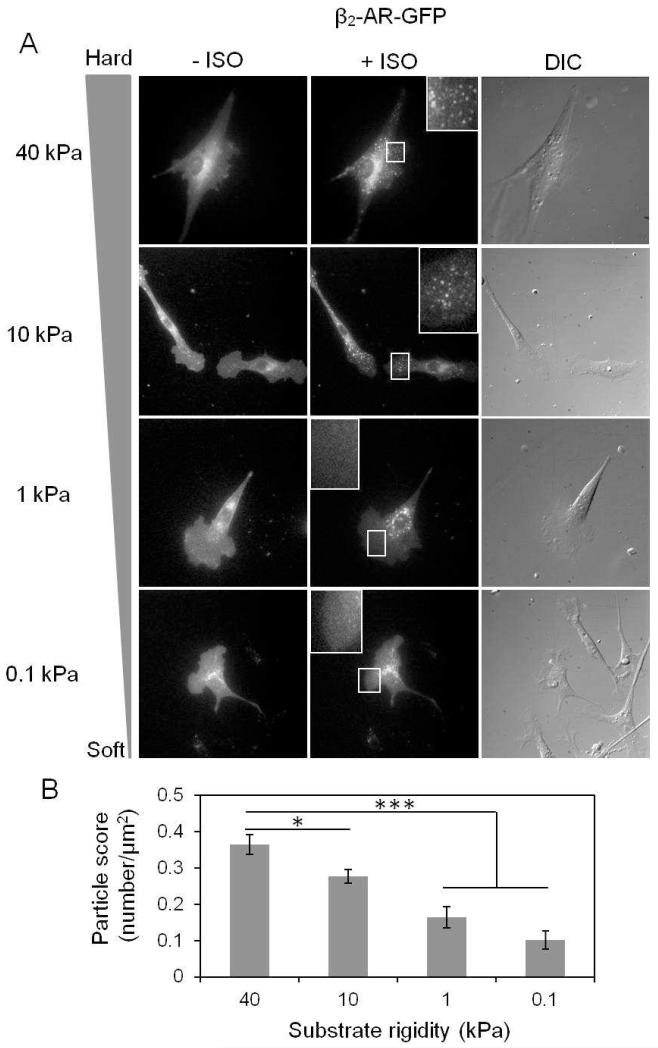
To carry out this study, we took advantage of a GFP-tagged β2-AR construct (β2-AR-GFP) and transfected it into the HMSCs. As shown in Figure 2A, there was an increasing degree of β2-AR internalization upon ISO treatment in HMSCs on gels with increasing stiffness. The quantification of internalized β2-AR-GFP particles showed that the ISO-induced internalization of β2-AR occurred strongly in cells on hard substrate (40 kPa) in accordance with the strongest PKA activity. In contrast, the number of internalized particles decreased in cells on soft substrates (Fig. 2B, n=10-12, 40 kPa vs 10 kPa, *P<0.05; 40 kPa vs 0.1 or 1 kPa, ***P<0.001). However, direct activation of adenylyl cyclase by FSK didn’t induce β2-AR internalization in HMSCs on either soft or hard gels (Fig. S3A). Therefore, our results indicate that substrate stiffness can regulate the degree of agonist-induced β2-AR internalization.
Figure 2. Effect of the substrate stiffness on the internalization of β2-AR in response to ISO.
(A) β2-AR-GFP construct was transfected into HMSCs and seeded on the substrate gels with different stiffness. Fluorescent images show the internalized particles before and after 20 min of ISO treatment. (B) The bar graphs represent the internalized particle quantified in the cells seeded on substrate gels with different stiffness in response to ISO. β2-AR internalization occurs in a stiffness-dependent manner in response to ISO (n=10-12, *P<0.05 and ***P<0.001).
3.3. Microtubules mediate the effect of substrate stiffness on β-AR agonist-induced PKA activity and β2-AR internalization
Since there are emerging evidences showing the structure of cellular cytoskeleton is strongly affected by the substrate stiffness to which a cell is seeded [17,31], we reasoned that the cytoskeleton would mediate the effect of the substrate stiffness on ISO-induced PKA activation and receptor internalization.
