Abstract
Genetic regulators and signaling pathways are important for the formation of blood vessels. Transcription factors controlling vein identity, intersegmental vessels (ISV) growth and caudal vein plexus (CVP) formation in zebrafish are little understood as yet. Here, we show the importance of the nuclear receptor subfamily member 1A (nr2f1a) in zebrafish vascular development. Amino acid sequence alignment and phylogenetic analysis of nr2f1a is highly conserved among the vertebrates. Our in situ hybridization results showed nr2f1a mRNA is expressed in the lateral plate mesoderm at 18 somite stage and in vessels at 24–30 hpf, suggesting its roles in vasculization. Consistent with this morpholino-based knockdown of nr2fla impaired ISV growth and failed to develop fenestrated vascular structure in CVP, suggesting that nr2f1a has important roles in controlling ISV and CVP growth. Consequently, nr2f1a morphants showed pericardial edema and circulation defects. We further demonstrated reduced ISV cells and decreased CVP endothelial cells sprouting in nr2f1a morphants, indicating the growth impairment of ISV and CVP is due to a decrease of cell proliferation and migration, but not results from cell death in endothelial cells after morpholino knockdown. To test molecular mechanisms and signals that are associated with nr2f1a, we examined the expression of vascular markers. We found that a loss of nr2f1a results in a decreased expression of vein/ISV specific markers, flt4, mrc1, vascular markers stabilin and ephrinb2. This indicates the regulatory role of nr2f1a in controlling vascular development. We further showed that nr2f1a likely interact with Notch signaling by examining nr2f1a expression in rbpsuh morphants and DAPT-treatment embryos. Together, we show nr2f1a plays a critical role for vascular development in zebrafish.
Introduction
Vertebrate blood vessels are critical for nutrient and oxygen supply and are developed in an evolutionarily conserved manner. The arteries and veins are formed from angioblast progenitors during vasculogenesis. After the establishment of these vessels, additional vessels develop from pre-existing vessels by angiogenesis. This process includes sprouting, migration, lumenization, branching and fusion to form a coordinated pattern of the vessel plexus, which is tightly controlled by growth factors, regulators and multiple signaling pathways. Ultimately, transcription factors play a central role in the regulation of the vascular development after receiving signals [1]–[5].
Zebrafish have been shown as a suitable vertebrate model to study developmental processes – so in vascular development [6]. In zebrafish, the dorsal aorta (DA) and posterior cardinal vein (PCV) form a primitive circulatory loop during vasculogenesis, then sprouting angiogenesis from the DA to form intersegmental vessels (ISVs) and from the axial vein to develop a honeycomb-like network called caudal vein plexus (CVP) via distinct signalling pathways. Specifically, the development of the ISV of the trunk begins with an angioblast sprouting from the DA and dorsad migration along the somite boundaries towards the midline where it undergoes a cell division. One daughter cell continues to migrate dorsally until it reaches the dorsal aspect of the embryo and fuses with angioblasts from anterior and posterior ISVs to form the dorsal longitudinal anastomotic vessels (DLAVs). The leading cells to migrate from the vessel are called tip cells. These are proliferative and show multiple filopodia, while the less proliferative, stationary cells which lumenize behind the tip cell are called stalk cells [7]. This process is largely controlled by VEGF and Notch signalling. However, Wiley and co-workers showed recently that BMP signalling pathways regulate sprouting angiogenesis from the axial vein to form CVP, which provides a distinct mechanism of angiogenic sprouting [8]. This processing is mediated by Dab2 to promote internalization of the BMP2 signaling complex and modulates SMAD phosphorylation [9]. In spite of this, knowledge related to molecules that are important for CVP angiogenesis is still limited. A complex coordination among these signaling pathways have a critical role in controlling endothelial tip cell fates during angiogenesis. Concerning the responsible factors, the orphan nuclear receptor chicken ovalbumin upstream promoter transcription factor II (CoupTFII; a.k.a. nr2f2) has been shown function in angiogenesis mediated upregulation of Angiopoietin-1 in mice [10], [11]. However, there is still limited understanding about the transcription factors that control the tip cell fate. In addition, the angiogenic function of nr2f2 is not very clear in zebrafish based on our study and recent discoveries [12], [13].
Studies of mNR2F (CoupTF) family have been demonstrated their physiological and pathologic importance. mNr2f2 have been identified their function in cell-fate specification, organogenesis, angiogenesis, lymphagenesis, metabolism and a variety of diseases [14]. In the field of mouse vascular development, mNr2f2 is a major determinant of venous identity and involved in angiogenesis [11]. In addition, mNr2f2 physically and functionally interact with Prox1 to specific lymphatic endothelial fate and promote the formation of lymphatic vasculature [15]. Our unpublished data also showed that nr2f2 in zebrafish plays a minor role in venous identity and functions critically in lymphangiogenesis, which is similar to recent reports [12], [13]. On the other hand, mammalian Nr2f1 has been showed as a critical regulator of CNS and peripheral nervous system development and controls cell differentiation in the inner ear [16], [17]. However, there is no document shown that mNr2f1 functions in vasculature.
In this study, we hypothesize the conserved NR2F family has an important function in zebrafish blood vessel formation. We showed nr2f1a mRNA is expressed in developing vessels, suggesting its roles in vasculature. We also showed loss of nr2f1a impairs ISV growth and CVP patterning. The impairment of vascular development results in pericardial edema and circulation defects at later developmental stage. We further demonstrated loss of nr2f1a results in a decreased expression of vascular specific markers, flt4, mrc1, ephrinb2 and stabilin, suggested the regulatory role of nr2f1a in controlling vascular development. Finally, we showed that nr2f1a likely interact with the Notch signalling. Together, we show nr2f1a plays a critical role for vascular development in zebrafish.
Materials and Methods
Zebrafish strains and husbandry
Zebrafish (Danio rerio) wild-type (AB) embryos and transgenic lines: Tg(kdrl:eGFP)la116, Tg(gata:dsRed) and Tg(fli1a:negfp)y7 [18] from Taiwan Zebrafish Core Facility at Academia Sinica or NTHU-NHRI were raised and maintained at the 28.5°C incubator according to The Zebrafish Book [19] with approval from the National Sun Yat-sen University Animal Care Committee. Embryos were treated with 0.003% 1-Phenyl-2-Thiourea (PTU) (Sigma, St. Louis, MO) at six hours post fertilization (hpf) to prevent pigment formation.
Morpholino and Tol2 DNA construct injections
Morpholinos for each gene were obtained from Gene-Tools, LLC (Philomath, OR), resuspended in ddH2O to 2 mM stock and further diluted to the working concentration with 0.5% phenol red (Sigma). To knockdown nr2f1a gene expression, we used nr2f1a-ATGMO (5′-CCAGACGCTAACTACCATTGCCATA-3′) to block translation and nr2f1a-e2i2MO (5′-GCCTCGTCTCACACACTCACCTGAC-3′) targeting the boundary of exon 2 and inton 2 to interfere with its splicing. Other morpholinos used in the paper are Rbpsuh ORFMO 5′- CAAACTTCCCTGTCACAACAGGCGC-3′ [2]. Microinjections were carried out according to Larmont et al [20]. Briefly, embryos were immobilized in an injection tray with 3% agar plate. MOs or expression vectors were injected into 1-2-cell-stage embryos. After injection, embryos were cultured in E3 buffer until being examined. To generate fli1:nr2f1a overexpressing embryos, we used the Tol2kit vectors with multisite Gateway cloning system (Invitrogen) to build expression constructs [21]. nr2f1a coding region franking with attb1/b2 sequences was amplified from cDNA using primers nr2f1a-f1-attb1: 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTCCACCATGGCAATGGTAGTTAGCGTC-3′ and nr2f1a-r1-attb2:
5′-GGGGACCACTTTGTACAAGAAAGCTGGGTTTCATTGAATGGACATGTAGGG-3′. A Gateway compatible 0.8 kb fli1 promoter has been described [30] Approximately 100 pg of plasmid DNA was co-injected with 40 pg of Tol2 mRNA into 1-cell embryos. Success of transgenesis can be verified in Tg(fli1:nr2f1a) transient transgenic, overexpressing embryos by expression of cmlc (cardiomyocyte light chain) driven GFP from the vector backbone.
