Abstract
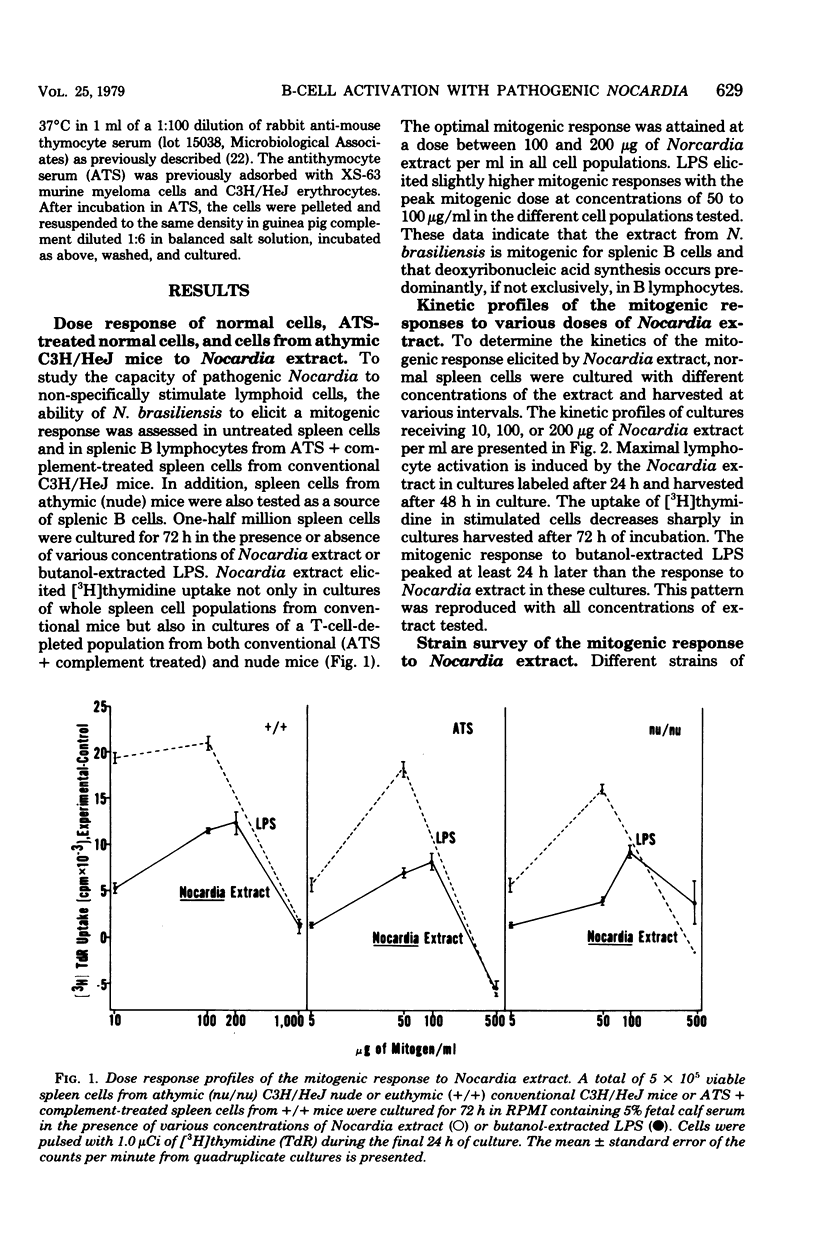
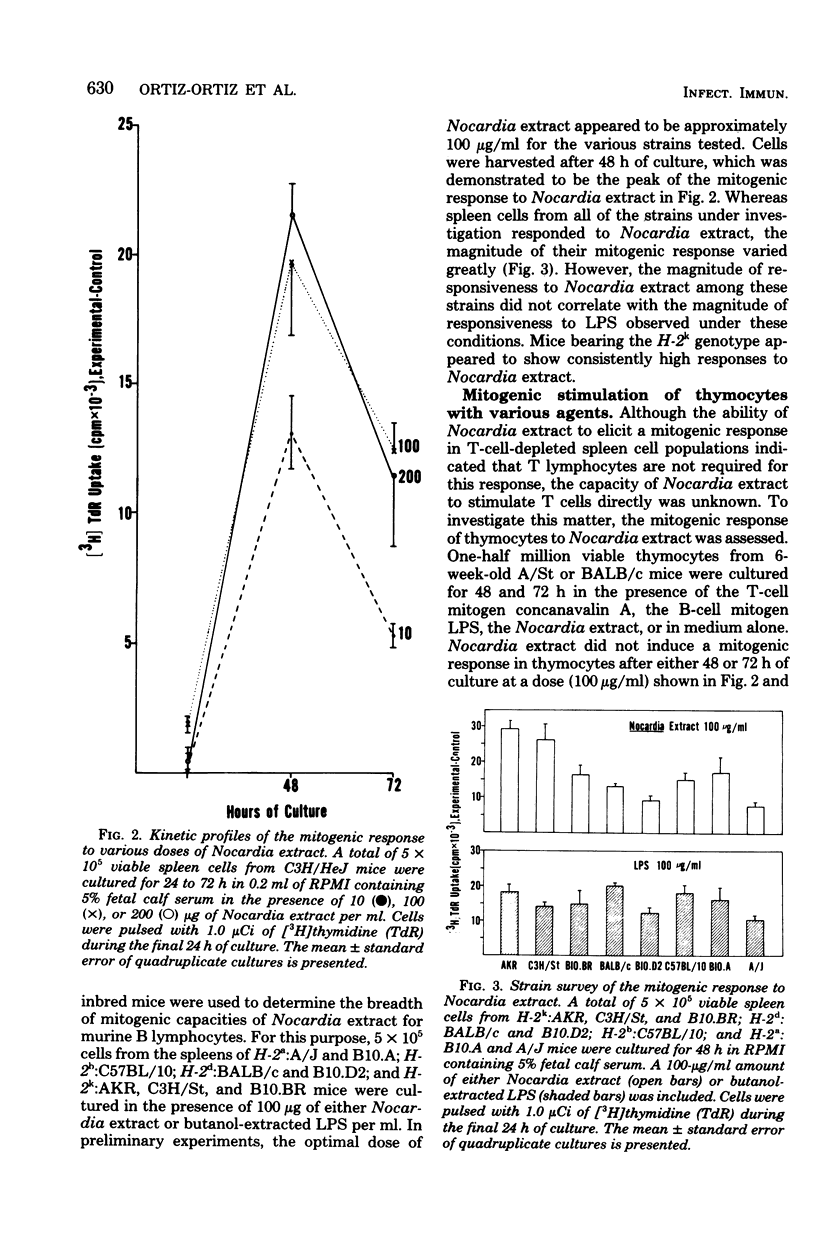
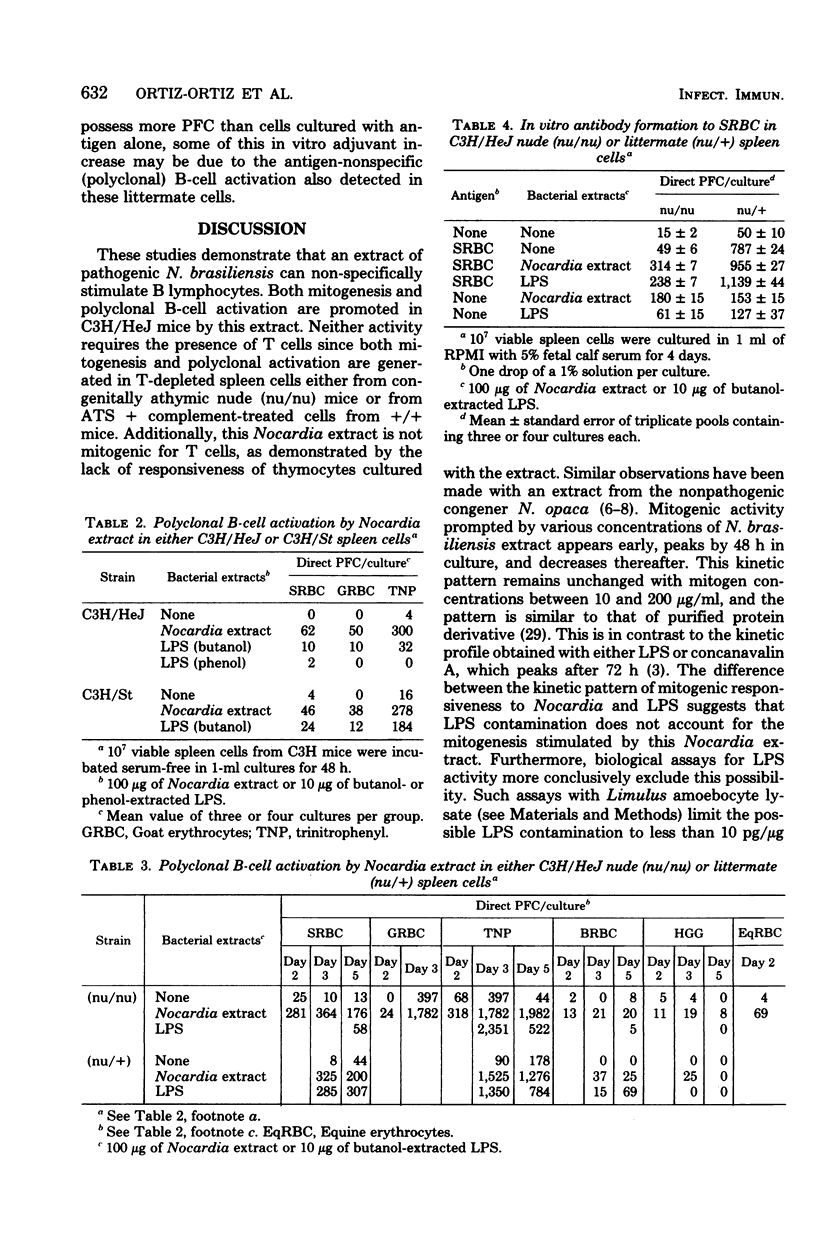
An extract from the pathogenic actinomycete Nocardia brasiliensis was mitogenic for murine lymphocytes. This deoxyribonucleic acid-synthetic response of whole spleen cells peaked after 48 h in culture at concentrations of Nocardia extract ranging from 10 to 200 micrograms/ml. The extract appeared to be a mitogen for B lymphocytes since cultures of spleen cells from congenitally athymic nude (nu/nu) mice and of antithymocyte serum plus complement-treated spleen cells from conventional (+/+) mice responded as well as untreated spleen cells from normal +/+ mice. Furthermore, thymocytes did not respond mitogenically to the extract. Mitogenic responses were stimulated in spleen cells from H-2(a), H-2(b), H-2(d), and H-2(k) mice, including lipopolysaccharide-nonresponder C3H/HeJ mice. This Nocardia extract also stimulated polyclonal B-cell activation to the hapten trinitrophenyl, serum protein human gamma globulin, and several mammalian erythrocytes in cultures of cells from both euthymic and nude mice. Additionally, the requirement for helper T cells in the primary in vitro immune response to sheep erythrocytes could be circumvented by the addition of this Nocardia extract. These results indicate that an extract from the pathogen N. brasiliensis can nonspecifically activate murine B lymphocytes and raise the possibility that polyclonal activation of B lymphocytes may contribute to the pathogenesis of nocardiosis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Ciorbaru R., Petit J. F., Lederer E., Chedid L., Lamensans A., Parant F., Parant M., Rosselet J. P., Berger F. M. Preparation and biological properties of water-soluble adjuvant fractions from delipidated cells of Mycobacterium smegmatis and Nocardia opaca. Infect Immun. 