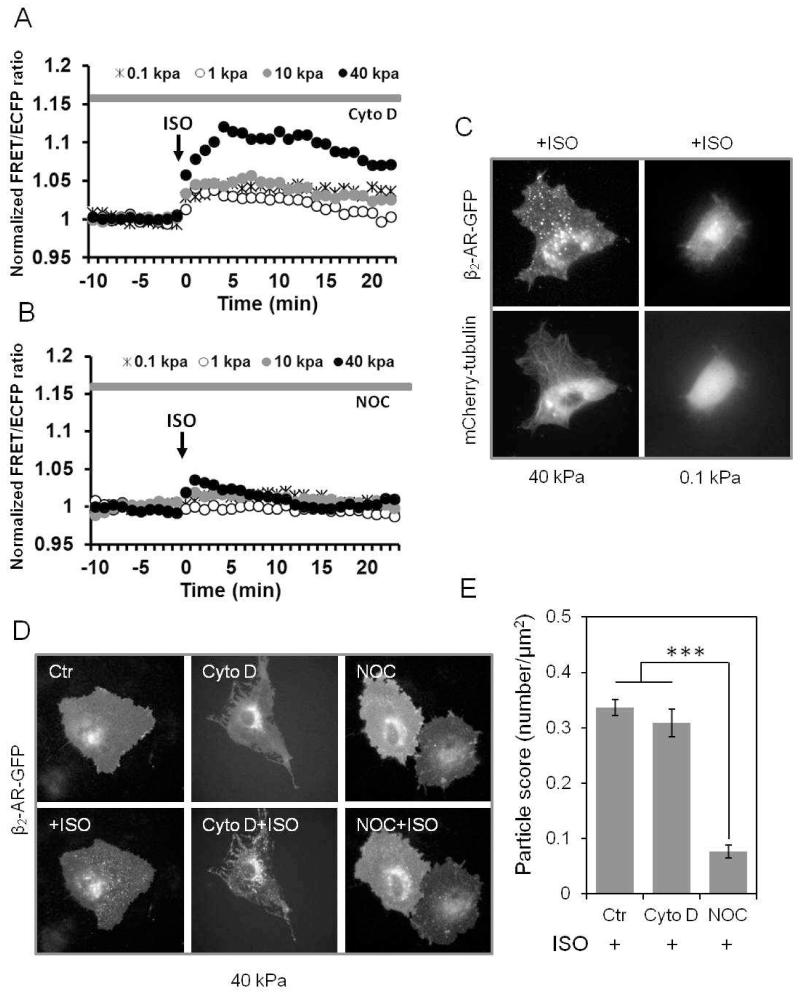
When cells were pretreated with Cytochalasin D (Cyto D) to inhibit the polymerization of actin filaments, the distinct responses of ISO-induced PKA activity between soft and hard substrates still remained (Fig. 3A). However, when pretreated with Nocodazole (NOC) to disrupt microtubules, the ISO-induced activation of PKA signals on hard substrate (40 kPa) was significantly reduced to a lower level, comparable to those from cells seeded on soft substrate (Fig. 3B). Notably, microtubules had no significant effect on the plateau of FSK-induced PKA signaling regardless of the magnitudes of substrate stiffness ranging from 0.1 to 40 kPa (Fig. S3B). These results indicate that microtubules, but not actin filaments, mediate the effect of substrate stiffness on the ISO-induced PKA activation, suggesting that substrate stiffness may affect ISO-induced PKA signals via a microtubule-dependent pathway.
Figure 3. Microtubules mediate the effect of substrate stiffness on ISO-induced PKA activation and receptor internalization.
Time courses represent the normalized FRET/ECFP emission ratio of ECFP-AKAR2-YPet sensor in response to ISO. The cells were cultured on gels with different stiffness (0.1-40 kPa) and pretreated with (A) Cytochalasin D (Cyto D, n=6), an inhibitor of actin polymerization or (B) Nocodazole (NOC, n=6), an inhibitor of microtubule polymerization. ISO-induced PKA activation is inhibited by NOC, but not Cyto D. (C) HMSCs cultured on hard gel (40 kPa) showed a large quantity of internalized particles of β2-AR and well organized microtubule structures (mCherry-tubulin) compared to the cells on soft gel (0.1 kPa). (D & E) Fluorescence images (D) and bar graphs (E) indicate that NOC, but not Cyto D treatment inhibited the ISO-induced β2-AR internalization in cells seeded on substrate gels with different stiffness (n=6, ***P<0.001).
