Abstract
Cyanobacteria require large quantities of iron to maintain their photosynthetic machinery; however, in most environments iron is present in the form of insoluble iron oxides. Whether cyanobacteria can utilize these sources of iron, and the potential molecular mechanisms involved remains to be defined. There is increasing evidence that pili can facilitate electron donation to extracellular electron acceptors, like iron oxides in non-photosynthetic bacteria. In these organisms, the donation of electrons to iron oxides is thought to be crucial for maintaining respiration in the absence of oxygen. Our study investigates if PilA1 (major pilin protein) may also provide a mechanism to convert insoluble ferric iron into soluble ferrous iron. Growth experiments supported by spectroscopic data of a strain deficient in pilA1 indicate that the presence of the pilA1 gene enhances the ability to grow on iron oxides. These observations suggest a novel function of PilA1 in cyanobacterial iron acquisition.
Introduction
In the oxidative environment of Earth, organisms must contend with the problem of accessing essential elements, which are locked into insoluble oxides. In particular, the bioavailability of iron is limited due to the tendency of Fe3+ to form insoluble minerals (i.e. goethite, hematite) [1].
Iron acquisition in bacteria
Given the limited bioavailability of iron, several acquisition strategies have evolved in bacteria. Siderophore-mediated iron uptake is one such strategy, involving the synthesis and secretion of low-molecular-weight iron chelators that tightly bind Fe3+. This results in a ferrisiderophore complex that is transported as a whole into the cell where the iron can then be removed for cellular utilization. This form of siderophore-mediated iron uptake has been studied extensively in Gram-negative bacteria such as Escherichia coli and Pseudomonas aeruginosa [2], [3], [4], [5]. Citrate also possesses Fe3+ chelating characteristics and many bacteria possess a citrate-mediated mechanism for iron uptake. The uptake of heme through hemophores (proteins that are synthesized and excreted) is another iron-acquisition strategy that has only been found in Gram-negative bacteria [6], [7].
Recently, the Type IV pili of non-photosynthetic soil bacteria have been shown to provide another strategy for iron acquisition. Observations using Scanning Tunneling Microscopy (STM) techniques demonstrated that these thick pili structures are electrically conductive, coining the name ‘bacterial nanowires’ [8], [9]. These pili are composed of pilins that form extracellular protein fibers and facilitate electron donation to external electron acceptors like metal oxides, thus facilitating respiration in anaerobic conditions [8], [9]. In addition to sustaining respiration, another - so far untested - consequence of reducing metal oxides is that this may also confer the ability to unlock iron that is essential for bacterial growth.
Iron utilization in cyanobacteria
Cyanobacteria employ a variety of strategies to access iron from the environment. Although siderophore production has been observed in several cyanobacteria species [10], detailed analysis has shown that genes for siderophores are not present in many cyanobacteria species, including Synechocystis sp. PCC 6803 [11]. Despite this, it has been suggested that Synechocystis sp. PCC 6803 can obtain iron bound to exogenous siderophores [12], [13] that are produced by other organisms in proximity. Citrate has the ability to chelate ferric iron, and ferric dicitrate uptake systems are present in the genomes of many cyanobacteria, including Synechocystis sp. PCC 6803. Alternatively, an iron uptake pathway for cyanobacteria involving a reductive step has been evaluated in recent studies, suggesting extracellular or periplasmic reduction occurs followed by transport of the iron through the plasma membrane into the cell [14].
Iron utilization in Synechocystis sp. PCC 6803
Several mechanisms for iron uptake are well-understood in Synechocystis sp. PCC 6803. In the plasma membrane of Synechocystis sp. PCC 6803 there are two transporters FutABC and FeoB, suggested to transport free Fe3+ and Fe2+, respectively [12], [13], [15]. A ferric dicitrate uptake system is coded by the genes slr1318–1319 and slr1491–1492 in Synechocystis sp. PCC 6803. Ferric ammonium citrate has been used as an iron source in cyanobacterial growth media, including BG-11 for Synechocystis sp. PCC 6803 [16].
Kranzler et al. [14] have presented data suggesting that Synechocystis sp. PCC 6803 is capable of acquiring iron through reduction of Fe3+ substrates before transport through the plasma membrane. Although the location of iron reduction is yet to be defined, it is proposed to be outside of the cell, on the surface of the outer membrane or in the periplasmic space [14]. This reductive two-step model for iron uptake allows for the utilization of a variety of inorganic and organic iron sources, thereby eliminating the presence for specific ferrisiderophore transporters. Following reduction, iron may be transported as Fe2+ or, in some cases, reoxidized to Fe3+ and then transported [17], [18], [19] across the cytoplasmic membrane. This ‘reductive activation’ may provide a mechanism for accessing the great variety of ferric iron conjugates and iron chelators cyanobacteria may encounter in their natural environment.
Iron-stress-induced chlorophyll-binding protein regulation
Iron limitation induces a remarkable remodeling of the photosynthetic machinery in cyanobacteria. Under iron stress IsiA (an iron-starvation-induced chlorophyll-binding protein), is expressed. IsiA can form ring structures around Photosystem I (PSI) [20], [21] and binds up to 50% of cellular chlorophyll [22]. Accumulation of IsiA leads to spectral changes that can be readily observed at room temperature and 77 K. The transcription of isiA in Synechocystis sp. PCC 6803 is regulated by transcriptional activation of the corresponding bi-cistronic isiAB operon [23] that is controlled by the ferric uptake regulator protein (Fur) [24]. Another regulation of the isiAB-operon involves an internal asRNA, isiR [25], [26]. A strain, in which the isiA gene has been inactivated, shows a transcriptional up-regulation of several pilin genes [27]. This observation indicates that the expression of pilins interfaces with a regulatory network that controls the expression of IsiA in response to stress conditions, including iron depletion.
Pilins in Synechocystis sp. PCC 6803
In Synechocystis sp. PCC 6803, functional pili are essential for twitching motility and DNA uptake [28], [29], [30], [31]. The genome of Synechocystis sp. PCC 6803 contains a large number of genes that show clear pilin characteristics. Four pilin genes are encoded individually (slr0079, slr1120, slr1456 and sll1359), but there are also three operons in which pilins are arranged in consecutive order. One operon consists of the genes sll1693–1696, where the protein encoded by sll1694 (the pilA1 gene) is the main constituent of the extracellular pili [28], [29], [30], [31]. Deletion of this pilin results in an inability to take up external DNA, impairment of mobility, and the absence of pili as shown by negatively stained electron micrographs [31]. The protein encoded by sll1695 (the pilA2 gene) is another pilin that has been suggested to be localized on the cytoplasmic surface of the cytoplasmic membrane [29], [30]. The function of proteins encoded by sll1693 and sll1696 are not known. The two additional operons containing pilin genes are slr1928–1931 and slr2015–2017. Unlike the pilins encoded by the operon slr1928–1931 [31], the pilins encoded within and the other operon (slr2015 and slr2016) are crucial for locomotion [29].
Aim of this study
There are several lines of evidence suggesting a role of pili in mediating the reduction of iron oxides in bacteria [8], [9], and reduction of iron has been implicated in making iron available for cyanobacteria [14]. As iron is an essential element and induces a strong phenotypic response in cyanobacteria, we decided to assess iron bioavailability as a function of the presence of PilA1 in Synechocystis sp. PCC 6803.
Results
Δsll1694 strain construction
The PilA pilin encoded by sll1694 is the main component of Type IV pili [29], [31]. A mutant of Synechocystis sp. PCC 6803 was generated in which sll1694 was replaced by a kanamycin-resistance cassette (Fig. S1 in File S1). Mutant segregation, i.e. the replacement of sll1694 in all copies of chromosomes in a Synechocystis sp. PCC 6803 strain, was achieved by plating out Synechocystis sp. PCC 6803 cells that were transformed with the Δsll1694 deletion plasmid on BG-11 media, supplemented with the antibiotic kanamycin. Complete segregation was confirmed by colony PCR (Fig. S2 in File S1).
