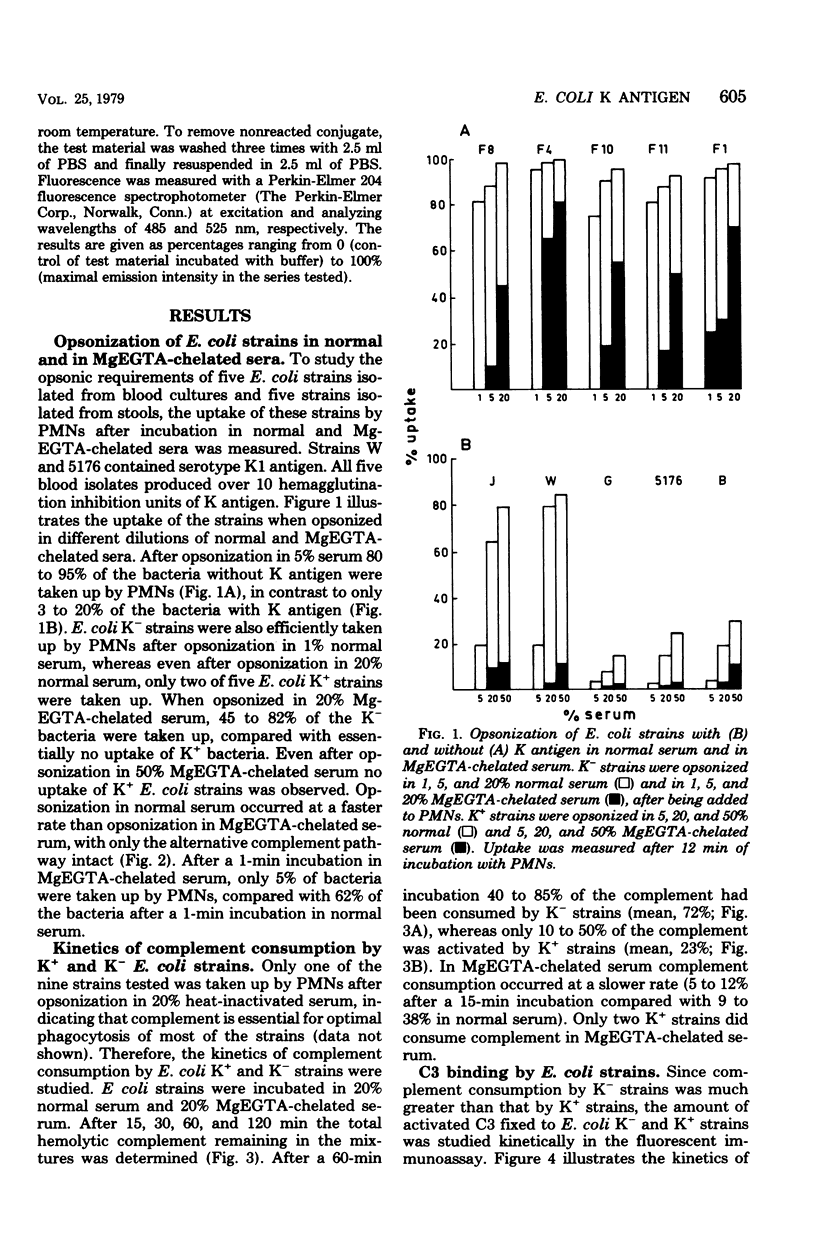
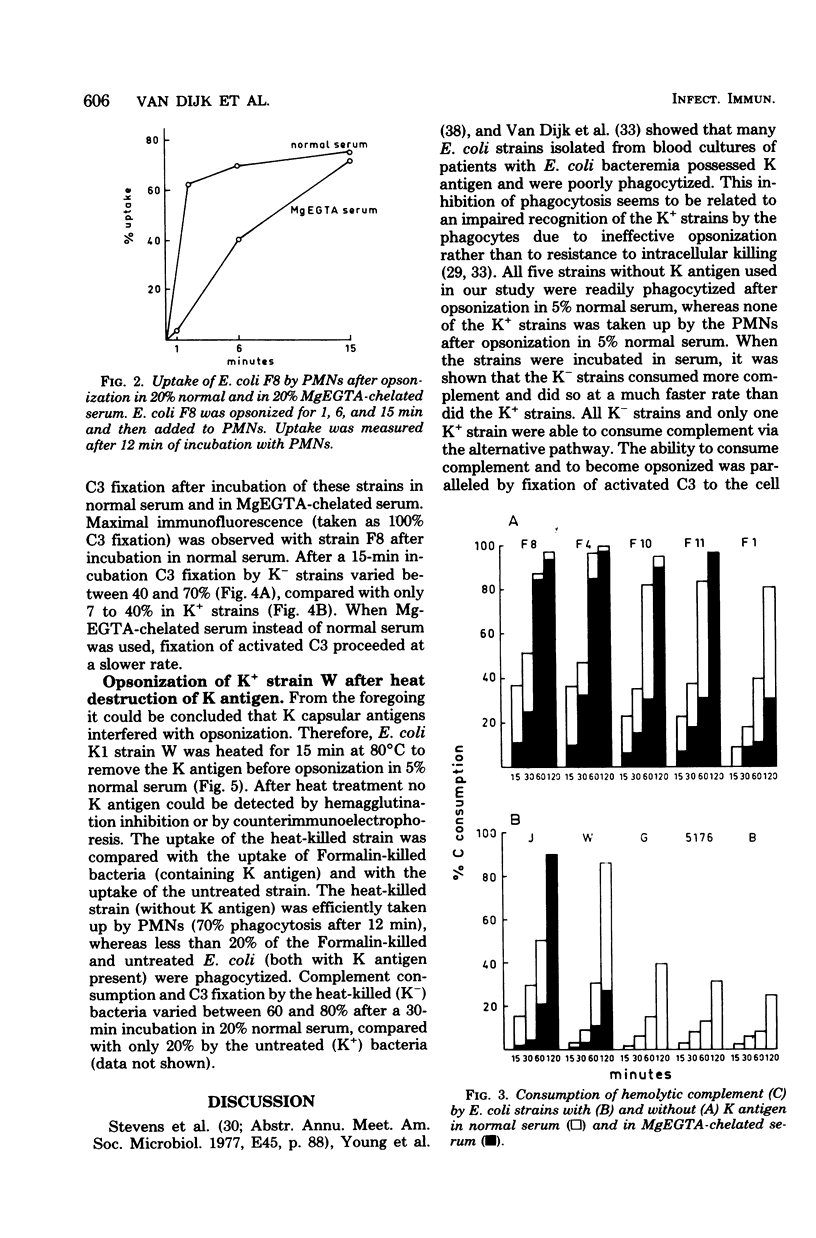
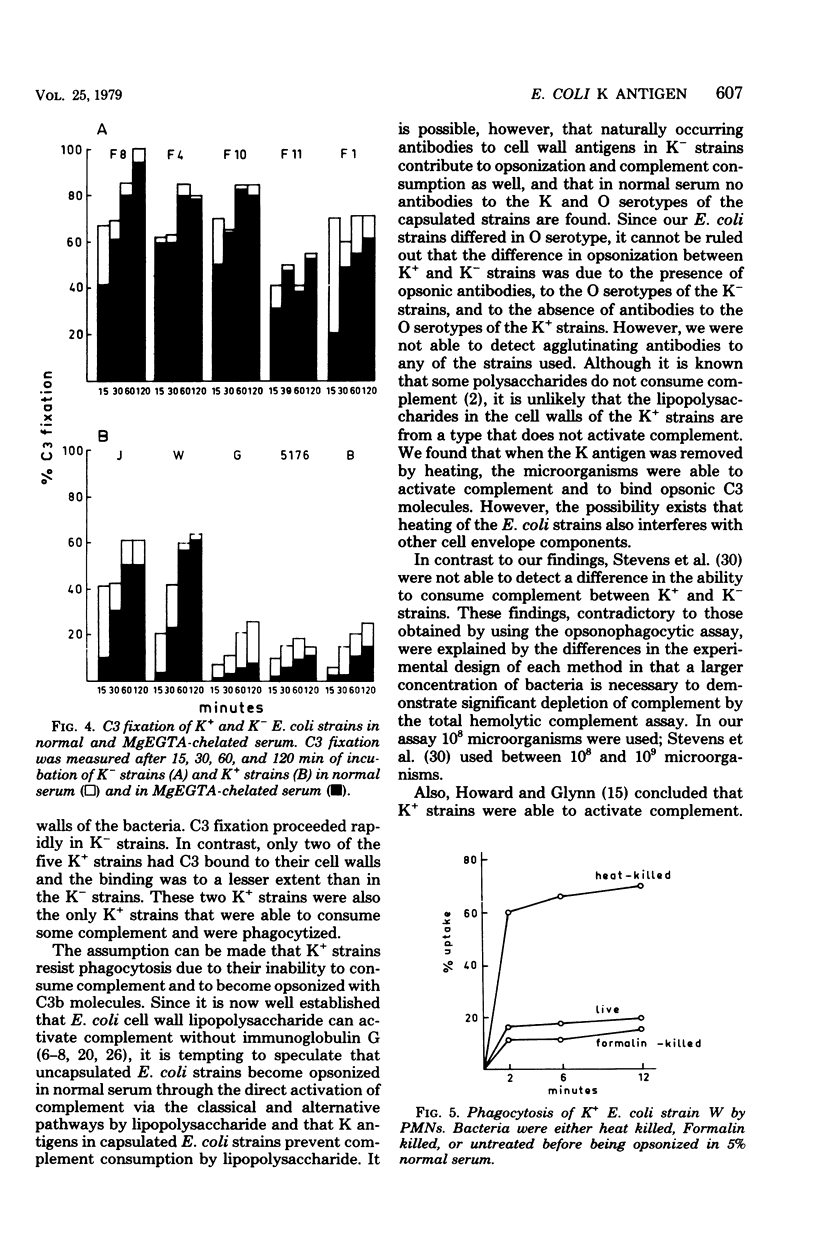
Abstract
Escherichia coli strains with K capsular polysaccharides are relatively resistant to phagocytosis by polymorphonuclear leukocytes, in contrast to E. coli strains without K antigens. This inhibition of phagocytosis is related to an impaired recognition of the K+ strains by the phagocytes due to ineffective opsonization. All five strains without K antigens were readily phagocytized after opsonization in 5% normal serum, compared with no uptake of the K+ strains. Evidence is presented that the decreased opsonization of the K+ strains in normal serum is caused by a low rate of complement activation of the strains, with subsequent absence of C3b fixation or C3d fixation or both to the cell wall of the bacteria. After removal of the K+ antigens by heating of a K+ E. coli strain, the strain was able to activate complement, to bind C3b or C3d or both, and to become opsonized. Complement was then activated via the classical and alternative pathways, which was comparable to the complement consumption by K- E. coli.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjornson A. B., Bjornson H. S. Activation of complement by opportunist pathogens and chemotypes of Salmonella minnesota. Infect Immun. 1977 Jun;16(3):748–753. doi: 10.1128/iai.16.3.748-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkstén B., Bortolussi R., Gothefors L., Quie P. G. Interaction of E. coli strains with human serum: lack of relationship to K1 antigen. J Pediatr. 1976 Dec;89(6):892–897. doi: 10.1016/s0022-3476(76)80592-6. [DOI] [PubMed] [Google Scholar]