We further found that β2-AR internalization was dependent on the support of microtubules. Co-transfection of mCherry-tubulin and β2-AR-GFP in HMSCs cultured on hard substrate showed well-organized microtubule networks and a high rate of occurrence in β2-AR internalization, which were all inhibited on soft substrate (Fig. 3C). Consistent with the ISO-regulated PKA pathway, disruption of microtubules by NOC, but not actin by CytoD, inhibited the ISO-induced β2-AR internalization on the hard substrate (Fig. 3D-E, n=6, ***P<0.001). Therefore, these results suggest that substrate stiffness regulates agonist-induced β2-AR internalization in a microtubule-dependent manner, similar to that of PKA signaling.
3.4. The β-AR agonist-induced PKA signaling and β2-AR internalization form a feedback loop
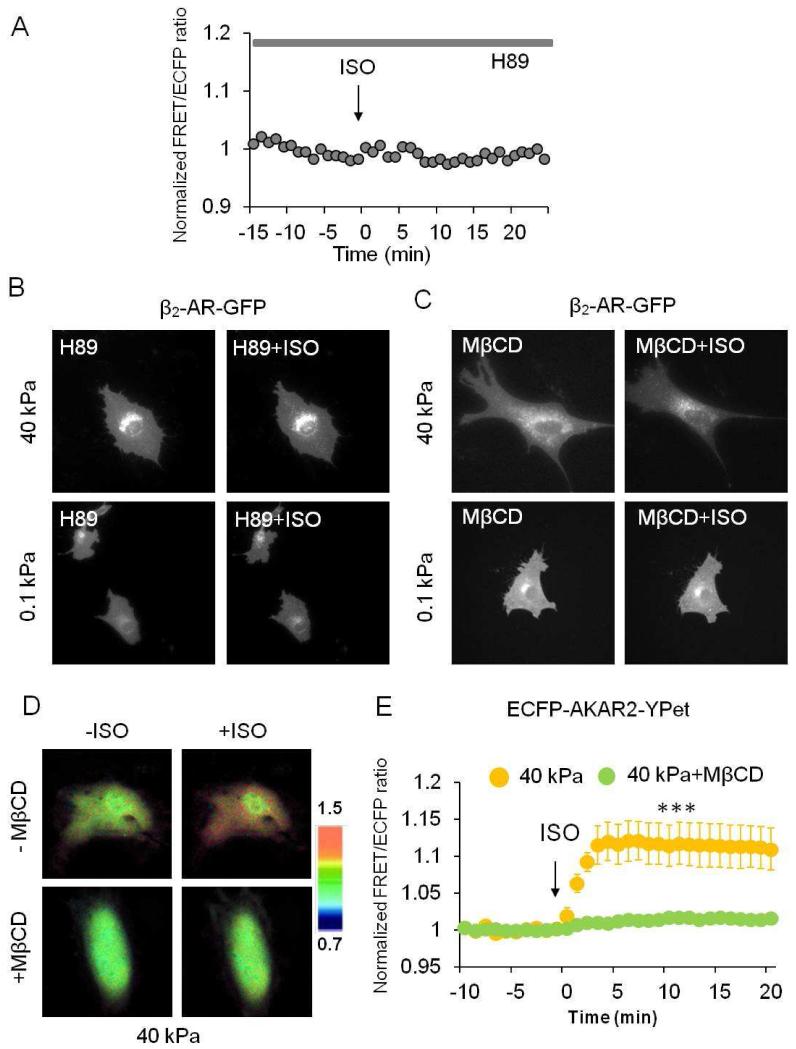
A potent selective inhibitor of PKA, H89 treatment, inhibited ISO and FSK-induced PKA activity (Fig. 4A, S3C). Interestingly, it also blocked the effect of substrate stiffness on β2-AR internalization upon ISO treatment (Fig. 4B), suggesting that β-AR agonist-induced PKA activation contributes to the internalization of β2-AR. Next we examined whether β2-AR internalization reciprocally affects β-AR agonist induced PKA signaling in response to substrate stiffness. To inhibit the internalization of β2-AR, we pretreated cells with MβCD that disrupts endocytosis of receptors. As shown in Figure 4C, MβCD treatment apparently inhibited ISO-induced β2-AR internalization on both hard (40 kPa) and soft (0.1 kPa) substrates. Further experiments revealed that the pretreatment of MβCD also significantly inhibited ISO-induced PKA activity on hard substrate (n=6, ***P<0.001) compared with that of untreated cells (n=7) (Fig. 4D-E). These results suggest that agonist-induced receptor internalization and PKA activity depend on each other and form a feedback loop in HMSCs seeded on substrates with different stiffness (Fig. 5).