Morpholino efficiency
The efficiency of morpholinos that cause mis-splicing was determined using reverse-transcriptase polymerase chain reaction (RT-PCR) with primers spanning either side of the targeted exon. RNAs from both injected and uninjected control were collected embryos using RNeasy mini kit (Qiagen). Single strand cDNA was synthesized with Re-Transcription kit (Roche) with reverse transcriptase and oligo-dT primer according to the manufacturer’s instructions. For nr2f1a MO efficiency and specificity test, primers nr2f1a_MO_f 5′-CTGCCTATTGACCAACACC-3′ and nr2f1a_MO_r 5′-CTCTCGATGTGTGCAGCATC-3′ were used, while for GAPDH (a loading control), primers GAPDH_F 5′- TGCTGTAACCGAACTCATTGTC-3′ and GAPDH_R 5′- CAAGCTTACTGGTATGGCCTTC-3′ were used. The results were visualized by running 5 µl of the PCR reaction on 1.0% agarose gel.
Whole-mount in situ hybridization
Whole-mount in situ hybridization was performed as described in [22], [23]. Probes for nr2f1a was obtained by PCR using primers described in Table S1 in File S1 and in vitro transcription using T7 Polymerase (Roche) with DIG-labeled UTP. The flt4, mrc1, stabilin, and ephrinb2a probes have been described [2], [24]. Embryos will be fixed using 4% paraformaldehyde and stored at −20°C in methanol for up to 6 months. After rehydration, permeabilization in 10 µg/mL Proteinase K, the probe against target mRNA will be added to embryos in hybridization buffer (Hyb) at 65°C overnight, then washed by 75%Hyb, 50%Hyb, 25%Hyb in 2X sodium citrate buffer (SSC) and 0.2X SSC for 1 hour. After blocking with 1%BSA in maleic acid buffer, an AP-conjugated anti-Dig antibody will be added and proceeded to react with NBT/BCIP substrate (Roche). The reaction was stopped by fixing for 15 minutes in 4% PFA, followed by two washes in PBT. Embryos were embedded in 3% methylcellulose and photographed.
Image and quantification
Whole mount embryos were mounted in 3% methyl cellulose (Sigma) or 1.5% low melt agarose (Invitrogen). White light or fluorescent images were photographed on a Zeiss Lumar V12 stereomicroscope equipped with an AxioCam HRc camera and AxioVision software (Carl Zeiss). Final figures were made using Adobe Photoshop. Confocal images were collected on a Zeiss LSM700 confocal microscope, and presented as maximal intensity projections generated in ImageJ (NIH, Bethesda, Maryland, USA). Embryos were immobilized and embedded in 5% Tricaine in 0.5% low melting point agarose (Invitrogen) immediately before the first time point of interest. For imaging at multiple time points, embryos were left embedded in 0.5% LMP agarose but were covered with E3 solution. This removed the paralyzing effect of Tricaine while ensuring the general position of the embryo did not change between time points. For cryosection, embryos were fixed with tissue freezing medium, Tek OCT Compound and sectioned at 10 µM using a Leica CM3050S cryostat and photographed on IX71 inverted microscope (Olympus) with an SPOT RT3 camera (DIAGNOSTIC Inc., Sterling heights, MI). The number of cells in the ISVs was determined by counting from individual slices of confocal stacks. The counting area is between 5–15 ISVs in the yolk extension. CVP loop number is counting the honeycomb structure from the end of yolk extension to the end of tail.
TUNEL staining
Embryos were fixed in 4% paraformaldehyde (PFA) and stored at −20°C in methanol. Embryos were washed with gradients of methanol/PBT starting with 3∶1 methanol:PBT (1X phosphate buffered saline, 0.1% Tween-20) and ending with 100% PBT. After digestion in 10 µg/mL Proteinase K for 20 minutes, embryos were fixed in 4% PFA for 15 minutes, then treated with 3% Hydrogen Peroxide in 0.1% PBT for 1 hour at room temperature in the dark and washed with PBT to eliminate endogenous peroxidase. For each sample group, 45 µl of TUNEL (TdT-mediated dUTP-X nick end labeling) label was pre-mixed with 5 µl of TUNEL enzyme and added to the embryos for 3 hours at 37°C in the dark. As a control, 50 µl of TUNEL label solution without enzyme was used. Embryos washed with PBT to remove unincorporated nucleotides and endogenous antibodies were blocked with 5% Normal Sheep Serum (NSS) in PBT for 2 hours at room temperature, then incubated with peroxidase (POD) conjugated anti-fluorescein antibody diluted 1∶2000 (Roche) in 5% NSS/PBT over night at 4°C in the dark, washed four times in PBT, and visualized using DAB kits (Roche).
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was purified using the RNeasy Mini kit (Qiagen). mRNA was reverse transcribed to cDNA using Reverse Transcriptase and oligo-dT primer (Roche) according to the manufacturer’s instructions. Quantitative RT-PCR was performed using a LightCycle 96 instrument (Roche) with SYBR Green I Master (Roche). qRT-PCR primers are listed in Table S1 in File S1. Relative gene expression levels were analyzed by the ΔΔ Ct method, with elongation factor 1α (EF1α) as a reference gene. All reactions were performed as biological triplicates.
DAPT treatment
A stock solution of 25 mM DAPT (Sigma) dissolved in DMSO was diluted to 75 µM for the working concentration and added to embryos E3 medium at 6 hpf. Control embryos were treated with an equivalent dose 0.3% of DMSO.
Results
Zebrafish nr2f1a is conserved among vertebrates and close to mammalian Nr2f2
The NR2F family consists of two highly homologous subtypes, nr2F1 and nr2F2. Pereira et al. showed that mammalian Nr2f2 (CoupTFII) is required for angiogenesis and heart development mediated by downregulating a proangiogenic soluble factor angiopoietin-1 [11]. You et al. showed that loss of mNr2f2 in mouse leads to an almost complete loss of the cardinal vein [10]. However, morpholino knockdown of zebrafish nr2f2 in embryos leads to a reduction in venous marker expression without gross vein and ISV anomalies [12] and our unpublished results. Thus, we went back to examine the phylogenetic relationship of nr2f1/2 in zebrafish and other species.
