Abstract
Purpose of review
Mantle cell lymphoma (MCL) is a mature B-cell malignancy that continues to have a high mortality rate. In this manuscript we discuss key pathogenic pathways in MCL biology and their possible therapeutic targeting.
Recent findings
In addition to Cyclin-D1 the transcription factor SOX-11 emerged as a common characteristic of MCL. Genomic studies have identified a number of recurrently mutated genes; in order of descending frequency these include ATM, CCND1, UBR5, TP53, BIRC3, NOTCH1/2 and TRAF2. However, no clear oncogenic driver has been identified. In contrast, several observations indicate that MCL cells are antigen-experienced cells and that the tumor microenvironment and B-cell receptor engagement are important. This is underscored by the impressive clinical responses achieved with the BTK inhibitor ibrutinib. Recently identified activating mutations in the non-canonical NF-κB pathway could give rise to ibrutinib resistance. PARP and aurora kinase inhibitors may be synthetic lethal with the common aberrations in DNA damage pathways found in MCL. Also, ABT-199, a potent and selective inhibitor of BCL-2 has promising activity in early studies.
Summary
MCL is a heterogeneous disease and no single Achilles heel has been identified. Nevertheless, genomic, molecular, and clinical studies have revealed vulnerabilities that can be exploited for effective therapy.
Keywords: Mantle cell lymphoma, Ibrutinib, Cyclin-D1, BCR, BCL-2
Introduction
Mantle cell lymphoma (MCL) is a mature CD5+ B-cell neoplasm accounting for ~6% of non-Hodgkin’s lymphomas (NHL) that contributes disproportionately to NHL-related mortality. MCL presents as a disseminated disease, with a leukemic component in ~20-30% of cases, and frequent involvement of the gastro-intestinal (GI) tract. Its hallmark translocation t(11;14)(q13;q32) puts the cyclin-D1 gene (CCND1) in close proximity of the immunoglobulin heavy locus (IGH) resulting in cyclin-D1 overexpression [1, 2]. No single genetic lesion that can give rise to MCL has been identified. The cyclin-D1 translocation is usually accompanied by additional genetic alterations affecting cell cycle, survival pathways, and DNA damage response. A relatively high degree of genomic instability characterizes MCL among B-cell NHL, and may contribute to its clinical and biological heterogeneity [2, 3].
The neural transcription factor SOX11 is expressed in most cases with MCL [4, 5]. Recent data show that SOX11 regulates PAX5 expression and its knockdown induces a shift toward plasmacytic differentiation. Thus, SOX11 may play a role in preventing maturation of MCL cells and play a role in the pathogenesis. Silencing SOX11 in MCL cell lines resulted in a slower tumor growth in xenograft models [6].
Treatment of MCL represents a challenge. Despite a high response rate to first line chemotherapy, the majority of patients relapses and succumbs to their disease. Even patients having remissions lasting beyond 5 years are not free of disease recurrence, as late relapses are well documented [7, 8].
Due to relatively low incidence of MCL, comparative trials have been rare, and no clear standard of care has been established [9]. Intensive combination of cytotoxic drugs followed by consolidation with high dose chemotherapy and autologous stem cell transplantation has been widely adopted for young and fit patients (recently reviewed in [7, 8]). However, the only potentially curative approach remains allogeneic stem cell transplantation. The high response rate with intensive regimens is typically achieved at the expense of increased toxicity and a significant risk of treatment related death, preventing their use in the frail and elderly who represent >50% of patients [9]. Moreover, a recent retrospective study reported that the survival benefit achieved by intensive chemotherapy over conventional treatment is lost when adjusted for clinical risk factors [10]. Unlike other B-cell NHL, single agent rituximab has limited activity in MCL, and using rituximab in combination with chemotherapy added only a modest benefit compared to chemotherapy alone [11].
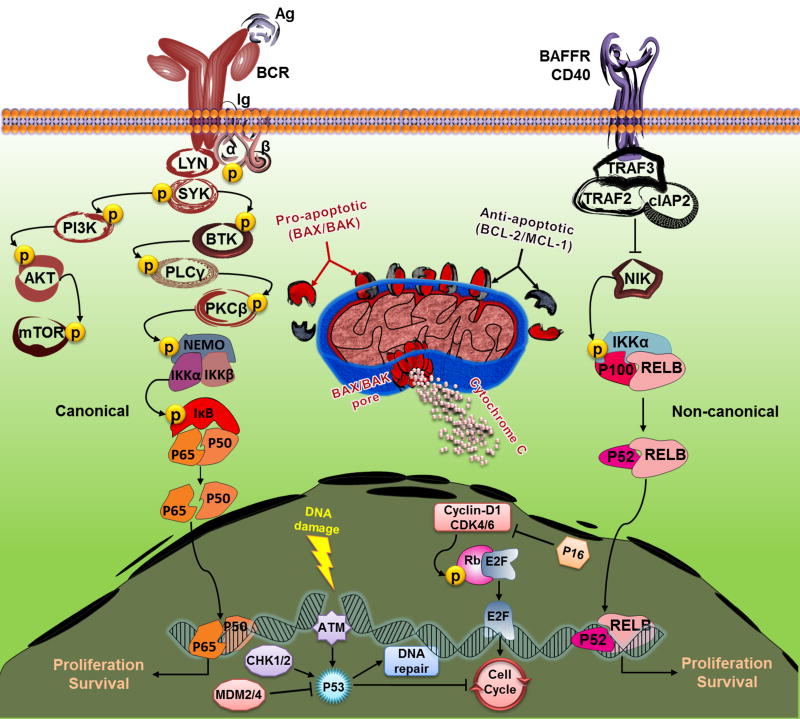
Here we discuss recent advances in MCL biology, with a particular focus on pathways critical for disease pathogenesis, and tumor proliferation, and survival. In particular, we will discuss cyclin-D1 and cell cycle control, DNA damage response, the B-cell receptor (BCR) and NF-κB pathways, tumor-microenvironment interactions, and finally BCL-2 and resistance to apoptosis (Fig. 1). These pathways may constitute the Achilles heel of MCL offering the prospect of targeted treatment approaches.