- Forsgren A., Quie P. G. Influence of the alternate complement pathway in opsonization of several bacterial species. Infect Immun. 1974 Aug;10(2):402–404. doi: 10.1128/iai.10.2.402-404.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Rietschel E. T., Lüderitz O., Westphal O. Interaction of lipopolysaccharides and lipid A with complement. Eur J Biochem. 1971 Mar 1;19(1):143–152. doi: 10.1111/j.1432-1033.1971.tb01298.x. [DOI] [PubMed] [Google Scholar]
- Gewurz H., Shin H. S., Mergenhagen S. E. Interactions of the complement system with endotoxic lipopolysaccharide: consumption of each of the six terminal complement components. J Exp Med. 1968 Nov 1;128(5):1049–1057. doi: 10.1084/jem.128.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebink G. S., Verhoef J., Peterson P. K., Quie P. G. Opsonic requirements for phagocytosis of Streptococcus pneumoniae types VI, XVIII, XXIII, and XXV. Infect Immun. 1977 Nov;18(2):291–297. doi: 10.1128/iai.18.2.291-297.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis T. P., Thompson J. J. Quantitative fluorescent immunoassay of antibodies to, and surface antigens of, Actinomyces viscosus. J Clin Microbiol. 1978 Feb;7(2):202–208. doi: 10.1128/jcm.7.2.202-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn A. A., Brumfitt W., Howard C. J. K antigens of Escherichia coli and renal involvement in urinary-tract infections. Lancet. 1971 Mar 13;1(7698):514–516. [PubMed] [Google Scholar]
- Glynn A. A., Howard C. J. The sensitivity to complement of strains of Escherichia coli related to their K antigens. Immunology. 1970 Mar;18(3):331–346. [PMC free article] [PubMed] [Google Scholar]
