Abstract
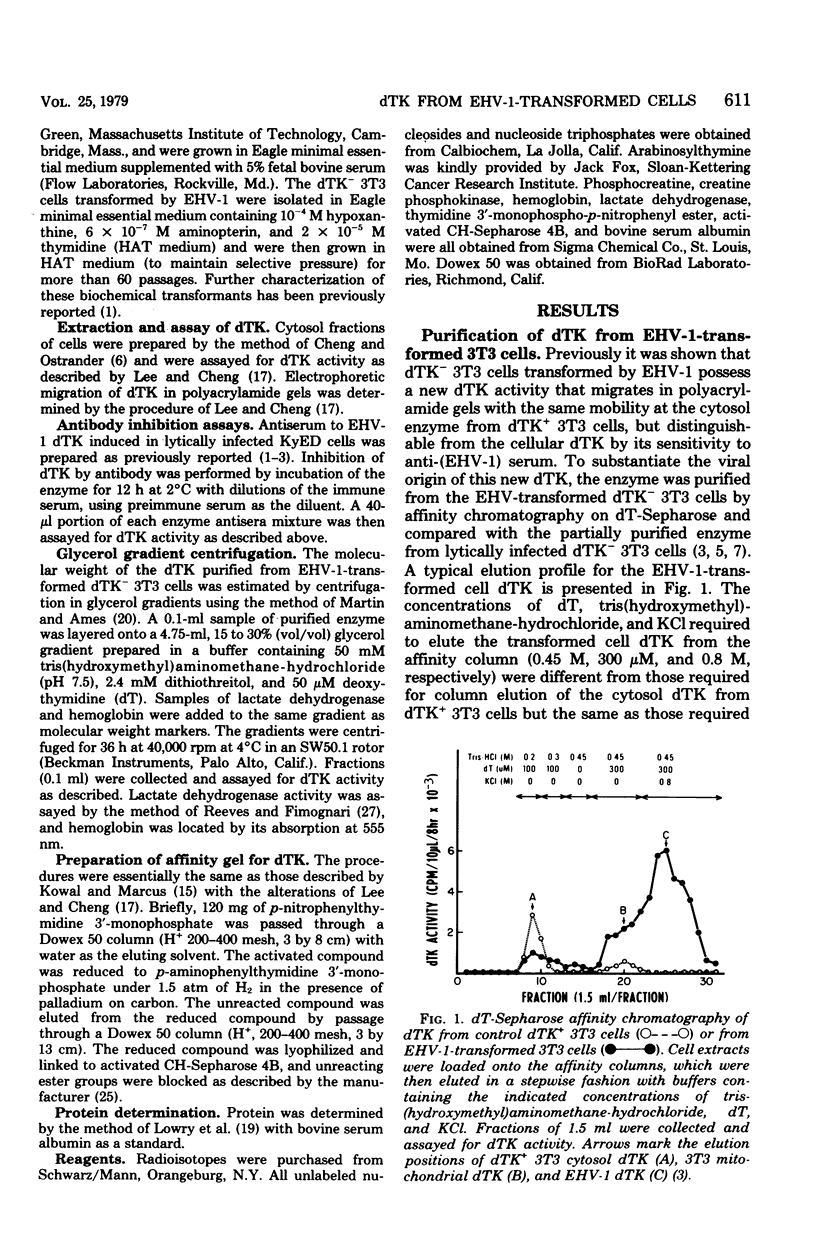
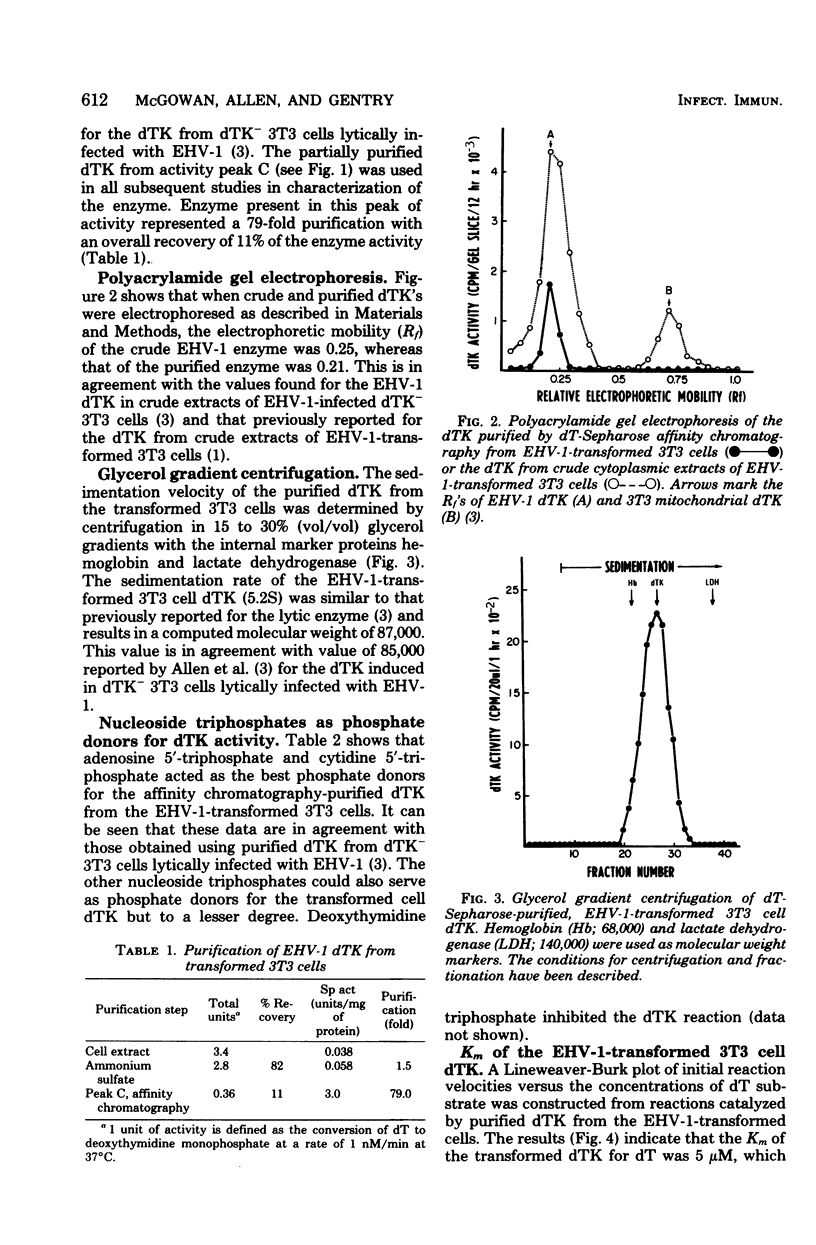
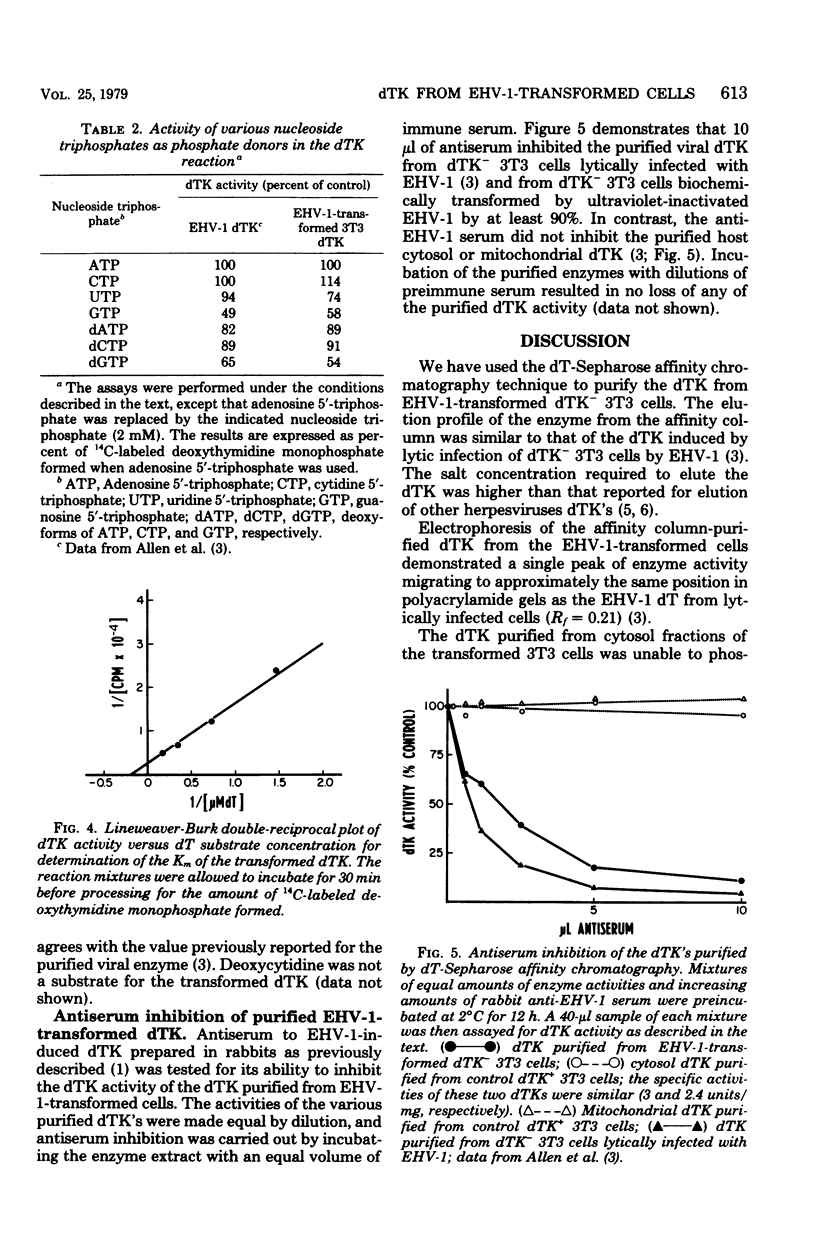
A line of mouse 3T3 cells lacking deoxythymidine kinase (dTK-) was stably transformed to see dTK+ phenotype after exposure to ultraviolet-irradiated equine herpesvirus type 1 (EHV-1). Deoxythymidine kinase (dTK) was purified from the biochemically transformed mouse cells by affinity chromatography on deoxythymidine-Sepharose. The purified dTK from EHV-1-transformed 3T3 cells was identical to the dTK purified from dTK- 3T3 cells lytically infected with EHV-1 with respect to its electrophoretic mobility, molecular weight, substrate specificity, phosphate donor specificity, and immunological specificity. The sedimentation velocity of the purified dTK from the transformed 3T3 cells was similar to that previously reported for the enzyme in lytically infected dTK- 3T3 cells, and its molecular weight was estimated to be 87,000. Antiserum prepared against the EHV-1 dTK induced in horse cells inactivated the dTK purified from the transformed mouse cells. The Km for deoxythymidine (5 micrometers) of purified dTK from the EHV-1-transformed cells was the same as that reported for the EHV-1-induced dTK. These results further support the notion that the dTK acquired by dTK- mouse 3T3 cells after transformation by EHV-1 is of viral and not of cellular origin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. P., McGowan J. J., Bryans J. T., Randall C. C., Gentry G. A. Induction of deoxythymidine kinase activity in several mammalian cell lines after infection with six different strains of equine herpesvirus type 1 (EHV-1). Virology. 1978 Oct 15;90(2):351–359. doi: 10.1016/0042-6822(78)90319-7. [DOI] [PubMed] [Google Scholar]