Fig. 1. Key pathways for targeted intervention in MCL.
BCR engagement induces a cascade of phosphorylation/activation of the pathway kinases (e.g. SYK, PI3Kδ, and BTK), leading to activation of the canonical NFκB pathway. Loss of function mutations in BIRC3 (encodes cIAP2) and TRAF2 release NIK from the inhibition, which leads to non-canonical NFκB pathway activation. Upon activation by apoptotic stimuli, the BH3-only proteins (e.g. BIM and BAD) inhibit the anti-apoptotic BCL-2 family members (e.g. BCL-2 and BCL-XL), thus releasing the pro-apoptotic members BAX and BAK and inducing apoptosis through caspase activation. Cyclin-D1 dimerizes with CDK4 and CDK6 to phosphorylate the Rb protein, thus releasing the transcription factor E2F and promoting cell cycle entry. Upon DNA damage, ATM activates P53 to initiate DNA repair, cell cycle inhibition, or even cell death. CHK1 and CHK2 kinases activate P53 while MDM2 and MDM4 E3-ubiquitin ligases inhibit P53. Illustration by authors.
Cyclin-D1
Cyclin-D1 over-expression plays a central role in MCL biology [2]. In addition to t(11;14)(q13;q32), cyclin-D1 levels may be increased due to deletions or point mutations in the 3’ untranslated region (3’UTR) that produce relatively shorter and more stable mRNAs [12, 13]. High cyclin-D1 mRNA levels correlate with increased tumor proliferation and inferior survival. Variable levels of cyclin-D2 and cyclin-D3 also reported in MCL cell lines, and cyclin-D1 knockdown resulted in minimal cytotoxicity possibly due to compensatory upregulation of cyclin-D2 [14]. Further, cyclin-D1-negative MCL exist that express the other D-type cyclins, cyclin-D2 or cyclin-D3 indicating that D-type cyclins may have redundant functions in MCL biology.
D-type cyclins dimerize with cyclin-dependent kinases CDK4 and CDK6 to phosphorylate retinoblastoma (Rb) proteins, thus promoting G1/S transition and cell cycle entry (Fig. 1). In addition, cyclin-D1/CDK4 complexes bind and titrate the cell cycle inhibitors p27 and p21 away from cyclin-E/CDK2 complexes, further enhancing cell cycle progression. Moreover, CDK4 and CDK6 activity in MCL can be enhanced through loss of their inhibitor, p16, secondary to CDKN2A (encodes p16) deletion or BMI-1 (transcriptional repressor of CDKN2A) amplification. Alvocidib (flavopiridol) is a pan-CDK inhibitor that also blocks RNA synthesis by inhibiting the CDK9/cyclin-T complex. The modest clinical activity of alvocidib has been linked to its complex pharmacokinetics [15]. Palbociclib (PD-0332991) is a reversible, specific inhibitor of CDK4 that is orally bioavailable and well tolerated in early studies. Seventeen relapsed MCL were treated in a phase I trial with 125 mg of palbociclib administered daily for 21 days of 28-day cycles [16]. Overall response rate (ORR) was 18% including one complete response (CR) (Table 1). Reduction in phospho-Rb and Ki67 was observed in most patients but did not predict response duration.
Table 1. Summary of recently published single agent targeted therapy in mantle cell lymphoma.
| Single agent | Target | Design | N | ORR (%) | CR (%) | mPFS (months) | mDOR (months) | mOS | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Palbociclib (PD-0332991) | CDK4/CDK6 | Phase I | 17 | 18 | 6 | N/A | N/A | N/A | Leonard, 2012 [16] |
| Idelalisib (GS-1101) | PI3Kδ | Phase I | 40 | 40 | 5 | 3.7 | 2.7 | N/A | Spurgeon, 2013 [17] |
| Everolimus (RAD-001) | mTOR | Phase II | 35 | 20 | 5.7 | 5.5 | N/A | N/A | Renner, 2012 [18] |
| Ibrutinib (PCI-32765) | BTK | Phase II | 111 | 68 | 21 | 13.9 | 17.5 | Not reached | Wang, 2013 [19] |
| ABT-199 (GDC-0199) | BCL-2 | Phase I | 32 (MCL, 8) | 53 (MCL, 100) | 6 (MCL, 0) | N/A | N/A | N/A | Davids, 2013 [20] |
| Alisertib (MLN-8237) | AAK | Phase II | 48 (MCL, 13) | 27 (MCL, 23) | 10 (MCL, 0) | N/A | N/A | N/A | Friedberg, 2014 [21] |
ORR, overall response rate; CR, complete remission; mPFS, median progression-free survival; mDOR, median duration of response; mOS, median overall survival; MCL, mantle cell lymphoma; BTK, Bruton’s tyrosine kinase; mTOR, mammalian target of rapamycin; BCL-2, B-cell lymphoma 2; CDK4/CDK6, cyclin-dependent kinase 4/6; PI3Kδ, Phosphatidylinositol 3-Kinase Delta; AAK, Aurora-A kinase; N/A, not available.