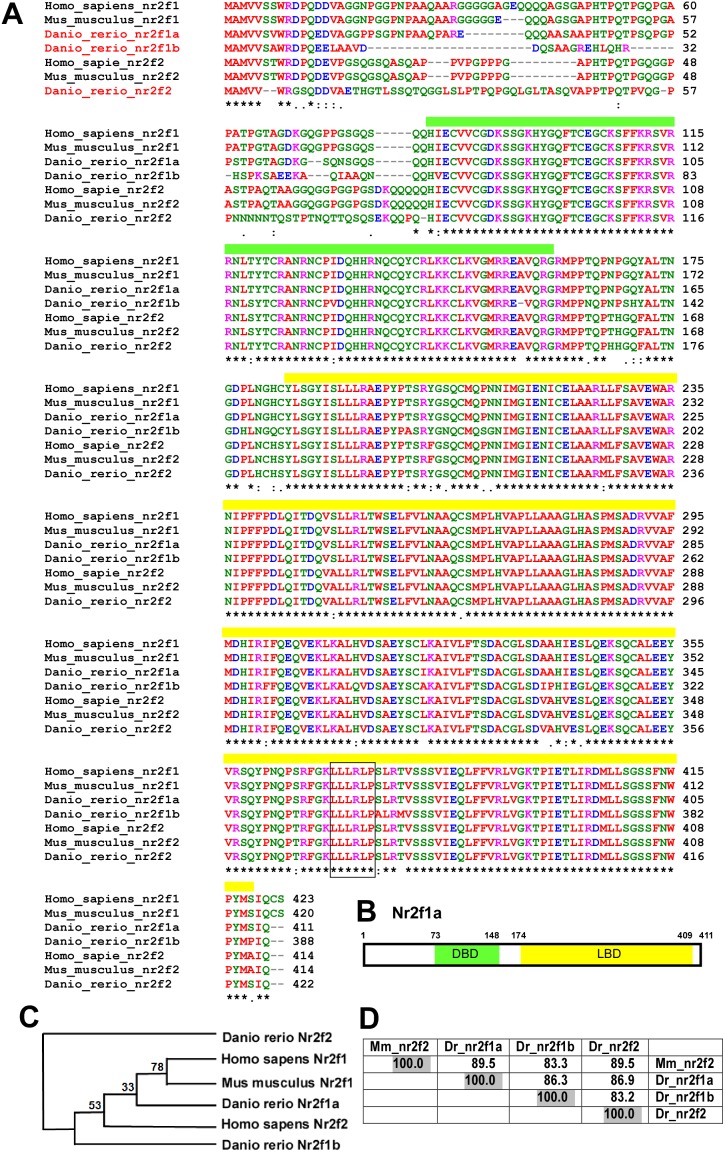
We started to analyse the amino acid sequences among those vertebrates, human, mouse and zebrafish. By comparing amino acid sequences of zebrafish nr2f1a with orthologs from other species, highly identical amino acids are showed (>83%) (Figure 1A, D). We also identify the putative ligand binding domain (similarity = 95%), a zinc-finger DNA binding domain (similarity = 98%) and a protein-protein interaction LLLRLP motif (Figure 1B). Those suggest the function of zebrafish nr2f1a as nuclear transcriptional factor and may play similar functions as mammalian Nr2f1/2. Phylogenetic analysis of nr2f1a orthologs in different species suggests the high evolutionary conservation of NR2F family members in different species (Figure 1C). Since mouse Nr2f2 showed significant vascular functions, we focus on the comparison of conservation among mouse nr2f2 and zebrafish nr2f1a, nr2f1b and nr2f2. As shown in Figure 1D, mouse Nr2f2 are highly conserved with zebrafish nr2f2, nr2f1a (both have score of 89.5) and nr2f1b (score of 83.3). Since our previous nr2f2 study in zebrafish showed only minor effects in vasculature, we hypothesize that nr2f1a may play important functions in vascularization similar to the mammalian Nr2f2 (CoupTFII).
Figure 1. nr2f1a is conserved among vertebrates.
(A) Comparison of the amino acid sequence of zebrafish nr2fIa to orthologs from other species by using ClustalW2 software. Identical amino acids are marked (*) below the sequence. The yellow bar represents a putative ligand binding domain and green box area indicates a zinc-finger DNA binding domain. Squared box indicates a conserved LLLRLP motif. (B) Schematic drawing shows that zebrafish nr2f1a protein contains 411 amino acids with the DNA binding domain (DBD) and ligand binding domain (LBD). (C) Cladograms provided from phylogenetic analysis of nr2f1a orthologs in different species using ClustalW2-phylogeny with the neighbor-joining method. The number (bootstrap values) at the nodes represent the possibility of groupings between 2 different nr2f members from different species. (D) Percent amino acid identity matrix among mouse (Mus_musculus) Nr2f2 and zebrafish (Danio_rerio) nr2f1a, nr2f1b and nr2f2.
nr2f1a is expressed in vasculature during development
To examine the potential role of nr2f1a in vascular development, we first determined its expression by whole-mount in sit hybridization during zebrafish embryonic development. At the 18 somite stage (S), nr2f1a is expressed in the lateral plate mesoderm (lpm), the telencephalon (t), diencephalon (d) and hindbrain (h). (Figure 2A). A dorsal view of embryos showed nr2f1a being expressed at lpm and axial vessels (Figure 2A’). Expression at the lateral plate mesoderm is corresponding to the location in the developing vasculature. At 24 hpf, nr2f1a is expressed in the telencephalon, diencephalon, hindbrain, as well as vessels of the trunk and caudal vein plexus (CVP) (Figure 2B, B’). Cross-sections of embryos show that nr2f1a is expressed in the dorsal aorta (da), posterior cardinal vein (pcv) and caudal vein plexus (CVP). At 30 hpf, nr2f1a expression continues in the head, vessels (v), intersegmental vessels (ISV) of the trunk and caudal vein plexus (CVP) (Figure 2E, E’). Those data show the expression of nr2f1a in vasculature during embryonic development and suggests the role of nr2f1a somewhere in vascular development. The expression pattern of nr2f1a in the head region from our study is similar to the previous studies by Bertrand et al and Love et al [25], [26] but the expression in vasculature is not described in previous publications. We found the expression level in the vasculature is much lower than in the head region. Thus, with shorter reaction time for in-situ staining, we can see clear expression pattern of nr2f1a in the head (data not shown) but no signals in the vasculature. Moreover, our nr2f1a expression is gene-specific because its expression shows different pattern from nr2f1b and nr2f2 compared to the previous studies [25], [26] and ZFIN database [27].
Figure 2. Spatiotemporal expression of nr2f1a during development.
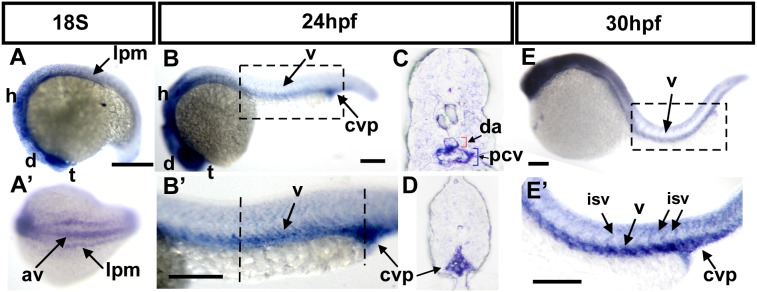
(A) nr2f1a expression can be observed at the 18S stage in the lateral plate mesoderm (lpm), the telencephalon (t), diencephalon (d) and hindbrain (h). (A’) Dorsal view of embryos show that nr2f1a is expressed at lpm and the axial vessels (av). (B, B’) At 24 hpf, nr2f1a is expressed in the telencephalon (t), diencephalon (d), hindbrain (h), as well as in vessels (v), and caudal vein plexus (CVP) of the trunk. B’ is an enlargement of B. (C, D) Cross sections of embryos from B’ show that nr2f1a is expressed in dorsal aorta (da), posterior cardinal vein (pcv), and caudal vein plexus (CVP). (E, E’) At 30 hpf, nr2f1a expression continues in the head, vessels (v), intersegmental vessels (ISV) and caudal vein plexus (CVP) of the trunk. E’ is an enlargement of E. Scale bars in all figures represent 100 µm.