- Guinée P. A., Agterberg C. M., Jansen W. H. Escherichia coli O antigen typing by means of a mechanized microtechnique. Appl Microbiol. 1972 Jul;24(1):127–131. doi: 10.1128/am.24.1.127-131.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinée P. A., Jansen W. H., Agterberg C. M. Detection of the K99 antigen by means of agglutination and immunoelectrophoresis in Escherichia coli isolates from calves and its correlation with entertoxigenicity. Infect Immun. 1976 May;13(5):1369–1377. doi: 10.1128/iai.13.5.1369-1377.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. J., Glynn A. A. The virulence for mice of strains of Escherichia coli related to the effects of K antigens on their resistance to phagocytosis and killing by complement. Immunology. 1971 May;20(5):767–777. [PMC free article] [PubMed] [Google Scholar]
- Jasin H. E. Human heat labile opsonins: evidence for their mediation via the alternate pathway of complement activation. J Immunol. 1972 Jul;109(1):26–31. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Klemperer M. R., Alper C. A., Rosen F. S. The enhancement of bacterial phagocytosis by serum. The role of complement components and two cofactors. J Exp Med. 1969 Jun 1;129(6):1275–1290. doi: 10.1084/jem.129.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaijser B., Ahlstedt S. Protective capacity of antibodies against Escherichia coli and K antigens. Infect Immun. 1977 Aug;17(2):286–289. doi: 10.1128/iai.17.2.286-289.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaijser B. Immunology of Escherichia coli: K antigen and its relation to urinary-tract infection. J Infect Dis. 1973 Jun;127(6):670–677. doi: 10.1093/infdis/127.6.670. [DOI] [PubMed] [Google Scholar]
- Loos M., Bitter-Suermann D., Dierich M. Interaction of the first (C1), the second (C2) and the fourth (C4) component of complement with different preparations of bacterial lipopolysaccharides and with lipid A. J Immunol. 1974 Mar;112(3):935–940. [PubMed] [Google Scholar]