Palbociclib induces early G1 arrest (pG1), and withdrawal results in S phase entry. Thus sequenced administration of palbociclib could be used to synchronize tumor proliferation and sensitize tumor cells to pG1- or S-specific targeted/cytotoxic agents [22]. This strategy is currently being tested by combining palbociclib with bortezomib [23].
The role of BCR signaling and the tissue microenvironment in MCL
The tumor microenvironment plays an important role in many B-cell malignancies [24, 25]. Similar to CLL, MCL typically involves blood, bone marrow, spleen and lymph nodes. In addition, MCL shows a propensity to involve gastrointestinal, oropharyngeal, and pulmonary sites as well as the skin. Interestingly, the primary site of disease presentation has some prognostic information, suggesting that different tissue tropism correlates with differences in disease biology [26]. A dependence on pro-survival factors in the tissue is supported by experiments demonstrating that co-culture of MCL cells with stromal cells extends their survival in vitro and can protect them from the cytotoxic effects of chemotherapy [27]. In addition, we recently showed that MCL cells in the lymph node show activation of the BCR and NF-κB pathways and are more proliferative than their counterparts in the peripheral blood [28].
MCL is often considered a transformation of pre-germinal center B-cells that are antigen-naïve. However, analysis of the clonal IGHV gene suggests that most MCL cells have undergone some antigen selection. Further, a subset of MCL shows evidence of early plasmacytic differentiation [29]. The IGHV gene encodes the immunoglobulin heavy chain variable region that is responsible in part for binding to antigen. In a recent study of 807 MCL samples, Hadzidimitriou and colleagues showed that the IGHV repertoire is biased toward frequent usage of IGHV3 and IGHV4 subsets [30]. Moreover, up to 40% of tumors expressed IGHV genes with somatic hypermutation, a characteristic of post-germinal B-cells indicating antigen selection. Further, it is not uncommon to encounter different MCL patients with virtually identical BCRs, so called stereotyped BCRs, suggesting exposure to the same antigen [30]. Akin to CLL, MCL tumors expressing mutated IGHV genes tend to have a somewhat more indolent course, albeit with less pronounced differences in outcome than what is seen in CLL [4]. These data suggest that in MCL as in CLL antigen selection plays an important role in disease biology [25]. This point is underscored by the observation that the BCR on MCL cells is activated in vivo and this activation, as previously shown for CLL [31], is primarily located to the lymph node [28].
The BCR comprises a transmembrane immunoglobulin (Ig) with an extracellular antigen binding domain, and an intracellular signaling domain linked to two co-receptors, Igα (CD79A) and Igβ (CD79B). Upon antigen binding, the BCR signals through a series of kinases, including LYN, SYK, PI3Kδ, and BTK among others (Fig. 1). A recent study reported that primary un-manipulated MCL cells have higher levels of p-NFκB and p-AKT compared to normal B-cells, and phosphorylation of SYK and PLCγ in response to BCR activation was more pronounced compared to normal B-cells and other NHL. Further, BCR-induced levels of p-SYK correlated with the degree of CD79B expression [32].
Among the different BCR pathway kinases, SYK, PI3Kδ, and BTK are of particular importance since they are essential for BCR signaling and normal B-cell development. SYK is sometimes amplified in MCL leading to SYK mRNA and protein overexpression [2]. PI3Kδ is the most abundant PI3K isoform expressed in MCL. However, PI3Kα and PI3Kγ isoforms are also expressed, and PI3Kα is often amplified. PI3K downstream of SYK is responsible for phosphorylating AKT and mTOR. Increased PI3K/AKT/mTOR signaling can be achieved through enhanced SYK phosphorylation or loss of the PTEN phosphatase [33]. Loss of function mutations in BTK result in X-linked agammaglobulinemia, an immunodeficiency syndrome characterized by the absence of mature B-cells. BTK is also expressed in neutrophils, monocytes, macrophages, and platelets, but not in T-lymphocytes or plasma cells; its role in these cells remains unclear. BTK is required for BCR-mediated survival signals, and inhibition of BTK can induce apoptosis of tumor cells from several B-cell malignancies including MCL, CLL, and diffuse large B-cell lymphoma [34-36]. Moreover, BTK is overexpressed in MCL when compared to normal B-cells [35], a finding shared with CLL. Notably, both CLL and MCL show high clinical response rates to the BTK inhibitor ibrutinib [19, 37-39].