Knockdown of nr2f1a results in vascular defects
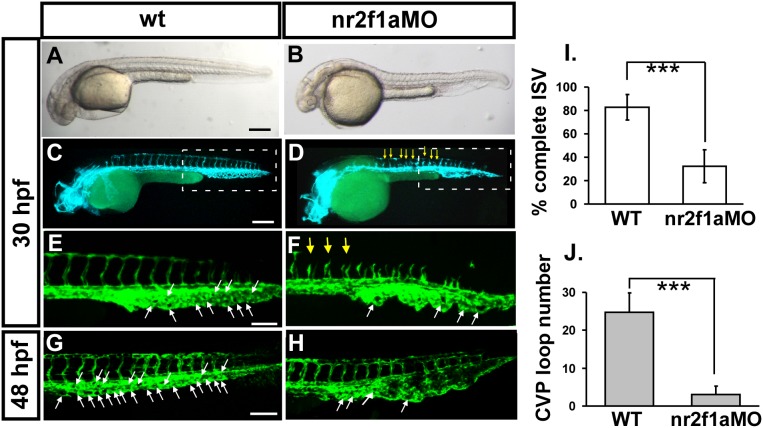
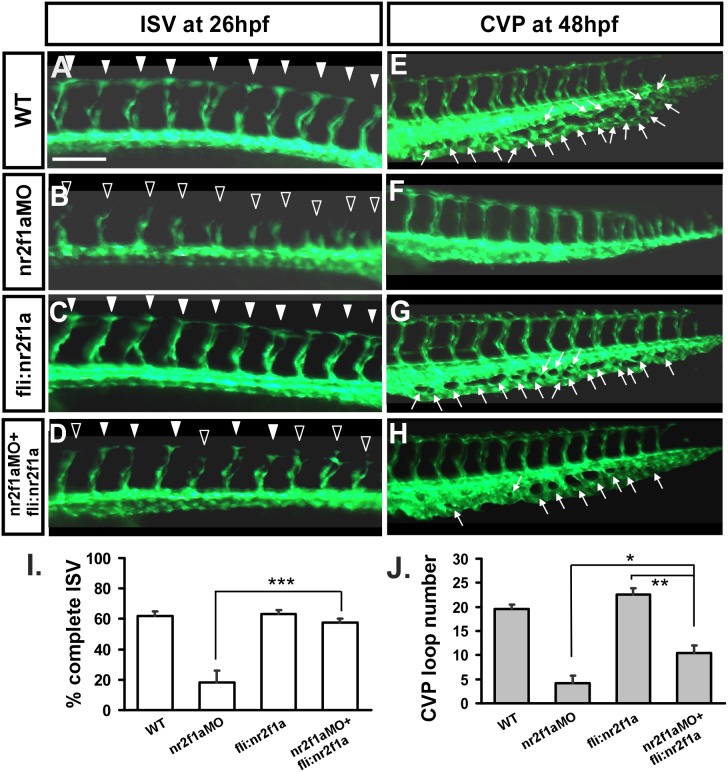
To identify the functional role of nr2f1a in vascular development, we knocked down its expression in Tg (kdrl:eGFP)la116 embryos which express GFP in endothelial cells. Injection of 4 ng of morpholino targeted against the exon 2-intron 2 splice junction (nr2f1ae2i2 MO) resulted in two obvious vascular phenotypes. Loss of nr2f1a showed ISV growth defect and mis-pattern plexus at CVP (caudal vein plexus) (Figure 3 C–H). At 30 hpf, a highly penetrate stalling of ISV growth was observed at the mid-somite at the midpoint of angioblast migration from the DA to the DLAV (Figure 3D, F) with only 30% of complete ISVs (n = 34 embryos) in nr2f1ae2i2 morphants compared to 80% of complete ISVs in uninjected controls (n = 26 embryos). The second phenotype we observed was the disruption of the honeycomb structure in the CVP as compared to uninjected controls at 30 hpf (Figure 3E, F). At 48 hpf, the swallowed plexus even became obvious (Figure 3H). Quantification of loop formation at CVP showed a 4-fold decrease in nr2f1a morphants (n = 10 in wt and in nr2f1aMO) at 48 hpf. During zebrafish embryonic angiogenesis, ISV and CVP formation were the two obvious vessel patterns to be observed. ISV angiogenesis is largely controlled by VEGF and Notch signalling. Recent studies showed that endothelial cells are also sprouting from the axial vein ventrally to form the CVP via BMP signalling pathways. Our data indicated that nr2f1a may play a critical role in controlling endothelial tip cell behaviors and in regulating ISV and CVP formation during angiogenesis.
Figure 3. Knockdown of nr2f1a causes defects in zebrafish vascular development.
(A–H) Loss of nr2f1a showing ISV growth defect (yellow arrows in F) and mis-pattern plexus at the CVP (caudal vein plexus) (white arrows) compared to wild-type control (E) at 30 hpf. E and F is enlargement of C and D, respectively. At 48 hpf, the swallowed plexus becomes obvious (H). At 30 hpf, in uninjected control embryos, intersegmental vessels (isv) have reached the DLAV at the dorsal aspect of the embryo (C, E) and the caudal vein plexus (cvp) formed honeycomb-like structures at the tail (E, white arrows). At the same stage ISVs are stalled at mid-somite in nr2f1ae2i2 morphants (D, F). (I) Quantification of percentage of completed ISV shows a ∼50% increase compared to nr2f1a morphants (n = 26 in wt and n = 34 in nr2f1aMO) at 30 hpf. (J) Quantification of loop formation at CVP shows a 4-fold decreased in nr2f1a morphants (n = 10 in wt and in nr2f1aMO) at 48 hpf. (*** refers to p<0.0001 by an unpaired student’s t-test. Scale bars are 200 µm for A–D, and 100 µm for E–H.