- McCabe W. R., Carling P. C., Bruins S., Greely A. The relation of K-antigen to virulence of Escherichia coli. J Infect Dis. 1975 Jan;131(1):6–10. doi: 10.1093/infdis/131.1.6. [DOI] [PubMed] [Google Scholar]
- McCabe W. R. Gram-negative bacteremia. Adv Intern Med. 1974;19:135–158. [PubMed] [Google Scholar]
- McCracken G. H., Jr, Sarff L. D., Glode M. P., Mize S. G., Schiffer M. S., Robbins J. B., Gotschlich E. C., Orskov I., Orskov F. Relation between Escherichia coli K1 capsular polysaccharide antigen and clinical outcome in neonatal meningitis. Lancet. 1974 Aug 3;2(7875):246–250. doi: 10.1016/s0140-6736(74)91413-5. [DOI] [PubMed] [Google Scholar]
- Medearis D. N., Jr, Camitta B. M., Heath E. C. Cell wall composition and virulence in Escherichia coli. J Exp Med. 1968 Sep 1;128(3):399–414. doi: 10.1084/jem.128.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977 Jan;118(1):362–368. [PubMed] [Google Scholar]
- Pitt J. Virulence of Escherichia coli K1 in adults. J Infect Dis. 1979 Jan;139(1):106–108. doi: 10.1093/infdis/139.1.106. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., McCracken G. H., Jr, Gotschlich E. C., Orskov F., Orskov I., Hanson L. A. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974 May 30;290(22):1216–1220. doi: 10.1056/NEJM197405302902202. [DOI] [PubMed] [Google Scholar]
- Rottini G., Dri P., Soranzo M. R., Patriarca P. Correlation between phagocytic activity and metabolic response of polymorphonuclear leukocytes toward different strains of Escherichia coli. Infect Immun. 1975 Mar;11(3):417–423. doi: 10.1128/iai.11.3.417-423.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens P., Huang S. N., Welch W. D., Young L. S. Restricted complement activation by Escherichia coli with the K-1 capsular serotype: a possible role in pathogenicity. J Immunol. 1978 Dec;121(6):2174–2180. [PubMed] [Google Scholar]