Inhibitors of PI3Kδ (idelalisib, ORR 40%) and mTOR (temsirolimus and everolimus) also showed clinical efficacy against MCL. However, responses were mostly partial and short-lived (Table 1) [17, 18, 40]. Similarly, fostamatinib an orally bioavailable inhibitor of SYK, had only limited activity in MCL [41]. The observed modest activity of idelalisib may be secondary to loss of PTEN, or redundancy in the pathway mediated by other expressed PI3K isoforms. Increased levels of PI3Kα was observed in resistant primary MCL cells, and at relapse compared to baseline [42]. Pan PI3K inhibitors (BKM120, XL147, BAY 80-6946, and GDC-0941) and the dual PI3Kγ/δ inhibitor (IPI-145) recently entered clinical testing in patients with hematological malignancies [43, 44]. So it will be interesting to see whether these drugs lead to more sustained and durable responses.
Ibrutinib, a first-in-class, orally bioavailable, covalent inhibitor of BTK, has recently shown striking clinical activity in many relapsed/refractory B-cell NHL [37, 39]. Ibrutinib binds covalently to Cys-481 on BTK and thereby irreversibly inactivates the kinase. A limited number of kinases possess a comparable cysteine residue, which confers considerable selectivity to ibrutinib. In a recent phase II trial, Wang and colleagues treated 111 relapsed/refractory MCL patients with 560 mg of ibrutinib once daily. Ibrutinib was overall well tolerated. ORR was 68% (CR, 21%), median progression-free survival was 13.9 months, and overall survival was 58% at 18 months (Table 1) [19]. Based on these data, the FDA granted accelerated approval to ibrutinib for relapsed/refractory MCL in November 2013. However, 32% of patients did not respond to ibrutinib, and 50% progressed within 14 months of therapy, respectively suggesting both intrinsic and acquired mechanisms of resistance. Rahal et al. reported on intrinsic resistance to ibrutinib in 6 of 10 MCL cell lines [45]. BCR pathway target genes and CARD11 were overexpressed in sensitive cells, and treatment with ibrutinib resulted in downregulation of NFκB target genes in sensitive cells only. NIK-dependent non-canonical NFκB pathway activation was reported in resistant cells, consistent with increased levels of RelB and p52. Moreover, targeted sequencing of 165 primary MCL samples revealed two mutually exclusive loss-of-function mutations, one in BIRC3 (10%) and the other in TRAF2 (6%). Both genes are known NIK inhibitors whose mutations lead to NIK-NFκB activation (Fig. 1) [45]. Taken together, this may explain at least in part the heterogeneity of patients’ response to ibrutinib, and suggest that targeting NIK may represent an alternative approach to treating MCL that are resistant to BCR pathway targeting.
BCL-2
The apoptosis regulator BCL-2 (B-cell lymphoma 2) family of proteins consists of anti-apoptotic members (e.g. BCL-2, MCL-1, BCL-W, and BCL-XL) and pro-apoptotic members (e.g. BAX, BAK, BIM, and BAD) (Fig. 1). Upregulation of BCL-2, MCL-1, and BCL-XL, and inactivation of BIM through genomic deletion, has been described in MCL [1]. Targeting pro-survival members of the BCL-2 family represents a promising approach to restore the apoptotic balance. Navitoclax (ABT-263) is an orally bioavailable molecule that targets both BCL-2 and BCL-XL. Navitoclax has shown encouraging results in early-phase clinical trials; however, it was associated with a dose limiting, concentration-dependent, rapid and profound thrombocytopenia, secondary to BCL-XL inhibition [46] [47]. This prompted an effort to reengineer into a BCL-2 selective molecule that spares BCL-XL [48]. The resultant ABT-199 (GDC-0199) is an orally bioavailable, first-in-class BCL-2 selective inhibitor. The high selectivity of ABT-199 for BCL-2 allows higher, more effective drug concentrations without thrombocytopenia [48, 49]. Davids and colleagues treated a cohort of relapsed/refractory NHL with single agent ABT-199 in a phase I, dose-escalation trial. 32 patients were enrolled, 8 of which had MCL [20]. The drug was overall well tolerated, and only two patients discontinued the drug due to G3/4 neutropenia. G3/4 thrombocytopenia occurred in 13% of patients, but was not dose-dependent or dose-limiting. All MCL patients responded to therapy (ORR 100%) across the different dosing regimens. All responders had partial response (PR) (Table 1). Dose escalation is still ongoing to determine the maximum tolerated dose for phase II. Touzeau et al. showed that not all MCL cell lines are sensitive to ABT-199, and that ME support through CD40 signaling can protect MCL cells against ABT-199 [50]. Thus, to unlock the full potential of ABT-199 it may be necessary to combine it with agents that can inhibit the prosurvival effects of the tissue microenvironment, such as ibrutinib or PI3K inhibitors [51, 52].
DNA damage response
In addition to cyclin-D1 overexpression the high degree of genomic instability represents a classic feature of MCL. These frequent recurrent genetic aberrations include losses, gains and copy number amplifications [1, 2]. Interestingly, MCL does not appear to carry a higher burden of somatic mutations than other B-cell malignancies [3, 53]. The reason remains unclear, but may be in part explained by increased stability of the DNA replication factor, CDT1, secondary to increased cyclin-D1/CDK4 activity, resulting in aberrant re-initiation of DNA replication during S phase [2].