Figure 4. The ISO-induced PKA activation and receptor internalization form a feedback loop.
(A) Time courses of emission ratio in the cells pretreated with H89 (20 μM), a PKA inhibitor, in response to ISO. H89 inhibits FRET emission ratio in ECFP-AKAR2-YPet transfected HMSCs. Similar results were observed in three independent experiments. (B) H89 inhibits ISO-induced internalization of β2-AR on both hard (40 kPa) and soft (0.1 kPa) gels. Similar results were observed in three independent experiments. (C) MβCD treatment inhibits ISO-induced β2-AR internalization in HMSCs cultured on both hard (40 kPa) and soft (0.1 kPa) gels. (D-E) FRET/ECFP emission ratio images and time courses revealed that the disruption of lipid rafts and receptor internalization by MβCD blocks ISO-induced PKA activation on hard gels (40 kPa) (n=6, ***P<0.001), whereas untreated cells still show the FRET increase in response to ISO (n=7).
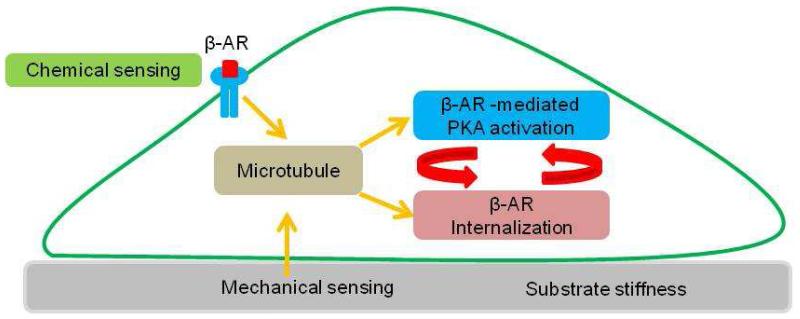
Figure 5. A proposed model of coordinated chemical and mechanical sensing in HMSCs.
Microtubules play a key role in integrating chemical and mechanical inputs to coordinate the β-AR agonist induced PKA activation and β-AR internalization, which forms a feedback loop.
4. Discussion
The landmark study of substrate stiffness directing HMSC differentiation established the mechanical environmental as one of the determining factors for stem cell functions (3). HMSCs on soft gels are prone to be neurogenic (0.1-1kPa) while they appear osteogenic on hard gels (25- 40 kPa). In our study, using substrate gels with a range of stiffness similar to this earlier work, we found that the agonist-induced GPCR-mediated PKA activity is significantly higher on hard than on soft gels. Further results indicate that this GPCR-mediated PKA activation and receptor internalization form a feedback loop, which is regulated by microtubules, but not by actin filaments.
Activated PKA can directly phosphorylate and activate its downstream effector, such as transcription factor CREB, to control gene expression therefore affect HMSC differentiation [32]. Indeed, cAMP/PKA pathway has been linked to the fate of HMSC differentiation [13,33]. For example, genes known to be activated by CREB, such as BMP2, ID2, FosB, promote osteogenesis both in vitro and in vivo [13,33-35]. Therefore, the higher PKA activity on hard gels observed with the FRET-based AKAR2 reporter in our study can have significant physiological consequences leading to osteogenesis, which corroborates the results from the landmark study [3]. As lower level of PKA activation on soft gels in our study is correlated with neurogenic fate [3], it is possible that the level of PKA activity at the initial stage is linked quantitatively with HMSC differentiation. In that case, PKA activity can be a marker for differentiation potential and therefore FRET-based AKAR2 reporter can be applied to screen drugs that can affect stem cell fate. Further systematic and quantitative study is needed to test this hypothesis.