- Stossel T. P. Phagocytosis (first of three parts). N Engl J Med. 1974 Mar 28;290(13):717–723. doi: 10.1056/NEJM197403282901306. [DOI] [PubMed] [Google Scholar]
- Van Dijk W. C., Verbrugh H. A., Peters R., Van Der Tol M. E., Peterson P. K., Verhoef J. Escherichia coli K antigen in relation to serum-induced lysis and phagocytosis. J Med Microbiol. 1979 Feb;12(1):123–130. doi: 10.1099/00222615-12-1-123. [DOI] [PubMed] [Google Scholar]
- Verbrugh H. A., Peters R., Peterson P. K., Verhoef J. Phagocytosis and killing of staphylococci by human polymorphonuclear and mononuclear leucocytes. J Clin Pathol. 1978 Jun;31(6):539–545. doi: 10.1136/jcp.31.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Kinetics of staphylococcal opsonization, attachment, ingestion and killing by human polymorphonuclear leukocytes: a quantitative assay using [3H]thymidine labeled bacteria. J Immunol Methods. 1977;14(3-4):303–311. doi: 10.1016/0022-1759(77)90141-7. [DOI] [PubMed] [Google Scholar]
- Verhoef J., Peterson P., Kim Y., Sabath L. D., Quie P. G. Opsonic requirements for staphylococcal phagocytosis. Heterogeneity among strains. Immunology. 1977 Aug;33(2):191–197. [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Jr, Quie P. G. Opsonic activity of agammaglobulinemic human sera. J Immunol. 1971 Jan;106(1):51–55. [PubMed] [Google Scholar]
- Young L. S., Stevens P., Ingram J. Functional role of antibody against "core" glycolipid of Enterobacteriaceae. J Clin Invest. 1975 Oct;56(4):850–861. doi: 10.1172/JCI108164. [DOI] [PMC free article] [PubMed] [Google Scholar]