1973 Jun;7(6):855–861. doi: 10.1128/iai.7.6.855-861.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspaugh M. A., Jensen F. C., Rabin H., Tan E. M. Lymphocytes transformed by Epstein-Barr virus. Induction of nuclear antigen reactive with antibody in rheumatoid arthritis. J Exp Med. 1978 Apr 1;147(4):1018–1027. doi: 10.1084/jem.147.4.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J., Möller G., Sjöberg O. Selective induction of DNA synthesis in T and B lymphocytes. Cell Immunol. 1972 Aug;4(4):381–393. doi: 10.1016/0008-8749(72)90040-8. [DOI] [PubMed] [Google Scholar]
- Andersson J., Sjöberg O., Möller G. Induction of immunoglobulin and antibody synthesis in vitro by lipopolysaccharides. Eur J Immunol. 1972 Aug;2(4):349–353. doi: 10.1002/eji.1830020410. [DOI] [PubMed] [Google Scholar]
- Armerding D., Katz D. H. Activation of T and B lymphocytes in vitro. I. Regulatory influence of bacterial lipopolysaccharide (LPS) on specific T-cell helper function. J Exp Med. 1974 Jan 1;139(1):24–43. doi: 10.1084/jem.139.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bona C., Damais C., Chedid L. Blastic transformtion of mouse spleen lymphocytes by a water-soluble mitogen extracted from Nocardia. Proc Natl Acad Sci U S A. 1974 May;71(5):1602–1606. doi: 10.1073/pnas.71.5.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bona C., Damais C., Dimitriu A., Chedid L., Ciorbaru R., Adam A., Petit J. F., Lederer E., Rosselet J. P. Mitogenic effect of a water-soluble extract of Nocardia opaca: a comparative study with some bacterial adjuvants on spleen and peripheral lymphocytes of four mammalian species. J Immunol. 1974 Jun;112(6):2028–2035. [PubMed] [Google Scholar]