- Allen G. P., McGowan J. J., Gentry G. A., Randall C. C. Biochemical transformation of deoxythymidine kinase-deficient mouse cells with UV-irradiated equine herpesvirus type 1. J Virol. 1978 Oct;28(1):361–367. doi: 10.1128/jvi.28.1.361-367.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G. P., McGowan J. J., Randall C. C., Mancini W., Cheng Y. C., Gentry G. A. Purification and characterization of deoxythymidine kinase (dTK) induced in dTK- 3T3 mouse cells by equine herpesvirus type 1 (EHV-1). Virology. 1979 Jan 30;92(2):367–374. doi: 10.1016/0042-6822(79)90141-7. [DOI] [PubMed] [Google Scholar]
- Chadha K. C., Munyon W. Presence of herpes simplex virus-related antigens in transformed L cells. J Virol. 1975 Jun;15(6):1475–1486. doi: 10.1128/jvi.15.6.1475-1486.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Chadha K. C., Hughes R. G., Jr Biochemical and immunological characterization of deoxythymidine kinase of thymidine kinaseless HeLa cells biochemically transformed by herpes simplex virus type. Infect Immun. 1977 May;16(2):486–492. doi: 10.1128/iai.16.2.486-492.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Ostrander M. Deoxythymidine kinase induced in HeLa TK- cells by herpes simplex virus type I and type II. II. Purification and characterization. J Biol Chem. 1976 May 10;251(9):2605–2610. [PubMed] [Google Scholar]
- Davidson R. L., Adelstein S. J., Oxman M. N. Herpes simplex virus as a source of thymidine kinase for thymidine kinase-deficient mouse cells: suppression and reactivation of the viral enzyme. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1912–1916. doi: 10.1073/pnas.70.7.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. B., Kingsbury D. T. Quantitation of the viral DNA present in cells transformed by UV-irradiated herpes simplex virus. J Virol. 1976 Mar;17(3):788–793. doi: 10.1128/jvi.17.3.788-793.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. B., Munyon W., Buchsbaum R., Chawda R. Virus type-specific thymidine kinase in cells biochemically transformed by herpes simplex virus types 1 and 2. J Virol. 