Characterization of pilA1 deletion strain
Liquid cultures of wild type, and the Δsll1694 strain were grown in liquid medium in order to assess the presence of PilA1 in these two strains. Once cultures reached an OD of 0.8, they were harvested and PilA were sheared off the cells by vortexing [32]. The concentrated supernatant was analyzed by SDS-PAGE (Fig. S3 in File S1). The main band visible in wild type (located at ∼20 kDa), was excised and characterized by mass spectrometry. Analysis of the sample protein resulted in the sequence of a 13 amino acid fragment (SMSGGTFYDSGTR). This sequence matched to the sll1694 gene product (PilA1), confirming that the gene product of sll1694 is the main constituent of the cell supernatant [28], [29], [30], [31] and the Δsll1694 strain had been inactivated.
Growth analysis
The growth characteristics of wild type and the Δsll1694 strain in liquid BG-11 medium (without added glucose or atrazine) on different iron sources were characterized. Cells grew fastest on ferric ammonium citrate, while slower growth was observed on ferric oxide, and the slowest growth occurred on goethite (Fig. 1). In all three media, the growth rate of the wild type exceeded the growth rate of the Δsll1694 deletion strain.
Figure 1. Liquid growth characteristics.
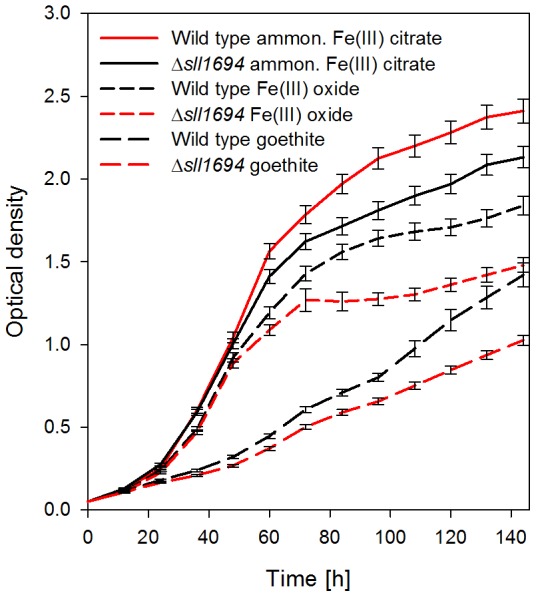
Photoautotrophic growth characteristics of wild type and the Δsll1694 strain in liquid BG-11 with ammonium iron(III) citrate, iron(III) oxide, or goethite as the exclusive iron source. Trend shown is the average of three separate experiments. Error bars indicate the standard error of the three experiments.
As vortexing is a very efficient method for removing pili from cells (as practiced for pili isolation), we suspected that pili may also be constantly sheared off in agitated liquid culture. To prevent the potential for shearing off of pilins and to allow for a persistent contact with iron oxide particles, we assessed growth of wild type and the Δsll1694 strain on agar plates.
For wild-type cells grown on plates, ferric ammonium citrate, ferric oxide, and goethite showed the same propensity for supporting growth as observed in liquid medium; however, on plates, the differences in growth between wild type and the Δsll1694 strain were more exaggerated than in liquid culture (Fig. 2). While ferric citrate supported growth of Δsll1694 cells, very slow growth was observed on ferric oxide and goethite.
Figure 2. Agar-plate growth characteristics.
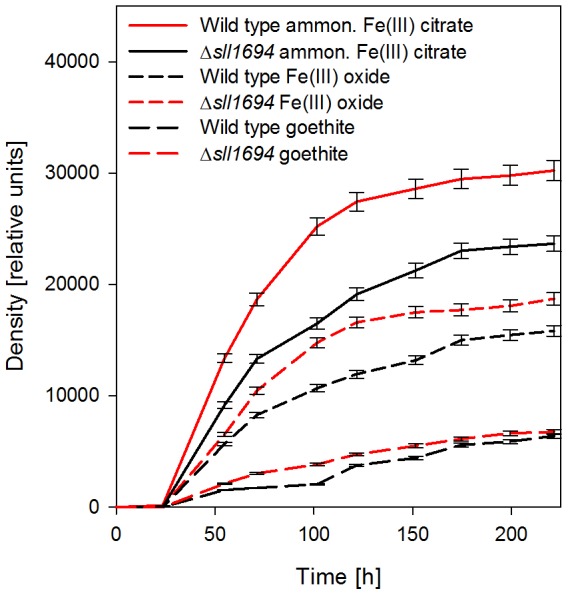
Photoautotrophic growth characteristics of wild type and the Δsll1694 strain on BG-11-contatining agar plates with ammonium iron(III) citrate, iron(III) oxide, or goethite as the exclusive iron source. Trend shown is the average of three separate experiments. Error bars showing the standard error of the three experiments.
Agar-grown Δsll1694 cells exhibited a dramatic change in coloration on all iron sources, including ferric ammonium citrate (Fig. 3), while no obvious difference in coloration between wild type and the Δsll1694 strain were observed in liquid culture. The speckled phenotype of the Δsll1694 strain grown on solid media may be indicative of the statistical nature of having access to iron oxide particles.
Figure 3. Agar-plate growth phenotype.
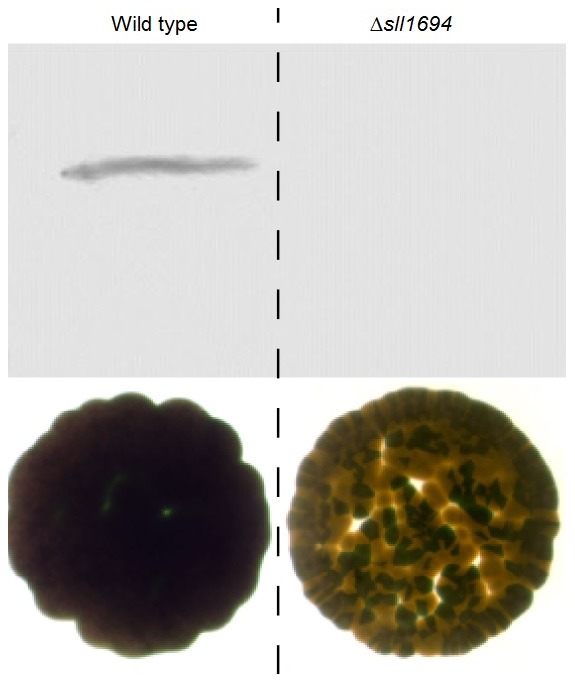
Images of wild type (left) and Δsll1694 strain (right) on petri dishes containing agar-solidified BG-11 medium with ammonium iron(III) citrate substituted by goethite. The extracellular protein harvested from wild type and Δsll1694 strains show the PilA protein (encoded by sll1694) in the wild type, but not the Δsll1694.