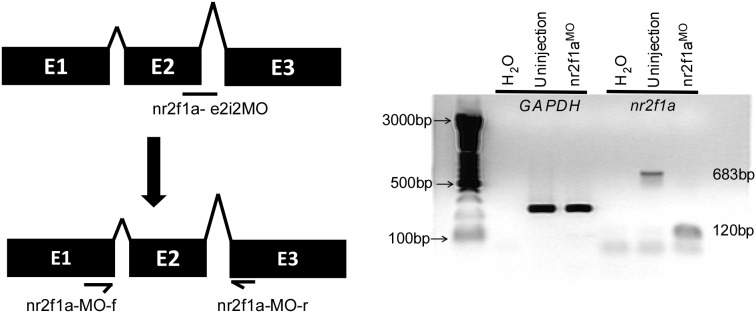
We further tested the efficiency of nr2f1ae2i2 morpholino knockdown. Injection of 4 ng of nr2f1ae2i2 morpholino completely disrupted normal splicing of nr2f1a as determined by RT-PCR (Figure 4). To test the specificity of nr2f1a morpholino targeting, we analyzed the nr2f1a splicing morpholino sequence (nr2f1ae2i2 MO) by nucleotide BLAST and showed high specificity (E-value = 10−5) compared to a 2nd target (E-value = 2.3). In addition, the sequence comparison to nr2f1b and nr2f2 showed only 36% identity and 44% identity, respectively, suggesting the morpholino targeting to nr2f1a is specific (Figure S1A in File S1). Moreover, injection of nr2f1ae2i2 morpholino completely disrupted the amount of the nr2f1a product, but nr2f1b and nr2f2 levels were unchanged (lane 5–8) compared to uninjected controls as determined by RT-PCR, indicating the specificity of the morpholino knockdown of nr2f1a (Figure S1B in File S1).
Figure 4. Morpholino-knockdown efficiency of nr2f1a in zebrafish embryos.
(A) Scheme shows nr2f1a morpholino targeting the pre-mRNA structure of nr2f1a and suggested mis-splicing fragment which can be detected by a nr2f1a_MO_f and nr2f1a_MO_r primer set. (B) cDNA from uninjected controls or nr2f1a morphants (injected with 4 ng morpholino) underwent PCR with primers for the housekeeping control gene (GAPDH), or for the nr2f1a gene. Morphants injected with nr2f1ae2i2, GAPDH levels are unchanged while the amount of wild-type nr2f1a product (683 bp) got diminished and a new lower molecular weight band appeared (120 bp), representing mis-splicing caused by the morpholino.
Loss of nr2f1a results in pericardial edema and defective circulation
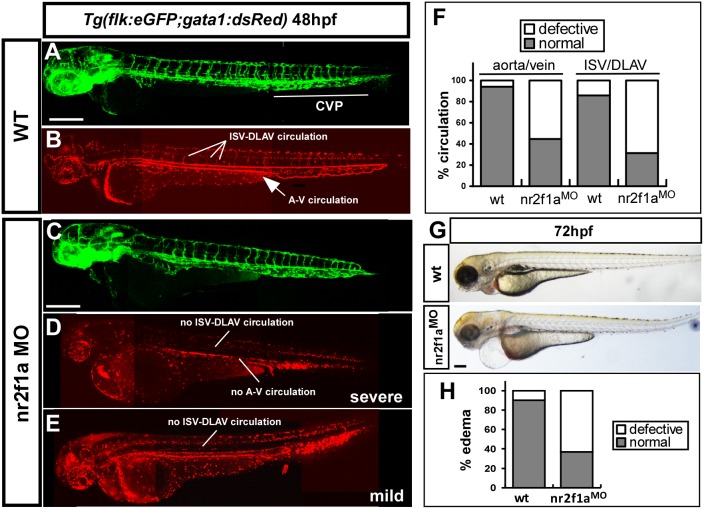
Edema and lack of circulation are common secondary consequences of defective blood vessel formation. We found that a loss of nr2f1a causes an increasing pericardial edema. Embryos showed ∼60% mild to severe edema at 72 hpf (n = 30 in wt and n = 69 in nr2f1a morphants) (Figure 5G–H). While we examined the circulation in wild-type and morphants, we noted deficient circulation in the trunk of nr2f1a morphants at 48 hpf (Figure 5A–F). nr2f1a morphants showed less than 50% of embryos with circulation in the axial vessels and less than 40% of the embryos with ISV circulation (n = 37 in wt and n = 42 in nr2f1a morphants) (Figure 5F). These data are consistent with the vasculature defects in the nr2f1a deficient embryos.
Figure 5. Loss of nr2f1a results in circulation defects and pericardial edema.
(A–E) nr2f1a e2i2 MO was injected into transgenic Tg (fli:eGFPy1; gata1:dsRed) embryos with GFP-labeled endothelial cells (A, C) and dsRed-labeled blood cells (B, D, E). Loss of nr2f1a showed a mis-pattern at ISV and at the caudal vein plexus (CVP) and results in circulation defects in severe (D, no circulation and blood stock) or mild (E, blood stock at CVP and shorter circulation) at 52 hpf compared to wild-type fish (B). (F) Circulation defects at the ISV/DLAV (∼68% in morphants) and slow to lose axial circulation of the aorta/vein in the trunk region (∼58% in morphants) are quantitated in wt (n = 37) and nr2f1aMO (n = 42) at 48–52 hpf. (G, H) Representative edema fish and quantitative results from three independent experiments showed 65% of nr2f1a morphants (n = 69) with mild to severe pericardial edema compared to wt (n = 30). The scale bar in A–E represent 200 µm and in G is 500 µm.
Phenotypic specificity of the nr2f1a morpholino knockdown
We showed knockdown of nr2f1a results in vascular defects (Figure 3) and determined the efficiency of the nr2f1ae2i2 morpholino knockdown (Figure 4). To test whether phenotypic specificity of the nr2f1a morpholino knockdown, we tested the effects of an additional morpholino targeting the translation initiation site (nr2f1aATG MO). Our results showed nearly identical vascular phenotypes as nr2f1ae2i2 morphants, including defects in ISV growth (70% decrease), ISV cell number, CVP formation (3-fold decrease), blood circulation and the development of edema occurrence (60% higher) (Figure S2 in File S1), suggesting that the phenotype of nr2f1a knockdown is gene-specific.
To further confirm the specificity of our morpholino experiments, we performed rescue experiments by overexpression of nr2f1a in wild-type and nr2f1ae2i2 morphant embryos. Transient transgenic overexpression of nr2f1a in endothelial cells using the fli1 promoter rescues ISV stalling by 40% in nr2f1a morphants compared to injection of nr2f1a morpholino alone (Figure 6A–D, I), while overexpression of nr2f1a in wild-type embryos has no obvious effect on the vascular development (Figure 6C). In addition, overexpression of nr2f1a restores the honeycomb-like structure in nr2f1a morphants for 50% at CVP (Figure 6H) compared to the nr2f1a morphants (Figure 6F). Finally, there was a slightly, but not significant increase in ISV completion and the CVP loop formation when overexpressing nr2f1a (Figure 6C, G) suggesting that nr2f1a is not sufficient for ISV and CVP growth, at least overexpression driven by fli promoter.
Figure 6. Knockdown of nr2f1a can be rescued by overexpression of nr2f1a.