The tumor suppressor ATM is the most commonly mutated gene in MCL, and also may be lost through the recurrent 11q22-23 deletion. ATM plays a critical role in DNA repair in response to double-strand breaks (DSB). Lymphoid cells need to undergo ordered double strand breaks to allow Ig rearrangements. Starczynski et al. characterized different NHL subtypes and B cells at different stages of differentiation for ATM expression [54]. They found high levels of ATM in B cells of the mantle zone and in plasma cells, but low levels in germinal center B cells and in immature B cells of the bone marrow. ATM levels in NHL subtypes mirror their cells of origin; low ATM levels were observed in most follicular lymphoma and diffuse large B-cell lymphoma, and ATM deletion or inactivating mutations were absent. In contrast, tumors originating from pre- and post-germinal center B-cells showed high ATM expression in the absence of ATM inactivating lesions, but these tumors displayed an increasing frequency of ATM inactivating mutations or deletion. Indeed, MCL has a higher frequency of ATM alterations compared to other NHL. This pressure to loose ATM in MCL may derive from the high ATM level in the cell of origin, and can be further enhanced by the high frequency of DSBs due to inappropriate re-initiation of DNA replication.
ATM functions in the DNA damage response pathway by activating P53 (Fig. 1). In turn, P53 may be inactivated by 17p deletion and TP53 mutations [3]. P53 function can also be affected by loss of CDKN2A locus that encodes the P53 stabilizer ARF, overexpression of the P53 inhibitors MDM2 and MDM4, and downregulation of the P53 activators CHK1 and CHK2. Further, UBR5, an E3 ubiquitin ligase required for effective CHK2 phosphorylation and subsequent P53 activation, is mutated in 18% of MCL tumors [55]. UBR5 also plays a critical role in the response to DNA double-strand breaks [56]. Interestingly, UBR5 and CCND1 mutations were reported to be mutually exclusive in a cohort of 102 patients [54], while other mutations often co-exist in the same tumor sample.
The degree of genomic instability and the high frequency of ATM and TP53 alterations may constitute an Achilles heel that can be exploited for “synthetic lethal” therapeutic approaches. Two genes are synthetic lethal if mutation of either alone is compatible with viability but mutation of both leads to death [57]. Thus, in the context of drug therapy of a tumor carrying a mutated gene, cell death may be achieved by inhibiting a synthetic lethal gene. The extensive alterations in the DNA damage response found in MCL can lead to “mitotic catastrophe”, the destruction of the cell during mitosis secondary to extensive DNA damage and/or defects in chromosome segregation. The poly-ADP ribose polymerase (PARP) is a critical enzyme involved in DNA repair for which small molecule inhibitors have been developed. ATM deficiency sensitizes MCL cell lines in vitro and in vivo to PARP inhibition, and this sensitivity is enhanced in the presence of a concomitant P53 deficiency [58]. Combining PARP inhibitors with DNA damaging agents may further increase the therapeutic effect [59].
Another enzyme important for successful mitosis is Aurora-A kinase (AAK), a serine/threonine kinase essential for centrosome maturation and separation. AAK is overexpressed in MCL and linked to inferior outcomes. AAK inhibition results in G2/M arrest, accumulation of mitotic cells, polyploidy and subsequent cell death. Alisertib (MLN-8237) is an oral, selective, ATP-competitive reversible inhibitor of AAK. A phase II study of alisertib in 48 NHL reported an ORR of 23% in the MCL group (3/13), with one CR (Table 1). The most common G3/4 adverse events were hematologic [21]. Alisertib synergizes in-vitro and in-vivo with the microtubule inhibitor vincristine against MCL, and rituximab enhances this synergism [60]. The combination of the three drugs was found to be safe and effective in a phase I study of relapsed/refractory NHL [61].
Conclusion
Identifying the specific vulnerabilities of cancer cells, it is hoped, can be used to develop targeted therapies. In some cases disease specific, genetic driver events have led the way. One of the most prominent and successful examples is chronic myelogenous leukemia. In contrast, MCL is a more heterogeneous disease, and a clear oncogenic driver remains elusive. The recent approval of ibrutinib for relapsed MCL constitutes an important milestone that in large part appears to have arisen from systematic clinical investigation. A number of new targeted drugs have entered clinical trials. Moving forward, incorporating the increasingly powerful genomic tools into clinical investigation may reveal the Achilles heel of MCL and will guide the most effective use of these agents.
Key Points.
The BCR signaling pathway and microenvironment support play an important role in MCL biology.
The BTK inhibitor ibrutinib induces high and durable responses in previously treated MCL (ORR 68%, mPFS 13.9 months), and is now FDA approved for this indication.
Activation of the non-canonical NIK-NFκB pathway in MCL represents a possible mechanism of resistance to ibrutinib.
Selective PI3Kδ inhibition induces short-lived responses possibly because of compensatory upregulation of other PI3K isoforms.
The BCL-2 selective inhibitor ABT-199 is highly active, and spares BCL-XL resulting in less thrombocytopenia.
The high degree of genomic instability makes MCL vulnerable to “synthetic lethal” approaches through inhibition of DNA repair mechanisms.
Acknowledgments
The authors are supported by the intramural research program of the Heart, Lung, and Blood Institute, National Institutes of Health.
