Abstract
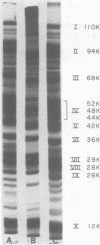
An antigenic complex has been isolated in a highly purified from from the Melvin strain of Neisseria gonorrhoeae. The complex has a molecular weight of 9.3 × 106 and on sodium dodecyl sulfate-polyacrylamide gel electrophoresis was found to consist of several subunits; the most predominant had the following molecular weights: 110,000, 94,000, 68,000, a smear containing (52,000, 48,000, and 44,000), 42,000, 36,000, 29,000, 28,000, 26,000, and 12,000 comprising 89% of the total protein. With the exception of the subunit of molecular weight 110,000, no change in the content or the mobility of other subunits was observed when β-mercaptoethanol was omitted from the denaturation solution of sodium dodecyl sulfate electrophoresis. Amino acid analysis of the complex showed a predominance of hydrophobic amino acids. These data implicated noncovalent interactions between the subunits. When the cells were labeled with fluorescamine it was possible to obtain a fluorescent complex with identical properties. Among several buffers used for the isolation of the complex, 0.2 M tris(hydroxymethyl)aminomethane buffer (pH 7.5) gave maximum yield with low amounts of lipopolysaccharide and phospholipid; the choice of the buffer for column chromatography did not seem to make any difference. The high protein content and low amounts of lipopolysaccharide and phospholipid are characteristic properties of the complex.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apicella M. A., Allen J. C. Isolation and characterization of the antigen of Neisseria gonorrhoeae. Infect Immun. 1973 Mar;7(3):315–321. doi: 10.1128/iai.7.3.315-321.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M. Antigenic heterogeneity of gonococcal pili. J Exp Med. 1975 Jun 1;141(6):1470–1475. doi: 10.1084/jem.141.6.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Arko R. J. Immunity to gonococcal infection induced by vaccination with isolated outer membranes of Neisseria gonorrhoeae in guinea pigs. J Infect Dis. 1977 Jun;135(6):879–887. doi: 10.1093/infdis/135.6.879. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M., Pearce W. A., Schoolnik G. K., Arko R. J. Protection against infection with Neisseria gonorrhoeae by immunization with outer membrane protein complex and purified pili. J Infect Dis. 1977 Aug;136 (Suppl):S132–S137. doi: 10.1093/infdis/136.supplement.s132. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M., Swanson J., Holmes K. K., Kraus S. J., Gotschlich E. C. Quantitative determination of antibody to gonococcal pili. Changes in antibody levels with gonococcal infection. J Clin Invest. 1973 Nov;52(11):2896–2909. doi: 10.1172/JCI107486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper W. A. The Serological Classification of Gonococci by Comparative Agglutination. J Bacteriol. 1937 Oct;34(4):353–379. doi: 10.1128/jb.34.4.353-379.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Frantz I. D. Growth Requirements of the Meningococcus. J Bacteriol. 1942 Jun;43(6):757–761. doi: 10.1128/jb.43.6.757-761.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckels J. E. The surface of Neisseria gonorrhoeae: isolation of the major components of the outer membrane. J Gen Microbiol. 1977 Apr;99(2):333–341. doi: 10.1099/00221287-99-2-333. [DOI] [PubMed] [Google Scholar]
- Johnston K. H., Gotschlich E. C. Isolation and characterization of the outer membrane of Neisseria gonorrhoeae. J Bacteriol. 1974 Jul;119(1):250–257. doi: 10.1128/jb.119.1.250-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Holmes K. K., Gotschlich E. C. The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotypic specificity. J Exp Med. 1976 Apr 1;143(4):741–758. doi: 10.1084/jem.143.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y. D., Carlo D. J., Zeltner J. A simplified procedure to determine tryptophan residues in proteins. Anal Biochem. 1975 Nov;69(1):55–60. doi: 10.1016/0003-2697(75)90565-5. [DOI] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maeland J. A. Antigenic determinants of aqueous ether extracted endotoxin from Neisseria gonorrhoeae. Acta Pathol Microbiol Scand. 1969;76(3):475–483. doi: 10.1111/j.1699-0463.1969.tb03277.x. [DOI] [PubMed] [Google Scholar]
- Maeland J. A. Immunochemical characterization of aqueous ether extracted endotoxin from Neisseria gonorrhoeae. Acta Pathol Microbiol Scand. 1969;76(3):484–492. doi: 10.1111/j.1699-0463.1969.tb03278.x. [DOI] [PubMed] [Google Scholar]
- Maeland J. A., Kristoffersen T., Hofstad T. Immunochemical investigations on neisseria gonorrhoeae endotoxin. 2. Serological multispecificity and other properties of phenol-water preparations. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(2):233–238. [PubMed] [Google Scholar]
- Maeland J. A., Kristoffersen T. Immunochemical investigations on Neisseria gonorrhoeae endotoxin. 1. Characterization of phenol-water extracted endotoxin and comparison with aqueous ether preparations. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(2):226–232. [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. II. Prog Allergy. 1962;6:30–154. doi: 10.1159/000313795. [DOI] [PubMed] [Google Scholar]
- Perry M. B., Daoust V. The lipopolysaccharides of Neisseria gonorrhoeae colony types 1 and 4. Can J Biochem. 1975 May;53(5):623–629. doi: 10.1139/o75-084. [DOI] [PubMed] [Google Scholar]
- Stokinger H. E., Ackerman H., Carpenter C. M. Studies on the gonococcus: II. Properties of an antigenic fraction isolated from cell-free gonococcal broth supernatants. J Bacteriol. 1944 Feb;47(2):141–147. doi: 10.1128/jb.47.2.141-147.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu S., Grosso L. Fluorescent labeling of proteins in sodium dodecyl sulfate complexes with fluorescamine. Biochem Biophys Res Commun. 1976 Sep 7;72(1):9–14. doi: 10.1016/0006-291x(76)90953-0. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]