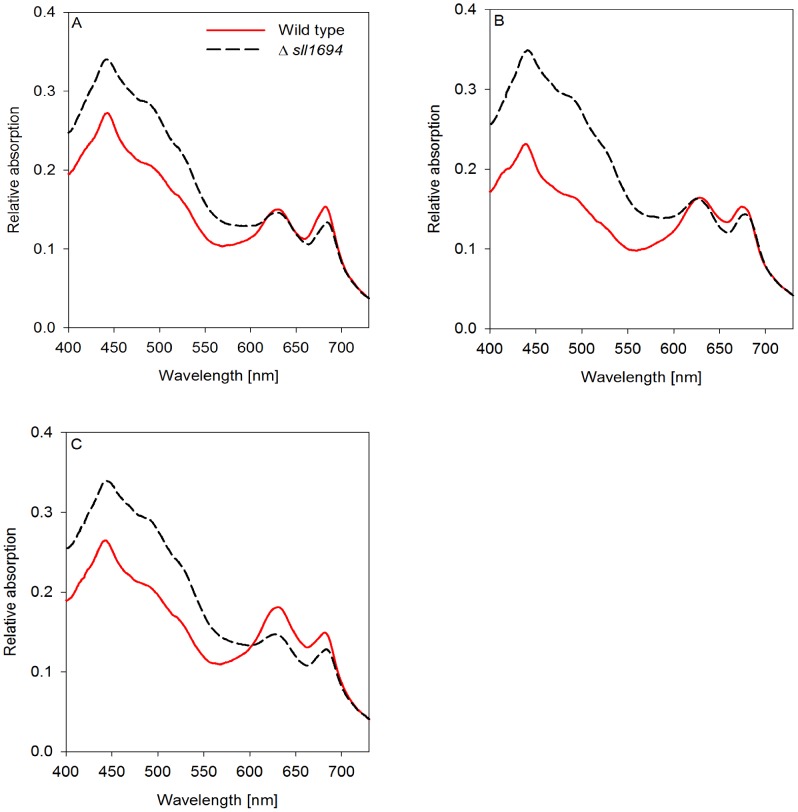
Whole cell absorption spectra
Absorption spectra of whole cells can provide information on the organization of the photosynthetic machinery of cyanobacteria. In Synechocystis sp. PCC 6803, absorption features can be readily assigned to chlorophyll a, carotenoids and phycobilins. In non-stress conditions, chlorophyll a is mainly associated with PSI and photosystem II (PSII) and exhibits absorption maxima around 435 nm (Soret band) and 680 nm. The main light-harvesting system of PSII is made up of phycobilisomes, which contain phycocyanin (absorption maxima at 570 nm and 620 nm) and to a lesser extent allophycocyanin (maxima at 650 nm), resulting in a composite absorption peak around 625 nm. It is possible to assess the relative chlorophyll / phycobilin ratio by evaluating the ratio of the 625 nm to 680 nm absorption peaks.
Synechocystis sp. PCC 6803 cells possess four main carotenoids [33] (β-carotene, zeaxanthin, myxoxanthophyll and echineone) that absorb light efficiently between 400 and 500 nm. The light absorption of the carotenoids therefore overlaps with the light absorption of chlorophyll a. An approximation of the amount of carotenoids within a cell can be obtained by assessing the peak ratio of chlorophylls at 435 nm and 680 nm. When Abs 435 nm / Abs 680 nm is small then a relatively small number of carotenoids are present. To unify replicated absorption spectra the statistical measurement of the standard error between the replicates was measured.
Carotenoids
The absorption ratio at 435 nm and 680 nm was used to assess the presence of carotenoids in relation to chlorophyll a in wild type and the Δsll1694 cells grown on agar plates and in liquid, which were supplied with different iron sources. Wild type and the Δsll1694 strain have a similar carotenoid to chlorophyll ratio when grown in liquid with ferric ammonium citrate as the iron source (Fig. 4A). An increase in the carotenoid to chlorophyll ratio can be deduced in the Δsll1694 mutant compared to the wild type when either iron oxide or goethite was the iron source in liquid grown cultures (Fig. 4B, C).
Figure 4. Absorption spectra of a photoautotrophically grown liquid culture.
Both wild type and Δsll1694 strains grown in BG-11 with ammonium iron(III) citrate (A), iron(III) oxide (B), or goethite (C) as the exclusive iron source. Samples were standardized to an OD800 of 0.3. Traces were baseline subtracted at 800 nm after acquisition. One data set is shown, which is representative of three separate experiments. The average standard error between triplicates was calculated. Wild type SE: ±3.0×10-3 (A), ±1.5×10−3 (B), ±2.9×10−3 (C); Δsll1694 SE: ±3.3×10−3 (A), ±3.9×10−3 (B), ±3.7×10−3 (C). (For non-baseline subtracted absorption spectra see Figure S4 in File S1).
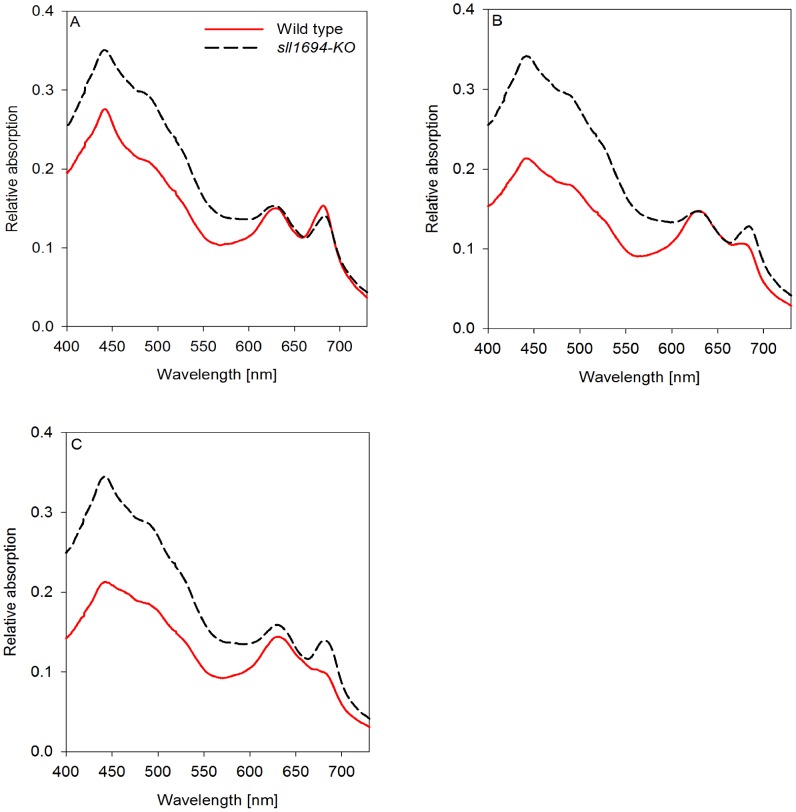
When grown on agar in the presence of ferric ammonium citrate, a similar carotenoid to chlorophyll ratio was present in the Δsll1694 strain and wild type (Fig 5A). In plate-grown cultures, the chlorophyll to carotenoid ratio was increased more in wild type than in the Δsll1694 strain grown on iron oxide and goethite (Fig 5B, C).
Figure 5. Absorption spectra of photoautotrophically grown plate cultures.
Both wild type and Δsll1694 cells grown on agar-solidified BG-11 with ammonium iron(III) citrate (A), iron(III) oxide (B), or goethite (C) as the exclusive iron source. Samples were standardized to an OD800 of 0.3. Traces were baseline subtracted at 800 nm after acquisition. One data set is shown, which is representative of three separate experiments. The average standard error between triplicates was calculated. Wild type SE: ±1.8×10−3 (A), ±1.3×10−3 (B), ±3.9×10−3 (C); Δsll1694 SE: ±4.0×10−3 (A), ±4.3×10−3 (B), ±3.0×10−3 (C). For non-baseline subtracted absorption spectra for plate grown cultures see Figure S5 in File S1).
Chlorophyll
The status of the photosynthetic machinery can be deduced from the amount of light-harvesting pigments that are present. In non-stress conditions, a relative high phycobilisome to chlorophyll ratio indicates active electron generation by PSII, while in stress conditions the number of phycobilisomes to chlorophyll is often reduced. The absorption at 625 nm and 680 nm can be used to assess changes in the phycobilisome to chlorophyll ratios in wild type and the Δsll1694 strain.
In liquid media, the Δsll1694 strain and wild type have similar chlorophyll to phycobilin ratios under two of the iron sources (ferric ammonium citrate, iron oxide) (Fig 4). When grown in liquid with goethite (Fig 4C), a small decrease in the chlorophyll to phycobilin ratio was observed in both wild type and Δsll1694 cells compared to cells grown on ferric ammonium citrate and ferric oxide.
On solid medium the Δsll1694 strain and wild type only have a similar chlorophyll to phycobilin ratio when grown on ferric ammonium citrate (Fig 5A). The wild type showed a substantial decrease in the chlorophyll to phycobilin ratio when grown on goethite and ferric oxide (Fig 5B & C).