- Bona C., Yano A., Dimitriu A., Miller R. G. Mitogenic analysis of murine B-cell heterogeneity. J Exp Med. 1978 Jul 1;148(1):136–147. doi: 10.1084/jem.148.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresser D. W. Most IgM-producing cells in the mouse secrete auto-antibodies (rheumatoid factor). Nature. 1978 Aug 3;274(5670):480–483. doi: 10.1038/274480a0. [DOI] [PubMed] [Google Scholar]
- Dresser D. W., Popham A. M. Induction of an IgM anti-(bovine)-IgG response in mice by bacterial lipopolysaccharide. Nature. 1976 Dec 9;264(5586):552–554. doi: 10.1038/264552a0. [DOI] [PubMed] [Google Scholar]
- Golub E. S., Mishell R. I., Weigle W. O., Dutton R. W. A modification of the hemolytic plaque assay for use with protein antigens. J Immunol. 1968 Jan;100(1):133–137. [PubMed] [Google Scholar]
- Gonzalez Ochoa A. Virulence of nocardiae. Can J Microbiol. 1973 Aug;19(8):901–904. doi: 10.1139/m73-144. [DOI] [PubMed] [Google Scholar]
- Goodman M. G., Parks D. E., Weigle W. O. Immunologic responsiveness of the C3H/HeJ mouse: differential ability of butanol-extracted lipopolysaccharide (LPS) to evoke LPS-mediated effects. J Exp Med. 1978 Mar 1;147(3):800–813. doi: 10.1084/jem.147.3.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerne N. K., Nordin A. A. Plaque Formation in Agar by Single Antibody-Producing Cells. Science. 1963 Apr 26;140(3565):405–405. doi: 10.1126/science.140.3565.405. [DOI] [PubMed] [Google Scholar]