1974 Jan;13(1):140–145. doi: 10.1128/jvi.13.1.140-145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobersen M. J., Jerkofsky M., Greer S. Enzymatic basis for the selective inhibition of varicella-zoster virus by 5-halogenated analogues of deoxycytidine. J Virol. 1976 Nov;20(2):478–486. doi: 10.1128/jvi.20.2.478-486.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner L., Dubbs D. R., Kit S. Chromosomal site(s) of integration of Herpes simplex virus type 2 thymidine kinase gene in biochemically transformed human cells. Int J Cancer. 1977 Aug 15;20(2):256–267. doi: 10.1002/ijc.2910200214. [DOI] [PubMed] [Google Scholar]
- Hackstadt T., Mallavia L. P. Deoxypyrimidine nucleoside metabolism in varicella-zoster virus-infected cells. J Virol. 1978 Feb;25(2):510–517. doi: 10.1128/jvi.25.2.510-517.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson A. T., Macnab J. C., Perbal B., Clements J. B. Virus specified enzyme activity and RNA species in herpes simplex virus type 1 transformed mouse cells. J Gen Virol. 1976 Sep;32(3):493–508. doi: 10.1099/0022-1317-32-3-493. [DOI] [PubMed] [Google Scholar]
- Kit S., Leung W. C., Jorgensen G. N., Trkula D., Dubbs D. R. Viral-induced thymidine kinase isozymes. Prog Med Virol. 1975;21:13–34. [PubMed] [Google Scholar]
- Kowal E. P., Markus G. Affinity chromatography of thymidine kinase from a rat colon adenocarcinoma. Prep Biochem. 1976;6(5):369–385. doi: 10.1080/00327487608061625. [DOI] [PubMed] [Google Scholar]
- Kraiselburd E., Gage L. P., Weissbach A. Presence of a herpes simplex virus DNA fragment in an L cell clone obtained after infection with irradiated herpes simplex virus I. J Mol Biol. 1975 Oct 5;97(4):533–542. doi: 10.1016/s0022-2836(75)80057-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee L. S., Cheng Y. C. Human deoxythymidine kinase. I. Purification and general properties of the cytoplasmic and mitochondrial isozymes derived from blast cells of acute myelocytic leukemia. J Biol Chem. 1976 May 10;251(9):2600–2604. [PubMed] [Google Scholar]