IsiA
In iron-limited conditions a shift in the red chlorophyll absorption peak to shorter wavelengths has previously been observed [34]. This shift has been attributed to the presence of IsiA [34], a chlorophyll-binding protein that is related to CP43. Unlike CP43, which is associated with PSII, IsiA has been shown to be primarily associated with PSI.
In liquid-grown wild-type cells and the Δsll1694 strain, a shift of the red chlorophyll peak to shorter wavelengths was observed when ferric ammonium citrate was replaced by ferric oxide (1–2 nm) and goethite (∼5–7 nm). The same trends were observed when wild type and the Δsll1694 strain were grown on plates with different iron sources (see Table 1).
Table 1. Absorption maxima of the red chlorophyll peak in wild type and Δsll1694 strain grown with different iron sources in liquid medium and on agar plates.
| wild type | Δsll1694 | |||
| liquid | plate | liquid | plate | |
| Iron(III) citrate | 682 nm | 682 nm | 685 nm | 685 nm |
| Iron oxide | 681 nm | 678 nm | 684 nm | 684 nm |
| Goethite | 675 nm | 675 nm | 680 nm | 682 nm |
77 K Fluorescence
The fluorescence spectra of whole cells at 77 K have been used to characterize the photosynthetic machinery of cyanobacteria [35]. Several fluorescence features can be assigned to specific photosynthetic complexes in Synechocystis sp. PCC 6803. The core light-harvesting complexes of PSII have characteristic fluorescence emission maxima at 685 nm (CP43) and 695 nm (CP47). Additionally, fluorescence emission at 685 nm has been found to arise from the terminal phycobilisome emitter, called Lcm [36], and IsiA [37]. PSI exhibits an emission maximum at 725 nm. In cyanobacteria, excitation with light at 440 nm preferentially excites chlorophyll a molecules that are part of PSI, PSII and IsiA, but not phycobilisomes (the main light-harvesting system associated with PSII of cyanobacteria). Validity of 77 K spectra is analyzed using the statistical measurement of the SE between replicates.
Spectral characteristics of cells grown in liquid and on agar
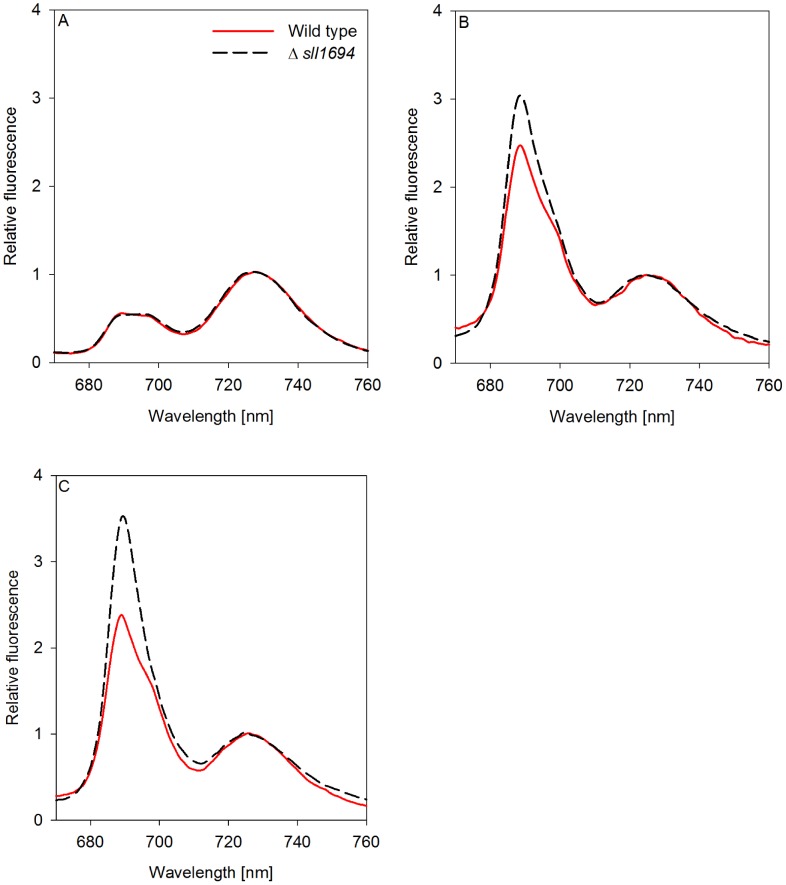
Cells grown in liquid medium with different iron sources were investigated using fluorescence emission spectra at 77 K (Fig. 6 A). When iron was supplied as ferric ammonium citrate, the wild type and the Δsll1694 strain show similar chlorophyll a emission spectra that conform with 77 K emission spectra generally reported for wild type grown in liquid. Interestingly, an increase in the 685 nm fluorescence emission in wild type and the Δsll1694 strain was observed when iron oxide or goethite were supplied (Fig. 6 B, C) in liquid culture.
Figure 6. 77 K fluorescence emission spectra of photoautotrophically grown liquid cultures excited by 440 nm light.
Both wild type and Δsll1694 cells were grown in BG-11 with ammonium iron(III) citrate (A), iron(III) oxide (B), or goethite (C) as the exclusive iron source. Samples were excited by a 440 nm light source. Traces were normalized to the PS I peak at 725 nm. Data shown is indicative of three separate experiments. The average standard error between triplicates was calculated. Wild type SE: ±1.3×10−2 (A), ±2.3×10−2 (B), ±1.8×10−2 (C); Δsll1694 SE: ±1.7×10−2 (A), ±1.2×10−2 (B), ±1.0×10−2 (C).
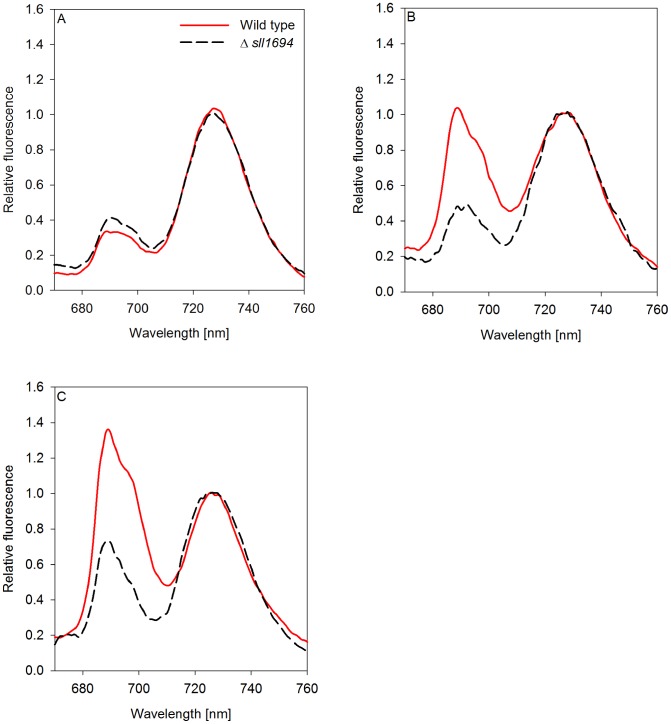
Under some conditions, the fluorescence emission spectra at 77 K of cells grown on agar (Fig. 7) contrast substantially to the spectra of the liquid-grown cells. In both, wild type and Δsll1694 strains, the fluorescence emission spectra from plate-gown cells looked similar to cells grown in liquid. In contrast, when goethite and iron oxide were supplied, the 685 nm and 695 nm peaks increased in the wild type, while a smaller increase was observed in the Δsll1694 strain.
Figure 7. 77 K fluorescence emission spectra of photoautotrophically grown plate cultures excited by 440 nm light.
Both wild type and Δsll1694 cells grown on BG-11 plates with ammonium iron(III) citrate (A), iron(III) oxide (B), or goethite (C) as the exclusive iron source. Samples were excited by a 440 nm light source. Traces were normalized to the PS I peak at 725 nm. One data set is shown, which is representative of three separate experiments. The average standard error between triplicates was calculated. Wild type SE: ±2.7×10−2 (A), ±3.2×10−2 (B), ±3.5×10−2 (C); Δsll1694 SE: ±4.4×10−2 (A), ±4.2×10−2 (B), ±3.1×10−2 (C).