- KUNKEL H. G., TAN E. M. AUTOANTIBODIES AND DISEASE. Adv Immunol. 1964;27:351–395. doi: 10.1016/s0065-2776(08)60711-7. [DOI] [PubMed] [Google Scholar]
- Kettman J., Dutton R. W. An in vitro primary immune response to 2,4,6-trinitrophenyl substituted erythrocytes: response against carrier and hapten. J Immunol. 1970 Jun;104(6):1558–1561. [PubMed] [Google Scholar]
- Luzzati A. L., Hengartner H., Schreier M. H. Induction of plaque-forming cells in cultured human lymphocytes by combined action of antigen and EB virus. Nature. 1977 Sep 29;269(5627):419–420. doi: 10.1038/269419a0. [DOI] [PubMed] [Google Scholar]
- Melchers F., Andersson J. Synthesis, surface deposition and secretion of immunoglobulin M in bone marrow-derived lymphocytes before and after mitogenic stimulation. Transplant Rev. 1973;14:76–130. doi: 10.1111/j.1600-065x.1973.tb00103.x. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Ortiz L., Contreras M. F., Bujalil L. F. Cytoplasmic antigens from Nocardia elicting a specific delayed hypersensitivity. Infect Immun. 1972 Jun;5(6):879–882. doi: 10.1128/iai.5.6.879-882.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D. E., Doyle M. V., Weigle W. O. Effect of lipopolysaccharide on immunogenicity and tolerogenicity of HGG in C57BL/6J nude mice: evidence for a possible B cell deficiency. J Immunol. 1977 Dec;119(6):1923–1932. [PubMed] [Google Scholar]
- Parks D. E., Weigle W. O. Regulation of B cell unresponsiveness by suppressor cells. Immunol Rev. 1979;43:217–240. doi: 10.1111/j.1600-065x.1979.tb00423.x. [DOI] [PubMed] [Google Scholar]
- Primi D., Smith C. I., Hammarström L., Möller G. Polyclonal B-cell activators induce immunological response to autologous serum proteins. Cell Immunol. 1977 Dec;34(2):367–375. doi: 10.1016/0008-8749(77)90258-1. [DOI] [PubMed] [Google Scholar]