Our results not only showed the effect of substrate stiffness on GPCR-mediated PKA activation but also pinpointed the molecular scenario of the signaling pathway affected. In general, ISO binding to β-AR can activate associated G proteins. In turn, G proteins activate adenylyl cyclase, which catalyze the conversion of ATP to cAMP. Increase cAMP binds to the regulatory subunits of PKA, resulting in the release and activation of the catalytic subunits. Our results indicate that the ISO-induced PKA activation was regulated by substrate stiffness. However, direct activation of adenylyl cyclase by FSK and subsequent PKA activation and receptor internalization was not affected by substrate stiffness (Fig. S2, S3A). Therefore, our results suggested that the signaling transduction regulated by substrate stiffness is upstream of the adenylyl cyclase activation. We further examined whether G-protein subunit Gαs was the point of regulation by substrate stiffness since a few previous reports have demonstrated that mechanical stress affects Gαs recruitment to focal adhesions, and Gαs appears to mediate mechanical force-induced cAMP signaling through integrins [36,37]. However, neither soft (0.1 kPa) nor stiff substrate (40 kPa) had any effect on Gαs recruitment and internalization in response to ISO (Fig. S4). Therefore, substrate stiffness may directly act at the adrenergic receptor level to modulate the cellular response to agonist stimulation.
In addition to the PKA activation, substrate stiffness also controls the agonist-induced receptor internalization. Inhibition of PKA activity by H89 treatment reduced the β2-AR internalization, suggesting that PKA may control receptor internalization by directly or indirectly phosphorylate GPCR through activation of GRKs [8,30]. The phosphorylation of GPCR likely recruits the scaffolding protein β-arrestin, which leads to receptor internalization [38]. Meanwhile, the inhibition of receptor internalization by MβCD treatment abolished the agonist-induced PKA activation, suggesting a feedback loop between PKA activation and receptor internalization. It is also possible that MβCD treatment itself extracting cholesterol out of lipid rafts, may affect lipid-raft mediated GPCR signaling.
We also found that microtubules are the possible mediators for the effect of substrate stiffness on both agonist-induced PKA activation and receptor internalization (Fig. 5). Substrate stiffness has been shown to affect cytoskeleton organization in various cell types including HMSCs [3,17]. Meanwhile, cytoskeleton is a key cellular component for membrane topography, trafficking and organelle movement. Therefore, cytoskeleton is a natural candidate to mediate the effect of substrate stiffness through regulating the function of cellular membrane signaling. Indeed, both microtubules and actin filaments have been indicated to regulate GPCR signaling and trafficking. While the involvement of actin filaments in β-AR signaling was reported [39,40], our result is consistent with a previous report that the microtubule inhibitor, NOC, but not an inhibitor of actin filaments, Latrunculin A, blocks the agonist-induced β2-AR internalization in epidermoid carcinoma A431 cells [12]. As such, our report provides evidence highlighting the importance of microtubule in mediating the effect of substrate-stiffness on GPCR signaling in HMSCs.
5. Conclusions
Our study reveals that the levels of agonist-induced PKA activation and β2-AR internalization in HMSCs were positively correlated with the stiffness of the substrate. These correlations are mediated by the integrity of microtubule dynamics, with the agonist-induced PKA activation and receptor internalization depending on each other to form a feedback loop. These findings not only shed lights on our understanding of how stem cells integrate the biophysical and biochemical cues in their microenvironment, but also provide instructive information for the potential application of GPCR-targeted drugs in tissue engineering and regenerative medicine.
Supplementary Material
Acknowledgements