- Leung W. C., Dubbs D. R., Trkula D., Kit S. Mitochondrial and herpesvirus-specific deoxypyrimidine kinases. J Virol. 1975 Sep;16(3):486–497. doi: 10.1128/jvi.16.3.486-497.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Matsuya Y., Green H. Somatic cell hybrid between the established human line D98 (presumptive HeLa) and 3T3. Science. 1969 Feb 14;163(3868):697–698. doi: 10.1126/science.163.3868.697. [DOI] [PubMed] [Google Scholar]
- Munyon W., Buchsbaum R., Paoletti E., Mann J., Kraiselburd E., Davis D. Electrophoresis of thymidine kinase activity synthesized by cells transformed by herpes simplex virus. Virology. 1972 Sep;49(3):683–689. doi: 10.1016/0042-6822(72)90525-9. [DOI] [PubMed] [Google Scholar]
- Munyon W., Kraiselburd E., Davis D., Mann J. Transfer of thymidine kinase to thymidine kinaseless L cells by infection with ultraviolet-irradiated herpes simplex virus. J Virol. 1971 Jun;7(6):813–820. doi: 10.1128/jvi.7.6.813-820.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicer A., Wigler M., Axel R., Silverstein S. The transfer and stable integration of the HSV thymidine kinase gene into mouse cells. Cell. 1978 May;14(1):133–141. doi: 10.1016/0092-8674(78)90308-2. [DOI] [PubMed] [Google Scholar]
- Rapp F., Buss E. R. Comparison of herpes simplex virus isolates using a quantitative selection assay for transformation. Intervirology. 1975;6(2):72–82. doi: 10.1159/000149458. [DOI] [PubMed] [Google Scholar]
- Thouless M. E., Chadha K. C., Munyon W. H. Serological specificity of thymidine kinase activity in herpes simplex virus-transformed L cells. Virology. 1976 Jan;69(1):350–351. doi: 10.1016/0042-6822(76)90225-7. [DOI] [PubMed] [Google Scholar]