- Rosén A., Gergely P., Jondal M., Klein G., Britton S. Polyclonal Ig production after Epstein-Barr virus infection of human lymphocytes in vitro. Nature. 1977 May 5;267(5606):52–54. doi: 10.1038/267052a0. [DOI] [PubMed] [Google Scholar]
- Shinohara N., Kern M. Differentiation of lymphoid cells: B cell as a direct target and T cell as a regulator in lipopolysaccharide-enhanced induction of immunoglobulin production. J Immunol. 1976 Jun;116(6):1607–1612. [PubMed] [Google Scholar]
- Sjöberg O., Andersson J., Möller G. Lipopolysaccharide can substitute for helper cells in the antibody response in vitro. Eur J Immunol. 1972 Aug;2(4):326–331. doi: 10.1002/eji.1830020406. [DOI] [PubMed] [Google Scholar]
- Slaughter L., Carson D. A., Jensen F. C., Holbrook T. L., Vaughan J. H. In vitro effects of Epstein-Barr virus on peripheral blood mononuclear cells from patients with rheumatoid arthritis and normal subjects. J Exp Med. 1978 Nov 1;148(5):1429–1434. doi: 10.1084/jem.148.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M., Nilsson B. S. PPD tuberculin--a B-cell mitogen. Nat New Biol. 1972 Dec 13;240(102):198–200. doi: 10.1038/newbio240198a0. [DOI] [PubMed] [Google Scholar]
- Yin E. T., Galanos C., Kinsky S., Bradshaw R. A., Wessler S., Lüderitz O., Sarmiento M. E. Picogram-sensitive assay for endotoxin: gelation of Limulus polyphemus blood cell lysate induced by purified lipopolysaccharides and lipid A from Gram-negative bacteria. Biochim Biophys Acta. 1972 Jan 28;261(1):284–289. doi: 10.1016/0304-4165(72)90340-6. [DOI] [PubMed] [Google Scholar]