In uninjected control embryos, intersegmental vessels (isv) have reached the DLAV at the dorsal region from 24–30 hpf (A, white arrowheads) and caudal vein plexus (cvp) were formed honeycomb-like structures at the tail around 48 hpf (E, white arrows). At the same stage, ISVs are stalled at mid-somite (B, hollow arrowheads) and less/no honeycomb structure is formed at CVP (F with no arrows) in nr2f1ae2i2 morphants. Overexpression of nr2f1a driven by fli promoter had no obvious defect in vasculature (C, G), but rescued the defect of ISV stalling (solid arrowhead) (D) and restored the honeycomb structure at CVP (H). (I) Quantification of percentage of completed ISV at 26 hpf shows a ∼40% increase in rescued embryos compare to nr2f1a morphants. Wild-type embryos had 62±3.1% complete ISV; nr2f1aMO had 18±8.0% complete ISV; fli:nr2f1a overexpression embryos had 63±2.6% complete ISV and rescued embryos had 57±2.7% complete ISV. (J) Quantification of loop formation at CVP showed a 2-fold increased in rescued embryos compared to nr2f1a morphants at 48 hpf. Wild-type embryos (19.6±0.9); nr2f1aMO (4.2±1.5); fli:nr2f1a overexpression embryos (22.6±1.3) and rescued embryos (10.6±1.6). (*** refers to p<0.0001, ** refers to p<0.001 and * refers to p<0.05 by an unpaired student’s t-test. Data are represented as means ± SEM). Scale bars are 100 µm for A–H.
nr2f1a is required for the growth of ISV cells
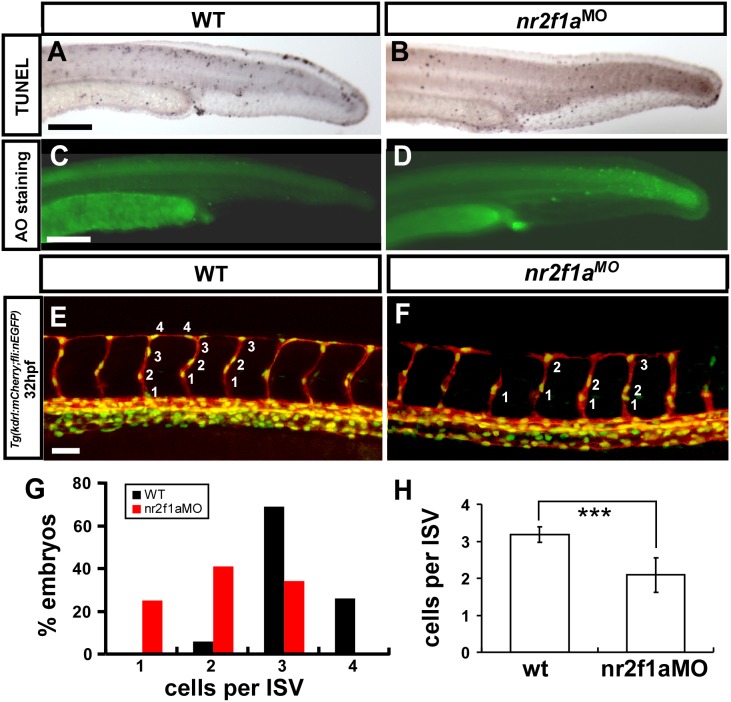
Loss of nr2f1a results in vascular growth defects suggests the impairment of endothelial cellular migration, proliferation or increase of cell death. To test these hypothesis, we first examine if more cell death in nr2f1a morphant? We showed nr2f1a morphant phenotypes do not result from morpholino- induced non-specific cell death as there is no significant increase in apoptosis in the trunk of morphants compared to wild type embryos by Acridine Orange staining and TUNEL assay (Figure 7). To test if loss of nr2f1a would decrease cell proliferation, we counted the numbers of endothelial cells per ISV in the Tg (kdrl:mCherry; fli1a:negfp)y7 embryos, where GFP was expressed in the nuclear of endothelial cells and the mCherry tag on endothelial ISV cells. Loss of nr2f1a showed significantly reduced ISV cells compared to uninjected wt in the control (n = 34 in nr2f1a morphants and n = 33 in wt control, p<0.0001). Those data suggested that nr2f1a was required for ISV cell growth to contribute to the vascular development, likely by regulation of the proliferation or migration of the cells.
Figure 7. nr2f1a is required for the growth of ISV cells.
(A–D) TUNEL labeling and AO staining was used to detect apoptotic cells in wild type, and nr2f1ae2i2 morphants. Although some apoptotic cells were observed on the skin, cell death in vascular regions was not elevated compared to wild type controls. (E–H) The number of cells forming each ISV counted in wild type control Tg(kdrl:mCherry; fli1a:negfp)y7 (E) and nr2f1a morphant embryos (F) at 32 hpf. (G) Proportional distribution of ISVs containing 1–5 cells and (H) average ISV cells per ISV counted in both wild type and nr2f1a morphant. (*** refers to p<0.0001 by an unpaired student’s t-test).
nr2f1a modulates vascular identity
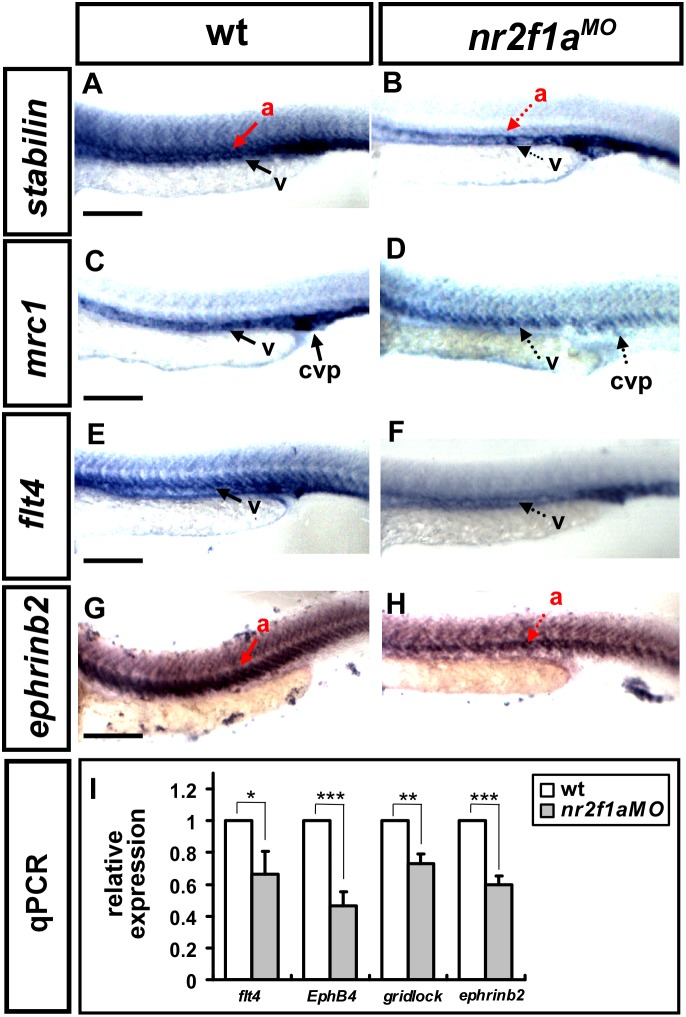
ISV growth defect and CVP mis-pattern suggested that nr2f1a is important for vascular development and likely modulates vascular identity. To test this hypothesis, we examined the expression of several vascular markers flt4, mrc1, stabilin, and ephrinb2 by in-situ hybridization. We found that the expression of the venous/ISV markers flt4 and mrc1 was decreased in nr2f1a morphants compared to the wild type controls at 24 hpf (Figure 8). The expression of arterial marker ephrinb2 and vascular marker stabilin are also reduced. To determine the extent of decreased marker expression, we quantified flt4, ephrinb2, ephrinb4 (EphB4) and gridlock transcript levels by qPCR and identified a 20–50% decrease in expression in nr2f1a morphants. These results suggest that nr2f1a regulates several vascular genes to promote vessel formation.