Footnotes
Disclosures:
Nothing to disclose
References
- 1.Jares P, Colomer D, Campo E. Molecular pathogenesis of mantle cell lymphoma. J Clin Invest. 2012;122:3416–3423. doi: 10.1172/JCI61272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117:26–38. doi: 10.1182/blood-2010-04-189977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3**.Bea S, Valdes-Mas R, Navarro A, et al. Landscape of somatic mutations and clonal evolution in mantle cell lymphoma. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1314608110. This manuscript highlights the degree of MCL heterogeneity at the genomic level. Using whole genome and/or exome sequencing, they identify new recurrent muations in MCL at the clonal or subclonal level. They also link some of the newly descibed muations to tumor behavior, such as NOTCH1/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navarro A, Clot G, Royo C, et al. Molecular subsets of mantle cell lymphoma defined by the IGHV mutational status and SOX11 expression have distinct biological and clinical features. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-12-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nygren L, Baumgartner Wennerholm S, Klimkowska M, et al. Prognostic role of SOX11 in a population-based cohort of mantle cell lymphoma. Blood. 2012;119:4215–4223. doi: 10.1182/blood-2011-12-400580. [DOI] [PubMed] [Google Scholar]
- 6**.Vegliante MC, Palomero J, Pérez-Galán P, et al. SOX11 regulates PAX5 expression and blocks terminal B-cell differentiation in aggressive mantle cell lymphoma. Blood. 2013;121:2175–2185. doi: 10.1182/blood-2012-06-438937. First manuscript showing that SOX11 plays an oncogenic role in MCL. By combining gene expression profiling with chromatin immunoprecipitation microarray analysis, they showed that SOX11 silencing shifts MCL cells toward plasmacytic differentiation away from the mature B-cell program. They also showed that SOX11 induces PAX5 upregulation, and subsequently BLIMP1 suppression. [DOI] [PubMed] [Google Scholar]
- 7.Vose JM. Mantle cell lymphoma: 2013 Update on diagnosis, risk-stratification, and clinical management. Am J Hematol. 2013;88:1082–1088. doi: 10.1002/ajh.23615. [DOI] [PubMed] [Google Scholar]
- 8.Rivera-Rodriguez N, Cabanillas F. Recent advances in the management of mantle cell lymphoma. Curr Opin Oncol. 2013;25:716–721. doi: 10.1097/CCO.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 9.Smith MR. Is Early Hematopoietic Stem-Cell Transplantation Necessary in Mantle-Cell Lymphoma? J Clin Oncol. 2013 doi: 10.1200/JCO.2013.53.2762. [DOI] [PubMed] [Google Scholar]
- 10.Budde LE, Guthrie KA, Till BG, et al. Mantle cell lymphoma international prognostic index but not pretransplantation induction regimen predicts survival for patients with mantle-cell lymphoma receiving high-dose therapy and autologous stem-cell transplantation. J Clin Oncol. 2011;29:3023–3029. doi: 10.1200/JCO.2010.33.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li ZM, Zucca E, Ghielmini M. Open questions in the management of mantle cell lymphoma. Cancer Treat Rev. 2013;39:602–609. doi: 10.1016/j.ctrv.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Wiestner A, Tehrani M, Chiorazzi M, et al. Point mutations and genomic deletions in CCND1 create stable truncated cyclin D1 mRNAs that are associated with increased proliferation rate and shorter survival. Blood. 2007;109:4599–4606. doi: 10.1182/blood-2006-08-039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slotta-Huspenina J, Koch I, de Leval L, et al. The impact of cyclin D1 mRNA isoforms, morphology and p53 in mantle cell lymphoma: p53 alterations and blastoid morphology are strong predictors of a high proliferation index. Haematologica. 2012;97:1422–1430. doi: 10.3324/haematol.2011.055715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klier M, Anastasov N, Hermann A, et al. Specific lentiviral shRNA-mediated knockdown of cyclin D1 in mantle cell lymphoma has minimal effects on cell survival and reveals a regulatory circuit with cyclin D2. Leukemia. 2008;22:2097–2105. doi: 10.1038/leu.2008.213. [DOI] [PubMed] [Google Scholar]
- 15.Jones JA, Rupert AS, Poi M, et al. Flavopiridol can be safely administered using a pharmacologically derived schedule and demonstrates activity in relapsed and refractory non- Hodgkin’s lymphoma. Am J Hematol. 2014;89:19–24. doi: 10.1002/ajh.23568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leonard JP, LaCasce AS, Smith MR, et al. Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma. Blood. 2012;119:4597–4607. doi: 10.1182/blood-2011-10-388298. [DOI] [PubMed] [Google Scholar]
- 17.Spurgeon SEF, Wagner-Johnston ND, Furman RR, et al. Final results of a phase I study of idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase P110{delta} (PI3K{delta}), in patients with relapsed or refractory mantle cell lymphoma (MCL). ASCO Meeting Abstracts; 2013. p. 8519. [Google Scholar]