To distinguish the contribution of IsiA and PSII to the 77 K fluorescence emission spectra, analysis of the ratio of the emissions specific for these complexes is required. The emission at 695 nm in relation to the emission peak at 685 nm indicates the presence of PSII and IsiA, respectively. This analysis revealed that slightly more IsiA is present in the Δsll1694 strain compared to the wild type in liquid-grown cells supplied with iron oxide and goethite (Fig. 6 B, C). Conversely, more IsiA is present in the wild type than in the Δsll1694 strain when grown on plates with iron oxide and goethite (Fig. 7 B, C).
The PSI and PSII ratio is another characteristic that can be evaluated by interpreting 77 K fluorescence emission spectra. Here the fluorescence emission at 695 nm indicates the presence of PSII, while the emission centered at 725 nm represents the presence of PSI. The spectra in Figs. 6 and 7 are normalized to the PSI emission peak at 725 nm. After taking into account of the contribution of IsiA to the spectra at 685 and 695 nm, it can be concluded that the ratio of PSII to PSI in liquid-grown cells is much higher than in the agar-grown cells for both wild type and the Δsll1694 strain on iron oxide and goethite. On agar, the wild type maintains a higher PSII to PSI ratio on iron oxide and goethite compared to the Δsll1694 strain.
Discussion
Our study investigated the role of PilA1 in the utilization of oxidized (ferric) iron sources. Iron is a crucial cofactor of many protein complexes that mediate photosynthetic electron transport in cyanobacteria. Moreover, iron is a growth-limiting factor in many environments. Utilization of iron oxides through electron donation to the iron may therefore be an important strategy for survival in otherwise iron-limited environments.
Electron donation to iron oxides as a mechanism for utilizing otherwise inaccessible sources of iron has recently been suggested to occur in cyanobacteria [14]. Extracellular or periplasmic reduction of ferric iron is thought to be followed by transport of the soluble ferrous iron through the cytoplasmic membrane into the cell. In the proposed scheme, a ferri-siderophore is thought to be disassembled via reduction outside of the plasma membrane. This disassembly is followed by the uptake of the iron through the cytoplasmic membrane [14]. The reduction of iron oxides also occurs in non-photosynthetic soil bacteria [9]. Several mechanisms have been proposed for this respiratory reduction including the use of electrically conductive pili that link cellular electron transport to extracellular electron acceptors [9]. Based on this information, we decided to investigate if PilA1, the main constituent of pilins [28], [29], [30], [31], [38], [39], has a role in iron acquisition in cyanobacteria.
Growth experiments
In our study, the lack of PilA1 has direct phenotypic consequences when cells are grown on plates, even when the biologically accessible iron source, ferric ammonium citrate, is present. Interestingly, the pilA1-deficient mutant strains exhibit a mosaic of pigmentation when grown on plates on ferric ammonium citrate. This phenotype may indicate that direct contact with iron oxide particles is required for iron utilization as proposed by Kranzler and colleagues [14]. Despite this, the difference between the Δsll1694 mutant and wild type indicates that another mechanism of iron reduction exists, as no change in pigmentation is observed in the wild type. In the wild type, extracellular PilA1 may enable the activation and utilization of oxidized iron sources while this is only possible when direct contact is made in the Δsll1694 mutant. Spectroscopic data reveals that this change in pigmentation is most likely the consequence of a decrease in phycobilisomes in the Δsll1694 strain.
Wild type and the Δsll1694 strain are able to grow on iron oxide and goethite in liquid cultures, although it is unlikely that they can make sustained contact with iron oxide mineral particles. Moreover, although the initial growth of wild type and Δsll1694 cells appears similar in liquid on iron oxide and goethite, the wild type shows more growth after several days. As pili may constantly be sheared off in liquid the ability to grow on iron in liquid culture indicates that in addition to the “cell-contact” iron utilization mechanism, an additional ferric iron uptake mechanism is also present, that cannot be engaged on plates.
Our growth experiments on agar plates show that iron oxide and goethite can be used by the Synechocystis sp. PCC 6803 wild-type strain, while a PilA1-deficient strain struggles to utilize these sources of iron. As the PilA1-deficient mutant grows slower than the wild type on oxidized iron minerals, it seems likely that pili play an important role in accessing this iron on agar plates. The ability to move that is connected to the presence of pili in some Synechocystis sp. PCC 6803 strains is lost in the strain we used for our experiments. Resequencing of this strain [40] revealed a frameshift mutation in slr0162, whose gene product, PilC, is required for twitching motility. Therefore locomotion by the wild-type cells is not the reason for the observed phenotype. The PilA1 protein may be essential in the transport of electrons from the electron transport chain to iron oxides, and the consequent reduction of ferric iron into soluble ferrous iron (Fig. 8); the electron carriers that mediate this reduction of iron sources remain to be defined.
Figure 8. Schematic of electron transport to extra-cellular particles containing ferric iron.
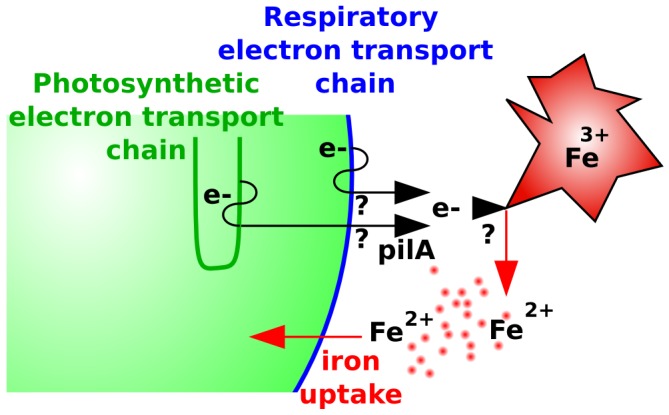
Ferric iron (Fe3+) is converted to ferrous iron (Fe2+) that can be taken up by a cyanobacterial cell. Electron transport indicated in black, thylakoid membranes indicated in green, respiratory membranes indicated in blue.
IsiA
IsiA is induced under iron-limited conditions and therefore the presence of IsiA may be a good indicator for the availability of iron [41]; however, although IsiA has originally been discovered during iron stress, recent experiments also indicate that IsiA is induced as a response to oxidative stress [42].
Spectroscopic data at room temperature and fluorescence emission spectra at 77 K of wild type and the Δsll1694 strains indicate the presence of IsiA when cells are grown on oxidized iron minerals in liquid; however, the shift to shorter wavelengths associated with the presence of IsiA is more exaggerated in the wild type than in Δsll1694 strain. It is furthermore notable that the Δsll1694 strain possesses an in vivo room temperature light absorption spectrum that is further red-shifted than the wild type when grown under every iron source tested, including the bio-available ferric ammonium citrate. Possible reasons for this red-shift are an increased amount of IsiA in wild type and/or an increased amount of PSI in the Δsll1694 strain. Indeed, 77 K fluorescence emission data indicate that this combination of factors is present in agar-grown cells. Moreover, 77 K fluorescence spectra also indicate that the origin of this shift is not necessarily due to changes in the amount of IsiA and PSI, as the Δsll1694 strain and wild type possesses almost identical 77 K fluorescence emission spectra when grown on ferric ammonium citrate on agar plates and in liquid.
We have therefore to accept – at first sight – a contradicting situation in regards to iron utilization and presence of IsiA: growth tests indicate that PilA1-deficient cells are not able to utilize oxidized iron minerals as efficiently as wild type, but in response to iron limitation, PilA1-deficient cells only produce elevated amounts of the stress-response protein IsiA in liquid culture, but not on plate. We think this behavior can be explained by the physiology of plate-grown colonies, and by attributing the production of IsiA to the presence of oxidative stress and not the absence of iron.