This work is supported in part by grants from NIH HL098472, HL109142, GM106403, NSF CBET 0846429 and 1344298 (Y.W.), Beckman Institute Graduate Student Fellowship (T.K.) and Beckman Institute Postdoctoral Fellowship (J.S.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Doze VA, Perez DM. GPCRs in stem cell function. Prog Mol Biol Transl Sci. 2013;115:175–216. doi: 10.1016/B978-0-12-394587-7.00005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rodrigues M, Griffith LG, Wells A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res Ther. 2010;1(4):32. doi: 10.1186/scrt32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- [4].Callihan P, Mumaw J, Machacek DW, Stice SL, Hooks SB. Regulation of stem cell pluripotency and differentiation by G protein coupled receptors. Pharmacol Ther. 2011;129(3):290–306. doi: 10.1016/j.pharmthera.2010.10.007. [DOI] [PubMed] [Google Scholar]
- [5].Mendez-Ferrer S, Battista M, Frenette PS. Cooperation of beta(2)- and beta(3)-adrenergic receptors in hematopoietic progenitor cell mobilization. Ann N Y Acad Sci. 2010;1192:139–44. doi: 10.1111/j.1749-6632.2010.05390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Takahata Y, Takarada T, Iemata M, Yamamoto T, Nakamura Y, Kodama A, et al. Functional expression of beta2 adrenergic receptors responsible for protection against oxidative stress through promotion of glutathione synthesis after Nrf2 upregulation in undifferentiated mesenchymal C3H10T1/2 stem cells. J Cell Physiol. 2009;218(2):268–75. doi: 10.1002/jcp.21594. [DOI] [PubMed] [Google Scholar]
- [7].Ma YC, Huang XY. Novel signaling pathway through the beta-adrenergic receptor. Trends Cardiovasc Med. 2002;12(1):46–9. doi: 10.1016/s1050-1738(01)00138-4. [DOI] [PubMed] [Google Scholar]
- [8].Chen-Izu Y, Xiao RP, Izu LT, Cheng H, Kuschel M, Spurgeon H, et al. G(i)-dependent localization of beta(2)-adrenergic receptor signaling to L-type Ca(2+) channels. Biophys J. 2000;79(5):2547–56. doi: 10.1016/S0006-3495(00)76495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang J, Hupfeld CJ, Taylor SS, Olefsky JM, Tsien RY. Insulin disrupts beta-adrenergic signalling to protein kinase A in adipocytes. Nature. 2005;437(7058):569–73. doi: 10.1038/nature04140. [DOI] [PubMed] [Google Scholar]
- [10].Costanzo V, Avvedimento EV, Gottesman ME, Gautier J, Grieco D. Protein kinase A is required for chromosomal DNA replication. Curr Biol. 1999;9(16):903–6. doi: 10.1016/s0960-9822(99)80395-9. [DOI] [PubMed] [Google Scholar]
- [11].Matyakhina L, Lenherr SM, Stratakis CA. Protein kinase A and chromosomal stability. Ann N Y Acad Sci. 2002;968:148–57. doi: 10.1111/j.1749-6632.2002.tb04333.x. [DOI] [PubMed] [Google Scholar]
- [12].Rosenberg D, Groussin L, Jullian E, Perlemoine K, Bertagna X, Bertherat J. Role of the PKA-regulated transcription factor CREB in development and tumorigenesis of endocrine tissues. Ann N Y Acad Sci. 2002;968:65–74. doi: 10.1111/j.1749-6632.2002.tb04327.x. [DOI] [PubMed] [Google Scholar]
- [13].Siddappa R, Martens A, Doorn J, Leusink A, Olivo C, Licht R, et al. cAMP/PKA pathway activation in human mesenchymal stem cells in vitro results in robust bone formation in vivo. Proc Natl Acad Sci U S A. 2008;105(20):7281–6. doi: 10.1073/pnas.0711190105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cohen DM, Chen CS. Mechanical control of stem cell differentiation. In: StemBook, editor. The stem cell Research Community. StemBook; 2008. http://dx.doi.org/10.3824/stembook.1.26.1. http://www.stembook.org. [PubMed] [Google Scholar]
- [15].Depry C, Allen MD, Zhang J. Visualization of PKA activity in plasma membrane microdomains. Mol Biosyst. 2011;7(1):52–8. doi: 10.1039/c0mb00079e. [DOI] [PubMed] [Google Scholar]
- [16].Pelham RJ, Jr., Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94(25):13661–5. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60(1):24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- [18].Ouyang M, Sun J, Chien S, Wang Y. Determination of hierarchical relationship of Src and Rac at subcellular locations with FRET biosensors. Proc Natl Acad Sci U S A. 2008;105(38):14353–8. doi: 10.1073/pnas.0807537105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Allen JA, Yu JZ, Donati RJ, Rasenick MM. Beta-adrenergic receptor stimulation promotes G alpha s internalization through lipid rafts: a study in living cells. Mol Pharmacol. 2005;67(5):1493–504. doi: 10.1124/mol.104.008342. [DOI] [PubMed] [Google Scholar]