- 18.Renner C, Zinzani PL, Gressin R, et al. A multicenter phase II trial (SAKK 36/06) of singleagent everolimus (RAD001) in patients with relapsed or refractory mantle cell lymphoma. Haematologica. 2012;97:1085–1091. doi: 10.3324/haematol.2011.053173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–516. doi: 10.1056/NEJMoa1306220. This phase II trial of single agent ibrutinib in relapsed/refractory MCL supported the accelerated FDA approval of ibrutinib. Wang and colleagues used a daily dose of 560 mg of ibrutinib, and reported an ORR of 68% and mPFS of 13.9 months. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davids MS, Seymour JF, Gerecitano JF, et al. The Single-Agent Bcl-2 Inhibitor ABT-199 (GDC-0199) In Patients With Relapsed/Refractory (R/R) Non-Hodgkin Lymphoma (NHL): Responses Observed In All Mantle Cell Lymphoma (MCL) Patients. Blood. 2013;122:1789. [Google Scholar]
- 21.Friedberg JW, Mahadevan D, Cebula E, et al. Phase II Study of Alisertib, a Selective Aurora A Kinase Inhibitor, in Relapsed and Refractory Aggressive B- and T-Cell Non-Hodgkin Lymphomas. J Clin Oncol. 2014;32:44–50. doi: 10.1200/JCO.2012.46.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.Chiron D, Martin P, Di Liberto M, et al. Induction of prolonged early G1 arrest by CDK4/CDK6 inhibition reprograms lymphoma cells for durable PI3Kdelta inhibition through PIK3IP1. Cell Cycle. 2013;12:1892–1900. doi: 10.4161/cc.24928. This manuscript shows an in-vitro evidence that palbociclib-induced early G1 arrest (through CDK4/6 inhibition) sensitizes MCL cells to the PI3Kδ inhibitor idelalisib. This may be a good combination to be tested in a clinical trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin P, DiLiberto M, Mason CE, et al. The Combination Of Palbociclib Plus Bortezomib Is Safe and Active In Patients With Previously Treated Mantle Cell Lymphoma: Final Results Of a Phase I Trial. Blood. 2013;122:4393. [Google Scholar]
- 24.Burger JA, Gribben JG. The microenvironment in chronic lymphocytic leukemia (CLL) and other B cell malignancies: Insight into disease biology and new targeted therapies. Semin Cancer Biol. 2014;24:71–81. doi: 10.1016/j.semcancer.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Herishanu Y, Katz BZ, Lipsky A, Wiestner A. Biology of chronic lymphocytic leukemia in different microenvironments: clinical and therapeutic implications. Hematol Oncol Clin North Am. 2013;27:173–206. doi: 10.1016/j.hoc.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ambinder AJ, Shenoy PJ, Nastoupil LJ, Flowers CR. Using primary site as a predictor of survival in mantle cell lymphoma. Cancer. 2013;119:1570–1577. doi: 10.1002/cncr.27898. [DOI] [PubMed] [Google Scholar]
- 27.Xargay-Torrent S, Lopez-Guerra M, Montraveta A, et al. Sorafenib inhibits cell migration and stroma-mediated bortezomib resistance by interfering B-cell receptor signaling and protein translation in mantle cell lymphoma. Clin Cancer Res. 2013;19:586–597. doi: 10.1158/1078-0432.CCR-12-1935. [DOI] [PubMed] [Google Scholar]
- 28.Saba NS, Liu D, Herman SEM, et al. Gene Expression Profiling Reveals The Lymph Node Microenvironment As a Niche For BCR Engagement, NFκB Pathway Activation, and Tumor Proliferation In Mantle Cell Lymphoma. Blood. 2013;122:82. [Google Scholar]
- 29.Perez-Galan P, Mora-Jensen H, Weniger MA, et al. Bortezomib resistance in mantle cell lymphoma is associated with plasmacytic differentiation. Blood. 2011;117:542–552. doi: 10.1182/blood-2010-02-269514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hadzidimitriou A, Agathangelidis A, Darzentas N, et al. Is there a role for antigen selection in mantle cell lymphoma? Immunogenetic support from a series of 807 cases. Blood. 2011;118:3088–3095. doi: 10.1182/blood-2011-03-343434. [DOI] [PubMed] [Google Scholar]
- 31.Herishanu Y, Perez-Galan P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117:563–574. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myklebust JH, Brody J, Alizadeh AA, et al. Potentiated B-Cell Antigen Receptor Signaling In Mantle Cell Lymphoma Is Associated With Overexpression Of Surface CD79B and IgM. Blood. 2013;122:1768. [Google Scholar]
- 33.Niemann CU, Wiestner A. B-cell receptor signaling as a driver of lymphoma development and evolution. Semin Cancer Biol. 2013;23:410–421. doi: 10.1016/j.semcancer.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–6296. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cinar M, Hamedani F, Mo Z, et al. Bruton tyrosine kinase is commonly overexpressed in mantle cell lymphoma and its attenuation by Ibrutinib induces apoptosis. Leuk Res. 2013;37:1271–1277. doi: 10.1016/j.leukres.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 36.Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI- 32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. First in human, phase 1 trial, testing the safety and efficacy of ibrutinib in a variety of Bcell malignancies including MCL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiestner A. Targeting B-Cell receptor signaling for anticancer therapy: the Bruton’s tyrosine kinase inhibitor ibrutinib induces impressive responses in B-cell malignancies. J Clin Oncol. 2013;31:128–130. doi: 10.1200/JCO.2012.44.4281. [DOI] [PubMed] [Google Scholar]