The physiological response of Synechocystis sp. PCC 6803 wild type and the Δsll1694 strain to growth on iron oxide minerals in liquid is somewhat surprising as the absorption spectra indicate that phycobilisomes are abundant and 77 K fluorescence emission spectra indicate the presence of IsiA in coordination with a high PSII to PSI ratio. This contrasts with the general observation of liquid-grown, iron-limited cyanobacteria cultures, where an increase in the amount of IsiA and a decrease in the amount of phycobilisomes and PSII are observed [43]. One interpretation of these data is that the wild-type cells experience some stress but are not iron-limited. This stress may originate from a higher oxygen concentration present within dense cyanobacterial colonies, e.g. bacterial mats [44].
Possible regulatory network
Recent transcriptome profiling has uncovered a wealth of expressed antisense and other noncoding RNAs in Synechocystis sp. PCC 6803 [45]. Within the pilA12 operon (sll1693, sll1694, sll1695 and sll1696), two asRNA molecules (sll1694-as and sll1695-as), along with three other non-coding RNA features have been detected [45]. The deletion and over-expression of these antisense RNA will aide in determining their function, including the regulation of the isiA operon.
A study that investigated the transcriptional response of an isiA deletion strain provides evidence that pilins are a component in a complex stress response network. An isiA deletion mutant exhibited an increase in the mRNA of the pilin-containing sll1693-sll1696 operon and two other pilin genes (slr1456 and slr0079) compared to wild type during standard growth conditions [27]. The isiA-isiB operon in turn also contains a regulatory mRNA (isiR) that would have been impaired by deleting isiA in the aforementioned study. The presence of regulatory RNAs in the pilin-containing operon sll1694-sll1695 and the isiA-isiB operon, in combination with the phenotypic coupling of these operons observed by Singh and colleagues [27] as well as our study, suggest that a complex regulatory framework coordinates PilA1 and IsiA expression. This assessment is in line with our observations, as iron metabolism and oxidative stress often interface with one another, and therefore may require complex regulation.
In our Δsll1694 deletion strain, the pilin sll1695 (PilA2) and a protein with an unknown function (sll1696) are likely not to be expressed due to the disruption of the operon. Inactivating each gene in this operon, while keeping the other genes intact will be crucial to pinpoint the component that is critical for the observed phenotype of the mutant we generated. This approach will also be useful to assess the function of regulatory elements that are present in the sll1693-sll1696 operon.
Conclusions
Our study provides evidence that Synechocystis sp. PCC 6803 is well adapted to growing on a solid medium. Synechocystis and Synechococcus species are part of microbial mats and therefore growing in a high-oxygen environment should be part of their physiological repertoire. In the case of Synechocystis sp. PCC 6803, the ability to form fast-growing colonies on plates is likely dependent on tolerating high oxygen concentrations.
The presented work shows that the absence of PilA1 in the cyanobacterium Synechocystis sp. PCC 6803 impairs growth on iron oxide minerals. This is the first report that connects pilins and by extension pili with iron oxide utilization in cyanobacteria. Presently, we cannot clearly distinguish if the observed phenotypic consequences are solely caused by the inability to utilize oxidized iron sources, or are also a consequence that arises due to the disruption of a regulatory network, or a combination of both. Nonetheless, if other bacteria can utilize pili to donate electrons to iron oxides, it would appear unlikely that cyanobacteria would not have developed this capability. The ancestors of modern cyanobacteria were surely the first to experience the consequences of elevated oxygen concentrations. It would therefore seem not surprising that cyanobacteria have developed or adapted a tool that allows them to unlock the non-bioavailable metal oxides their oxygen produces.
Materials and Methods
Growth and maintenance of stock cultures
Cells were maintained on BG-11 agar plates containing 5 mM glucose, 20 µM atrazine and appropriate antibiotics where applicable [46]. Liquid cultures were established in 300 mL Erlenmeyer flasks that have been specifically modified as described by Eaton-Rye [46]. Cultures containing BG-11, 5 mM glucose and appropriate antibiotics were grown mixotrophically. Both plates and liquid cultures were grown under constant illumination (30 µE.m−2.s−1), at 30°C. Liquid cultures were provided with filtered aeration via small aquarium pumps. The air was additionally bubbled through ddH2O to prevent dehydration of cell cultures.
Alternative iron sources
BG-11 media without ammonium iron(III) citrate was supplemented with either 1.84 µg.mL−1 (23 µM Fe) of iron(III) oxide, or 2.04 µg.mL−1 (23 µM Fe) of goethite. BG-11.
Experimental liquid-media growth curve
Liquid cultures of Synechocystis sp. PCC 6803 were grown in the presence of 5 mM glucose to an OD of 1.0. Cells were centrifuged at 2760 g for 10 min at 25°C and washed twice in BG-11. A flask containing 150 mL BG-11 (without glucose or atrazine) was inoculated with washed cells to give a starting OD of 0.05. Cultures were maintained under constant temperature (30°C), illumination (30 µE.m−2.s−1) and aeration. The OD of each culture was recorded every 12 h over 148 h period using a custom built spectrophotometer [47]. To measure the OD accurately, a small sample of the culture was taken and diluted 5 – fold to yield the true culture OD.
Experimental solid-media growth curve
Liquid cultures of Synechocystis sp. PCC 6803 were grown in the presence of 5 mM glucose to an OD of 1.0. Cells were centrifuged at 2760 g for 10 min at 25°C followed by two washing steps in sterile water and resuspended to an OD of 1.0. A small amount of this liquid culture was used to inoculate 2 mL of sterile water to an OD of 0.1. Serial dilutions of this culture were subsequently made to attain two further OD aliquots of 0.01 and 0.001. Two micro liters of each of the three culture samples were spotted onto BG-11 (without glucose or atrazine) agar growth plates with appropriate growth conditions (specific iron source). Cultures were maintained under constant temperature (30°C), illumination (30 µE.m−2.s−1) and aeration. Raw images of each culture were taken every 24 h over a 220 h period using a custom built plate imager. The integrated intensity of the cultures was compared to the agar background to give a relative growth parameter.
Polymerase chain reaction
Primers for PCR analysis were ordered as needed in the 5′ to 3′ orientation with specific restriction enzyme cut sites upstream of the 5′ end (Table. S1 in File S1), through Sigma-Aldrich (Sigma-Aldrich, NSW, Australia).
The reactions were performed in 1x Phusion amplification buffer containing 0.4 mM of each dNTP (dATP, dCTP, dGTP and dTTP), 0.4 mM of each primer and 0.05 units/µL Phusion polymerase (Thermo Fisher Scientific, USA). PCR conditions were: (1) initial denaturing step at 98°C for 30 s; (2) 14 cycles of 98°C for 7 s, annealing at 62°C (−1°C per cycle) for 20 s, extension at 72°C for 30 s/kb; (3) 16 cycles of 98°C for 7 s, annealing at 62°C for 20 s, extension at 72°C for 30 s/kb; then (4) final extension at 72°C for 5 min. PCR products were cleaned using a QIAquick PCR purification kit (Qiagen, Duesseldorf, Germany).
Separation of DNA samples by gel electrophoresis
DNA samples were separated by electrophoresis using 0.8% agarose gels in the presence of 10 mM NaOH and 73 mM boric acid at pH 8.0. The running condition was 150 V for 25 min at room temperature. Prior to loading, samples were mixed with 10× loading buffer (0.25% bromophenol blue, 0.25% xylene cyanol FF and 30% glycerol). DNA was visualized by exposure to a UV light after soaking the gel in ddH2O containing 1 mg/mL ethidium bromide for 10 min. Gel images were captured using a GelDoc (BioRad, USA).
Plasmid construction
Ligation reactions were carried out at a vector to insert ratio of 3∶1 with ∼100 ng DNA in total. The reaction contained 50 mM Tris-HCl (pH7.5), 10 mM MgCl2, 10 mM dithiothreitol (DTT), 1 mM ATP, and 3 U of T4 DNA ligase (Roche, Mannheim, Germany). The reaction mixture was incubated at 22°C overnight.