- [20].Tao J, Wang HY, Malbon CC. Protein kinase A regulates AKAP250 (gravin) scaffold binding to the beta2-adrenergic receptor. Embo J. 2003;22(24):6419–29. doi: 10.1093/emboj/cdg628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ouyang M, Huang H, Shaner NC, Remacle AG, Shiryaev SA, Strongin AY, et al. Simultaneous visualization of protumorigenic Src and MT1-MMP activities with fluorescence resonance energy transfer. Cancer Res. 2010;70(6):2204–12. doi: 10.1158/0008-5472.CAN-09-3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kim TJ, Seong J, Ouyang M, Sun J, Lu S, Hong JP, et al. Substrate rigidity regulates Ca2+ oscillation via RhoA pathway in stem cells. J Cell Physiol. 2009;218(2):285–93. doi: 10.1002/jcp.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Titushkin I, Cho M. Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. Biophys J. 2007;93(10):3693–702. doi: 10.1529/biophysj.107.107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhong W, Tian K, Zheng X, Li L, Zhang W, Wang S, et al. Mesenchymal stem cell and chondrocyte fates in a multishear microdevice are regulated by Yes-associated protein. Stem Cells Dev. 2013;22(14):2083–93. doi: 10.1089/scd.2012.0685. [DOI] [PubMed] [Google Scholar]
- [25].Sadoshima J, Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. Embo J. 1993;12(4):1681–92. doi: 10.1002/j.1460-2075.1993.tb05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hughes-Fulford M. Signal transduction and mechanical stress. Sci STKE. 2004;(249):RE12. doi: 10.1126/stke.2492004re12. 2004. [DOI] [PubMed] [Google Scholar]
- [27].McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483–95. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- [28].Elliott WC, Gorlin R. Isoproterenol in treatment of heart disease. Hemodynamic effects in circulatory failure. Jama. 1966;197(5):315–20. [PubMed] [Google Scholar]
- [29].Gupta PR, Jain S. Stepping down in asthma. Indian J Chest Dis Allied Sci. 2013;55(2):117–9. [PubMed] [Google Scholar]
- [30].Penela P, Ribas C, Mayor F., Jr. Mechanisms of regulation of the expression and function of G protein-coupled receptor kinases. Cell Signal. 2003;15(11):973–81. doi: 10.1016/s0898-6568(03)00099-8. [DOI] [PubMed] [Google Scholar]
- [31].Bhadriraju K, Hansen LK. Extracellular matrix- and cytoskeleton-dependent changes in cell shape and stiffness. Exp Cell Res. 2002;278(1):92–100. doi: 10.1006/excr.2002.5557. [DOI] [PubMed] [Google Scholar]
- [32].Sands WA, Palmer TM. Regulating gene transcription in response to cyclic AMP elevation. Cell Signal. 2008;20(3):460–6. doi: 10.1016/j.cellsig.2007.10.005. [DOI] [PubMed] [Google Scholar]
- [33].Kim JM, Choi JS, Kim YH, Jin SH, Lim S, Jang HJ, et al. An activator of the cAMP/PKA/CREB pathway promotes osteogenesis from human mesenchymal stem cells. J Cell Physiol. 2013;228(3):617–26. doi: 10.1002/jcp.24171. [DOI] [PubMed] [Google Scholar]
- [34].Kurabayashi M, Dutta S, Jeyaseelan R, Kedes L. Doxorubicin-induced Id2A gene transcription is targeted at an activating transcription factor/cyclic AMP response element motif through novel mechanisms involving protein kinases distinct from protein kinase C and protein kinase A. Mol Cell Biol. 1995;15(11):6386–97. doi: 10.1128/mcb.15.11.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ionescu AM, Drissi H, Schwarz EM, Kato M, Puzas JE, McCance DJ, et al. CREB Cooperates with BMP-stimulated Smad signaling to enhance transcription of the Smad6 promoter. J Cell Physiol. 2004;198(3):428–40. doi: 10.1002/jcp.10421. [DOI] [PubMed] [Google Scholar]
- [36].Meyer CJ, Alenghat FJ, Rim P, Fong JH, Fabry B, Ingber DE. Mechanical control of cyclic AMP signalling and gene transcription through integrins. Nat Cell Biol. 2000;2(9):666–8. doi: 10.1038/35023621. [DOI] [PubMed] [Google Scholar]
- [37].Alenghat FJ, Tytell JD, Thodeti CK, Derrien A, Ingber DE. Mechanical control of cAMP signaling through integrins is mediated by the heterotrimeric Galphas protein. J Cell Biochem. 2009;106(4):529–38. doi: 10.1002/jcb.22001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002;115(Pt 3):455–65. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- [39].Lee HJ, Wall B, Chen S. G-protein-coupled receptors and melanoma. Pigment Cell Melanoma Res. 2008;21(4):415–28. doi: 10.1111/j.1755-148X.2008.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Volovyk ZM, Wolf MJ, Prasad SV, Rockman HA. Agonist-stimulated beta-adrenergic receptor internalization requires dynamic cytoskeletal actin turnover. J Biol Chem. 2006;281(14):9773–80. doi: 10.1074/jbc.M511435200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.