- 40.Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27:3822–3829. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- 41.Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115:2578–2585. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Iyengar S, Clear A, Bödör C, et al. P110α-mediated constitutive PI3K signaling limits the efficacy of p110δ-selective inhibition in mantle cell lymphoma, particularly with multiple relapse. Blood. 2013;121:2274–2284. doi: 10.1182/blood-2012-10-460832. This manuscript shows evidence of possible mechanisms of resistance to idelalisib (PI3Kδ inhibitor) by highlighting the importance of PI3Kα in MCL biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horwitz SM, Flinn I, Patel MR, et al. Preliminary safety and efficacy of IPI-145, a potent inhibitor of phosphoinositide-3-kinase-{delta},{gamma}, in patients with relapsed/refractory lymphoma. ASCO Meeting Abstracts; 2013. p. 8518. [Google Scholar]
- 44.Jabbour E, Ottmann OG, Deininger M, Hochhaus A. Targeting the phosphoinositide 3- kinase pathway in hematologic malignancies. Haematologica. 2014;99:7–18. doi: 10.3324/haematol.2013.087171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45**.Rahal R, Frick M, Romero R, et al. Pharmacological and genomic profiling identifies NFkappaB- targeted treatment strategies for mantle cell lymphoma. Nat Med. 2014;20:87–92. doi: 10.1038/nm.3435. This study provides insights into the mechanism of MCL resistance to ibrutinib. Authors were able to identify a subset of MCL that is dependent on non-canonical NFκB signaling through NIK. Targeting this pathway may represent an alternative approach to treating ibrutinibresistant MCL. [DOI] [PubMed] [Google Scholar]
- 46.Wilson WH, O’Connor OA, Czuczman MS, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts AW, Seymour JF, Brown JR, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 49.Davids MS, Letai A. ABT-199: taking dead aim at BCL-2. Cancer Cell. 2013;23:139–141. doi: 10.1016/j.ccr.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Touzeau C, Brosseau C, Dousset C, et al. Mantle-Cell Lymphoma (MCL) Cells Are Highly Sensitive To ABT-199 But Their Sensitivity May Be Altered By The Microenvironment Via The Up-Regulation Of Bcl-Xl and Bcl2A1. Blood. 2013;122:4285. [Google Scholar]
- 51.Davids MS, Deng J, Wiestner A, et al. Decreased mitochondrial apoptotic priming underlies stroma-mediated treatment resistance in chronic lymphocytic leukemia. Blood. 2012;120:3501–3509. doi: 10.1182/blood-2012-02-414060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao X, Bodo J, Sun D, et al. Combination Of Ibrutinib With ABT-199, a BCL-2 Pathway Inhibitor: Effective Therapeutic Strategy In a Novel Mantle Cell Lymphoma Cell Line Model. Blood. 2013;122:645. [Google Scholar]
- 53.Villamor N, Lopez-Guillermo A, Lopez-Otin C, Campo E. Next-generation sequencing in chronic lymphocytic leukemia. Seminars in hematology. 2013;50:286–295. doi: 10.1053/j.seminhematol.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Starczynski J, Simmons W, Flavell JR, et al. Variations in ATM protein expression during normal lymphoid differentiation and among B-cell-derived neoplasias. Am J Pathol. 2003;163:423–32. doi: 10.1016/S0002-9440(10)63672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55**.Meissner B, Kridel R, Lim RS, et al. The E3 ubiquitin ligase UBR5 is recurrently mutated in mantle cell lymphoma. Blood. 2013;121:3161–3164. doi: 10.1182/blood-2013-01-478834. Identification of the recurrent UBR5 mutation in 18% of MCL. UBR5 may contribute to MCL biology through its involvement in the DNA damage response pathway. [DOI] [PubMed] [Google Scholar]
- 56.Gudjonsson T, Altmeyer M, Savic V, et al. TRIP12 and UBR5 suppress spreading of chromatin ubiquitylation at damaged chromosomes. Cell. 2012;150:697–709. doi: 10.1016/j.cell.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 57.Kaelin WG., Jr The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5:689–698. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- 58.Williamson CT, Kubota E, Hamill JD, et al. Enhanced cytotoxicity of PARP inhibition in mantle cell lymphoma harbouring mutations in both ATM and p53. EMBO Mol Med. 2012;4:515–527. doi: 10.1002/emmm.201200229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwartz GK, Carvajal R, Fury M, et al. ABT-888 Plus Bendamustine Is Safe and Has Shown Preliminary Efficacy In Patients With Lymphoma. Blood. 2013;122:4366. [Google Scholar]
- 60.Mahadevan D, Stejskal A, Cooke LS, et al. Aurora A inhibitor (MLN8237) plus vincristine plus rituximab is synthetic lethal and a potential curative therapy in aggressive B-cell non- Hodgkin lymphoma. Clin Cancer Res. 2012;18:2210–2219. doi: 10.1158/1078-0432.CCR-11-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kelly KR, Persky DO, Mahadevan D, et al. Phase 1 Study Of Investigational Agent MLN8237 (Alisertib) + Rituximab ± Vincristine In Patients (Pts) With Relapsed/Refractory (Rel/Ref) Aggressive B-Cell Lymphomas. Blood. 2013;122:3027. [Google Scholar]