Transformation of Synechocystis sp. PCC 6803
Liquid cultures were grown in the presence of glucose to an OD of approximately 0.5. Cells were then centrifuged at 2760 g for 10 min and suspended in 0.5 mL BG-11 media to a final OD of 2.5 in a sterile glass tube. Approximately 5 µg of plasmid DNA was added to tubes and incubated at 30°C under 30 µE.m−2.s−1 of illumination for 6 h with gentle shaking at the 3 h mark. Negative controls with no DNA were also included. Samples were spread over sterile nitrocellulose paper on BG-11 plates supplemented with glucose and incubated for 12 h. The filters, with cells attached, were then transferred to plates containing glucose, atrazine and appropriate antibiotics. After two weeks, single colonies were picked and streaked out weekly for three weeks to ensure complete segregation. Colony PCR was used to verify complete segregation using the sll1694 left flank forward (NcoI) primer and the sll1694 right flank reverse (PstI) primer.
Whole cell absorption spectra
Whole cell absorption spectra were measured using a Jasco V-550 spectrophotometer. Cells were suspended in BG-11 containing HEPES-NaOH, pH 7.5, to an OD of 0.3. Translucent cellotape was fixed on both sides of the cuvette holder during measurements. Spectra were baseline subtracted after acquisition, during the data analysis.
77 K fluorescence
Cells were diluted with BG-11 containing HEPES/NaOH, pH 7.5, to a chlorophyll concentration of 2 µg.mL−1 measured using a custom built fluorometer [48]. Cells were then transferred into glass tubes, frozen in liquid nitrogen and fluorescence emission assessed using a fluorescence spectrometer (Perkin-Elmer, MPF-3L). The emission slit was set to 4 nm for each measurement. The excitation slit for samples was 12 nm and 10 nm for 440 nm and 580 nm, respectively. Traces were normalized to a PSI peak at 725 nm in the emission spectra.
PilA protein harvest
Cells were grown in 200 mL of BG-11 to an OD of 0.8. They were then centrifuged at 2760 g for 8 min and the supernatant was discarded. The cell pellet was then re-suspended in 5 mL of BG-11. The cell suspension was subjected to 2 min of thorough vortexing followed by another centrifugation at 2760 g for 8 min. The supernatant was carefully removed without disrupting the cell pellet and concentrated using a Vivaspin 500 column with a 5 kDa molecular weight cut off (GE Healthcare, UK) to a final volume of 50 µL.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
In this study, 12% SDS-PAGE using a Tris-glycine buffer system was used. The running condition for Tris-glycine was 200 V for 55 min at room temperature. Directly after completion of electrophoresis, the gels were removed from the glass cassette and soaked in a gel-fixing solution (50% (v/v) ethanol and 10% (v/v) acetic acid in 18 MΩ resistant purified milli Q water) for 1 h. The gel was then incubated at room temperature overnight with slight agitation in gel-washing solution (50% (v/v) methanol and 10% (v/v) acetic acid in ddH2O). For visualization of protein bands the gel was covered with Coomassie stain (0.1% (w/v) Coomassie blue R350, 20% (v/v) methanol and 10% (v/v) acetic acid in 18 MΩ resistant purified milli Q water) for 3 to 4 h with slight agitation. Destain solution (50% (v/v) methanol and 10% (v/v) acetic acid in 18 MΩ resistant purified milli Q water) was then used several times to remove excess Coomassie stain. The gel was then stored indefinitely in a storage solution (5% (v/v) acetic acid in 18 MΩ resistant purified milli Q water.
Mass spectrometry analysis
Samples were excised carefully from the SDS-PAGE gel and analyzed at the Centre for Protein Research at the University of Otago in order to determine identity of the protein bands.
Supporting Information
Contains the following files: Figure S1. Genomic DNA sll1694-deletion through homologous recombination. Figure S2. Gel showing colony PCR products. Figure S3. SDS-PAGE Analysis of extracellular proteins. Figure S4. Absorption spectra of liquid grown culture. Figure S5. Absorption spectra of plate grown culture. Table S1. PCR primers including specific cut sites.
(DOC)
Acknowledgments
We would like to thank to Thorsten Kleffmann at the Centre for Protein Research at the University of Otago for analyzing the supernatant concentrates.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by a Postdoctoral Fellowship to MHM from the New Zealand Foundation for Research and Technology, succeeded by the Ministry of Business, Innovation and Employment; (http://www.msi.govt.nz/). Additional funding was provided through the PhD position awarded to JL by the Norwegian University of Science and Technology, Trondheim, Norway. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Frausto da Silva JJR, Williams RJP (2001) The Biological Chemistry of the Elements: The Inorganic Chemistry of Life. New York: Oxford University Press. 561 p. [Google Scholar]
- 2. Braun V, Killmann H (1999) Bacterial solutions to the iron-supply problem. Trends Biochem Sci 24: 104–109. [DOI] [PubMed] [Google Scholar]
- 3. Faraldo-Gomez JD, Sansom MSP (2003) Acquisition of siderophores in gram-negative bacteria. Nature Rev Mol Cell Biol 4: 105–116. [DOI] [PubMed] [Google Scholar]
- 4. Sandy M, Butler A (2009) Microbial iron acquisition: marine and terrestrial siderophores. Chem Rev 109: 4580–4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Braun V, Hantke K (2011) Recent insights into iron import by bacteria. Curr Opin Chem Biol 5: 328–334. [DOI] [PubMed] [Google Scholar]
- 6. Létoffé S, Ghigo JM, Wandersman C (1994) Iron acquisition from heme and hemoglobin by a Serratia marcescens extracellular protein. Proc Natl Acad Sci USA 91: 9876–9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghigo JM, Létoffé S, Wandersman C (1997) A new type of hemophore-dependent heme acquisition system of Serratia marcescens reconstituted in Escherichia coli . J Bacteriol 179: 3572–3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, et al. (2005) Extracellular electron transfer via microbial nanowires. Nature 435: 1098–1101. [DOI] [PubMed] [Google Scholar]
- 9. Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, et al. (2006) Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR – 1 and other microorganisms. Proc Natl Acad Sci USA 103: 11358–11363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hopkinson BM, Morel FMM (2009) The role of siderophores in iron acquisition by photosynthetic marine microorganisms. Biometals 22: 659–669. [DOI] [PubMed] [Google Scholar]
- 11. Ehrenreich IM, Waterbury JB, Webb EA (2005) Distribution and diversity of natural product genes in marine and freshwater cyanobacterial cultures and genomes. Appl Environ Microbiol 71: 7401–7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Katoh H, Hagino N, Grossman AR, Ogawa T (2001) Genes essential to iron transport in the cyanobacterium Synechocystis sp. strain PCC 6803. J Bacteriol 183: 2779–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katoh H, Hagino N, Ogawa T (2001b) Iron-binding activity of FutA1 subunit of an ABC-type iron transporter in the cyanobacterium Synechocystis sp. Strain PCC 6803. Plant Cell Physiol 42: 823–827. [DOI] [PubMed] [Google Scholar]
- 14. Kranzler C, Lis H, Shaked Y, Keren N (2011) The role of reduction in iron uptake processes in a unicellular, planktonic cyanobacterium. Environ Microbiol 13: 2990–2999. [DOI] [PubMed] [Google Scholar]
- 15. Badarau A, Firbank SJ, Waldron KJ, Yanagisawa S, Robinson NJ, et al. (2008) FutA2 is a ferric binding protein from Synechocystis PCC 6803. J Biol Chem 283: 12520–12527. [DOI] [PubMed] [Google Scholar]