Figure 8. nr2f1a modulates the expression of vascular markers.
(A–H) Compared to wild type controls (A, C, E, G), expression of the venous markers mrc1 (D) and flt4 (F) were reduced in the trunk of nr2f1a morphants at 24 hpf. In addition, expression of the arterial marker ephrinb2 (H) and pan-vascular marker stabilin decreased (B). (I) Quantification of the relative expression level by qPCR assay showed a ∼25% to 50% reduced expression in vascular markers flt4 (0.66±0.14), EphB4 (0.46±0.08), gridlock (0.73±0.06) and ephrinb2 (0.59±0.05) in nr2f1a morphants. *** refers to p<0.0001, ** refers to p<0.001 and * refers to p<0.05 by an unpaired student’s t-test. Data are represented as means ± SEM. Scale bars represent 100 µm in A–H.
Regulatory relationships between nr2f1a and Notch
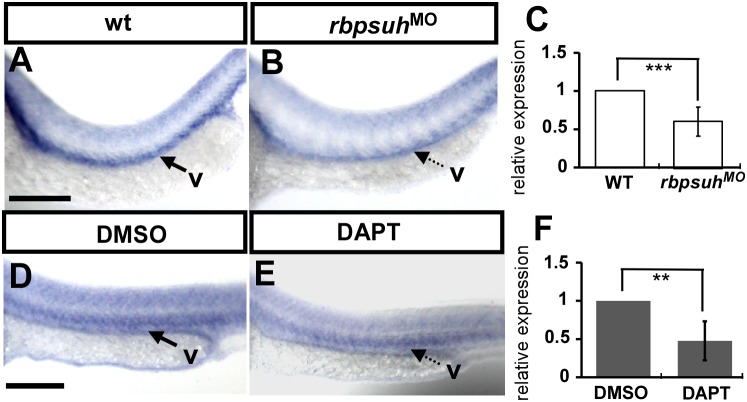
We demonstrated that interruption of nr2f1a expression by morpholino injection results in ISV stalling at the midline with a decrease in the number of cells per ISV. In addition, loss of nr2f1a causes the deformation of CVP. Notch signalings has been shown to be important for vein identity and the interaction of ISV tip cell with VEGF signaling during angiogenesis. Activation of Notch signaling provides ISV stalling at the midline, while loss of notch signaling leads to an increasing number of cells per ISV [7]. Thus, we hypothesize that inactivation of Notch signalings will increase nr2f1a expression. To test if nr2f1a interacts with notch signalings, we suppressed Notch signaling by rbpsuhMO injection (Figure 9A–C) or DAPT treatment (Figure 9D–F). Surprisingly, we found nr2f1a expression is downregulated when Notch signals were inhibited, with a 50–60% reduction by using in-situ hybridization and qPCR (Figure 9). These data suggest upon the genetic interaction between nr2f1a and Notch signals. However, the results contradict our hypothesis that Notch inactivation negatively regulated nr2f1a expression. Other regulators that promote angiogenesis, thus, may mask or dominate the function of nr2f1a during the Notch inactivation.
Figure 9. Regulatory relationships between nr2f1a and Notch.
(A–B) nr2f1a expression is downregulated in rbpsuh morphants (B) as compared to untreated control embryos (A). (D–E) nr2f1a expression is downregulated after treatment with DAPT (E) as compared to DMSO control embryos (D). (C, F) Quantification of the relative nr2f1a expression level by real-time qPCR assay shows a ∼50% reduced expression in rbpsuh morphants (C) and DAPT-treated embryos (F) compared to uninjected embryos and DMSO-treated controls. Scale bars represent 100 µm in A, B, D and E.
Discussion
In the present study we examined the role of nr2f1a in angiogenesis in vivo during zebrafish development. We first showed nr2f1a is conserved among the vertebrates (Figure 1) and its expression in the developing vessels in zebrafish (Figure 2). We found that knockdown of zebrafish nr2f1a by morpholinos led to growth impairment of intersegmental vessels (ISV) and defects in the angiogenic sprouting and formation of CVP (Figure 3), which results in the pericardial edema and circulation defect (Figure 5). TUNEL assay and ISV cells examination suggested vascular defect in nr2f1a morphants was associated with impaired proliferation and/or migration of ISV tip cells (Figure 7). Furthermore, genetic evidence suggested that nr2f1a interact with Notch signalings (Figure 9). Taken together, these results demonstrate zebrafish nuclear receptor nr2f1a ortholog to mammalian coupTFI/II is essential for vascular patterning in vivo. The cooperative function and combinatory effects between nr2f1a and its paralog nr2f1b and nr2f2 in zebrafish will be tested in the future.
The angiogenic sprouting of zebrafish ISVs and CVPs is regulated by different signaling mechanisms and endothelial cells respond to angiogenic cues is critical to coordinate distinct cellular behaviors to form an organized vessel network. Molecular mechanisms that transcription factors promote angiogenesis of ISV and CVP are still not well-understood. To reveal the molecular mechanisms that control vascular patterning, we first focused on the roles transcription factors play in vascular patterning. We previously identified LIM-transcription factor islet2 functions in the specification of venous and tip cells in zebrafish (unpublished results). Other research groups and we also found that in zebrafish nr2f2 plays only a minor role in early vascular development, but have a significant function in venous angiogenesis and lymphogenesis [12], [28], [29] and our unpublished results. In this study, we emphasize the role nr2f1a has in ISVs growth and CVP sprouting and extension. It is likely involved in cell proliferation and migration during the angiogenic process. Meanwhile, our data suggested that nr2f1a likely affects ISV and CVP angiogenesis directly because we found nr2f1a mainly expressed in vascular derivatives and no mural cells are present at the early stages of development.
ISVs cells are derived from angioblasts that are sprouting from the dorsal aorta and vein. Stalling of intersegmental vessel growth at the mid somite might, therefore, either occur through defective proliferation or defective migration of cells. We here demonstrated that a knockdown of nr2f1a in zebrafish leads to ISV growth stalling and a decreased number of ISV cells. TUNEL analysis suggests that cell death is not increased in the trunk region, suggesting that nr2f1a is necessary to promote a proliferation of ISV cells. However, the ultimate fate of these cells as tip or stalk cells remains unknown.
The process of ISV formation is largely controlled by VEGF and Notch signaling [4]. In this study we test the interaction between nr2f1a and Notch signaling. Surprisingly, we observed positively regulation between Notch signaling and nr2f1a expression. These data suggest on a genetic interaction between nr2f1a and Notch signals. However, these results contradict to our hypothesis that Notch inactivation negatively regulated nr2f1a expression. Thus, other regulators that promote angiogenesis mask or take over the function of nr2f1a during the Notch inactivation. On the other hand, the requirement of nr2f1a for vascular integrity maybe mediated by other signals. It would be interesting if other signaling molecules, such as Wnt/β-catenin or BMP/smad interact with nr2f1a. We will address this question in the future.