- 16. Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111: 1–61. [Google Scholar]
- 17. Askwith C, Eide D, Vanho A, Bernard P S, Li LT, et al. (1994) The Fet3 gene of Saccharomyces cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell 76: 403–410. [DOI] [PubMed] [Google Scholar]
- 18. Stearman R, Yuan DS, YamaguchiIwai Y, Klausner RD, Dancis A (1996) A permease-oxidase complex involved in high-affinity iron uptake in yeast. Science 271: 1552–1557. [DOI] [PubMed] [Google Scholar]
- 19. Maldonado MT, Allen AE, Chong JS, Lin K, Leus D, et al. (2006) Copper-dependent iron transport in coastal and oceanic diatoms. Limnol Oceanogr 51: 1729–1743. [Google Scholar]
- 20. Boekema EJ, Hifney A, Yakushevska AE, Piotrowski M, Keegstra W, et al. (2001) A giant chlorophyll-protein complex induced by iron deficiency in cyanobacteria. Nature 412: 745–748. [DOI] [PubMed] [Google Scholar]
- 21. Bibby TS, Nield J, Barber J (2001) Iron deficiency induces the formation of an antenna ring around trimeric photosystem I in cyanobacteria. Nature 412: 743–745. [DOI] [PubMed] [Google Scholar]
- 22. Falk S, Samson G, Bruce D, Huner NPA, Laudenbach DE (1995) Functional analysis of the iron-stress induced CP43' polypeptide of PS II in the cyanobacterium Synechococcus sp. PCC 7942. Photosynth Res 45: 51–60. [DOI] [PubMed] [Google Scholar]
- 23. Vinnemeier J, Kunert A, Hagemann M (1998) Transcriptional analysis of the isiAB operon in salt-stressed cells of the cyanobacterium Synechocystis sp. PCC 6803. FEMS Microbiol Lett 169: 323–330. [DOI] [PubMed] [Google Scholar]
- 24. Kunert A, Vinnemeier J, Erdmann N, Hagemann M (2003) Repression by Fur is not the main mechanism controlling the iron-inducible isiAB operon in the cyanobacterium Synechocystis sp. PCC 6803. FEMS Microbiol Lett 227: 255–262. [DOI] [PubMed] [Google Scholar]
- 25. Dühring U, Axmann IM, Hess WR, Wilde A (2006) An internal antisense RNA regulates expression of the photosynthesis gene isiA . Proc Nat Acad Sci USA 18: 7054–7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Legewie S, Dienst D, Wilde A, Herzel H, Axmann IM (2008) Small RNAs establish delays and temporal thresholds in gene expression. Biophys J 7: 3232–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singh A, Li H, Bono L, Sherman L (2005) Novel adaptative responses revealed by transcription profiling of a Synechocystis sp. PCC 6803 ΔisiA mutant in the presence and absence of hydrogen peroxide. Photosynth Res 84: 65–70. [DOI] [PubMed] [Google Scholar]
- 28. Bhaya D, Watanabe N, Ogawa T, Grossman A (1999) The role of an alternative sigma factor in motility and pilus formation in the cyanobacterium Synechocystis sp. strain PCC6803. Proc Natl Acad Sci USA 96: 3188–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bhaya D, Bianco NR, Bryant D, Grossman A (2000) Type IV pilus biogenesis and motility in the cyanobacterium Synechocystis sp. PCC6803. Mol Microbiol 37: 941–951. [DOI] [PubMed] [Google Scholar]
- 30. Bhaya D, Takahashi A, Grossman A (2001) Light regulation of Type IV pilus-dependent motility by chemosensor-like elements in Synechocystis sp. PCC 6803. Proc Natl Acad Sci USA 13: 7540–7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yoshihara S, Geng XX, Okamoto S, Yura K, Murata T, et al. (2001) Mutational analysis of genes involved in pilus structure, motility and transformation competency in the unicellular motile cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol 42: 63–73. [DOI] [PubMed] [Google Scholar]
- 32. Nakasugi K, Svenson CJ, Neilan BA (2006) The competence gene, comF, from Synechocystis sp. strain PCC 6803 is involved in natural transformation, phototactic motility and piliation. Microbiology 152: 3623–3631. [DOI] [PubMed] [Google Scholar]
- 33. Lagarde D, Vermaas W (1999) The zeaxanthin biosynthesis enzyme β-carotene hydroxylase is involved in myxoxanthophyll synthesis in Synechocystis sp. PCC 6803. FEBS Lett 454: 247–251. [DOI] [PubMed] [Google Scholar]
- 34. Odom WR, Hodges R, Chitnis PR, Guikema JA (1993) Characterization of Synechocystis sp. PCC 6803, in iron-supplied and iron deficient media. Plant Mol Biol 23: 1255–1264. [DOI] [PubMed] [Google Scholar]
- 35. Murakami A (1997) Quantitative analysis of 77K fluorescence emission spectra in Synechocystis sp. PCC 6714 and Chlamydomonas reinhardtii with variable PS I/PS II stoichiometries. Photosynth Res 53: 141–148. [Google Scholar]
- 36. Wilson A, Boulay C, Wilde A, Kerfeld CA, Kirilovsky D (2007) Light-induced energy dissipation in iron-starved cyanobacteria: roles of OCP and IsiA proteins. Plant Cell 19: 2656–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Burnap RL, Troyan T, Sherman LA (1993) The highly abundant chlorophyll protein complex of iron-deficient Synechococcus sp. PCC7942 (CP43') is encoded by the isiA gene. Plant Physiol 103: 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meyer T, Billyard E, Haas R, Storzbach S, So M (1984) Pilus genes of Neisseria gonorrheae: Chromosomal organization and DNA sequence. Proc Natl Acad Sci USA 81: 6110–6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fussenegger M, Rudel T, Barten R, Ryll R, Meyer T (1997) Transformation competence and type-4 pilus biogenesis in Neisseria gonorrhoeae – a review. Gene 192: 125–134. [DOI] [PubMed] [Google Scholar]
- 40. Morris JN, Crawford TS, Jeffs A, Stockwell PA, Eaton-Rye JJ, et al. (2014) Whole genome re-sequencing of two ‘wild type’ strains of the model cyanobacterium Synechocystis sp. PCC 6803. New Zealand J Bot 52: 36–47. [Google Scholar]
- 41. Riethman HC, Sherman LA (1988) Purification and characterization of an iron stress-induced chlorophyll-protein from the cyanobacterium Anacystis nidulans R2. Biochim Biophys Acta 2: 141–151. [DOI] [PubMed] [Google Scholar]
- 42. Singh AK, Sherman LA (2007) Reflections on the function of IsiA, a cyanobacterial stress-inducible, Chl-binding protein. Photosynth Res 9: 17–25. [DOI] [PubMed] [Google Scholar]
- 43. Guikema JA, Sherman LA (1983) Organization and function of chlorophyll in membranes of cyanobacteria during iron starvation. Plant Physiol 73: 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Revsbech NP, Jørgensen BB, Blackburn TH, Cohen Y (1983) Microelectrode studies of the photosynthesis and O2, H2, S, and pH profiles of a microbial mat. Limnol Oceanogr 28: 1062–1074. [Google Scholar]
- 45. Mitschke J, Georg J, Scholz I, Sharma CM, Dienst D, et al. (2011) An experimentally anchored map of transcriptional start sites in the model cyanobacterium Synechocystis sp. PCC 6803. Proc Natl Acad Sci USA 5: 2124–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eaton-Rye JJ (2011) The construction of gene knock-outs in the cyanobacterium Synechocystis sp. PCC 6803. Methods Mol Biol 684: 309–324. [DOI] [PubMed] [Google Scholar]
- 47. Lamb JJ, Eaton-Rye JJ, Hohmann-Marriott MF (2013) A cost-effective solution for the reliable determination of cell numbers of microorganisms in liquid culture. Curr Microbiol 67: 123–129. [DOI] [PubMed] [Google Scholar]
- 48. Lamb JJ, Eaton-Rye JJ, Hohmann-Marriott MF (2012) An LED-based fluorometer for chlorophyll quantification in the laboratory and in the field. Photosynth Res 114: 59–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contains the following files: Figure S1. Genomic DNA sll1694-deletion through homologous recombination. Figure S2. Gel showing colony PCR products. Figure S3. SDS-PAGE Analysis of extracellular proteins. Figure S4. Absorption spectra of liquid grown culture. Figure S5. Absorption spectra of plate grown culture. Table S1. PCR primers including specific cut sites.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.