As for our previous findings and our current study, we would like to ask whether zebrafish nr2f1a plays a conserved role in vasculature similar to Nr2f2 in mice? Our previous study suggested that nr2f2 plays a minor role in vein and tip cell differentiation but acts as a major determinant of lymphogenesis in zebrafish (unpublished results). In this study, we showed that zebrafish nr2f1a is the ortholog to mouse Nr2f2 and plays a role in ISV and CVP angiogenesis, which is consistent with the partial function of Nr2f2 in mice [11]. Those data indicate the conserved vascular function of the NR2F family among vertebrates. Our phylogenetic analysis of NR2F members among vertebrates suggests that zebrafish nr2f1a and nr2f2 are closer to mammalian nr2f2. On the other hand, zebrafish nr2f1b is much different from the zebrafish paralogs nr2f2/nr2f1a but evolutionary conserved to its ortholog mammalian Nr2f1. The function of mouse Nr2f1 has been identified and the transcriptional targets of Nr2f1 are identified in inner ear tissue of mice [31]. Their gene ontology categories are cell adhesion, cartilage development, cell cycle regulation, myeloid cell differentiation and lipid/steroid metabolism. Thus, there is so far no evidence showing a mammalian Nr2f1 function in vasculature. Since mice Nr2f1 is a critical regulator for neural development and controls cell differentiation in the inner ear, it would be interesting to test whether zebrafish nr2f1a has other functions that might be similar to mammalian Nr2f1. The same holds for zebrafish nr2f1b which might have functions similar to mammalian Nr2f1.
In conclusion, we demonstrate a new transcription factor nr2f1a participate in ISV growth, and reveal a novel role of nr2f1a in vascular biology. Finally, to understand the molecular mechanisms that nr2f1a control the pattering of ISV and CVP, transcriptome analysis of nr2f1a downstream targets could be very interesting and will be pursued in the near future. The potential downstream targets of nr2f1a will be interesting to characterize and may be served potential targets to suppress angiogenesis, offering important therapeutic implications, such as cancer therapy.
Supporting Information
Combined Supporting Information file.
(DOC)
Acknowledgments
We thank Dr. Sarah Childs for providing the primers, plasmids and in-situ hybridization probes used in this work. We also thank Dr. Tai Ming-Hong for useful comments on the manuscript. We thank Taiwan Zebrafish Core Facility at Academia Sinica (TZCAS) and at NTHU-NHRI (TZeTH), which is supported by National Science Council (NSC-102-2321-B-001-038) in Taiwan for providing wild-type TL or AB strains and transgenic fish.
Funding Statement
This work was supported by the grants from National Science Council, Taiwan (NSC101-2311-B-110-002, NSC102-2311-B-110-002) and from the NSYSU-KMU Joint Research Project (#NSYSUKMU102-P028) to CYW; the grant from Zuoying Branch of Kaohsiung Armed Forces General Hospital, Taiwan to BJW (ZAFGH 102-03). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Risau W (1997) Mechanisms of angiogenesis. Nature 386: 671–674. [DOI] [PubMed] [Google Scholar]
- 2. Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, et al. (2001) Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128: 3675–3683. [DOI] [PubMed] [Google Scholar]
- 3. Childs S, Chen J-N, Garrity D, Fishman M (2002) Patterning of angiogenesis in the zebrafish embryo. Development 129: 973–982. [DOI] [PubMed] [Google Scholar]
- 4. Lamont RE, Childs S (2006) MAPping out arteries and veins. Sci STKE 2006: pe39. [DOI] [PubMed] [Google Scholar]
- 5. Hamik A, Wang B, Jain MK (2006) Transcriptional regulators of angiogenesis. Arterioscler Thromb Vasc Biol 5: 797–808. [DOI] [PubMed] [Google Scholar]
- 6. Patterson C (2009) Torturing a blood vessel. Nat Med 15: 137–138. [DOI] [PubMed] [Google Scholar]
- 7. Siekmann AF, Lawson ND (2007) Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 445: 781–784. [DOI] [PubMed] [Google Scholar]
- 8. Wiley D, Kim J, Hao J, Hong C, Bautch V, et al. (2011) Distinct signalling pathways regulate sprouting angiogenesis from the dorsal aorta and the axial vein. Nat Cell Biol 13: 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, Kang H, Larrivée B, Lee M, Mettlen M, et al.. (2012) Context-dependent proangiogenic function of bone morphogenetic protein signaling is mediated by disabled homolog 2. Dev Cell 23. [DOI] [PMC free article] [PubMed]
- 10. You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, et al. (2005) Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 435: 98–104. [DOI] [PubMed] [Google Scholar]
- 11. Pereira FA, Qiu Y, Zhou G, Tsai MJ, Tsai SY (1999) The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev 13: 1037–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aranguren XL, Beerens M, Vandevelde W, Dewerchin M, Carmeliet P, et al. (2011) Transcription factor COUP-TFII is indispensable for venous and lymphatic development in zebrafish and Xenopus laevis. Biochem Biophys Res Commun 410: 121–126. [DOI] [PubMed] [Google Scholar]
- 13. Swift MR, Pham VN, Castranova D, Bell K, Poole RJ, et al. (2014) SoxF factors and Notch regulate nr2f2 gene expression during venous differentiation in zebrafish. Developmental Biology 390: 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin F, Qin J, Tang K, Tsai S, Tsai M (2011) Coup d’Etat: an orphan takes control. Endocr Rev 32: 404–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Srinivasan RS, Geng X, Yang Y, Wang Y, Mukatira S, et al. (2010) The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev 24: 696–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou C, Tsai S, Tsai M (2001) COUP-TFI: an intrinsic factor for early regionalization of the neocortex. Genes Dev 15. [DOI] [PMC free article] [PubMed]
- 17. Tang K, Xie X, Park J, Jamrich M, Tsai S, et al. (2010) COUP-TFs regulate eye development by controlling factors essential for optic vesicle morphogenesis. Development 137: 725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choi J, Dong L, Ahn J, Dao D, Hammerschmidt M, et al. (2007) FoxH1 negatively modulates flk1 gene expression and vascular formation in zebrafish. Dev Biol 304: 735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westerfield M (1995) The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio Rerio). Eugene, OR: University of Oregon Press.
- 20. Lamont RE, Vu W, Carter AD, Serluca FC, MacRae CA, et al. (2010) Hedgehog signaling via angiopoietin1 is required for developmental vascular stability. Mech Dev 127: 159–168. [DOI] [PubMed] [Google Scholar]
- 21. Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, et al. (2007) The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn 236: 3088–3099. [DOI] [PubMed] [Google Scholar]
- 22. Jowett T, Lettice L (1994) Whole-mount in situ hybridizations on zebrafish embryos using a mixture of digoxigenin- and fluorescein-labelled probes. Trends Genet 10: 73–74. [DOI] [PubMed] [Google Scholar]
- 23. Thisse C, Thisse B (2008) High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc 3: 59–69. [DOI] [PubMed] [Google Scholar]
- 24. Wong KS, Proulx K, Rost MS, Sumanas S (2009) Identification of vasculature-specific genes by microarray analysis of Etsrp/Etv2 overexpressing zebrafish embryos. Dev Dyn 238: 1836–1850. [DOI] [PubMed] [Google Scholar]
- 25. Bertrand S, Thisse B, Tavares R, Sachs L, Chaumot A, et al. (2007) Unexpected novel relational links uncovered by extensive developmental profiling of nuclear receptor expression. PLoS Genet 3: e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Love CE, Prince VE (2012) Expression and retinoic acid regulation of the zebrafish nr2f orphan nuclear receptor genes. Dev Dyn 241: 1603–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thisse B, Thisse C (2005) High Throughput Expression Analysis of ZF-Models Consortium Clones ZFIN Direct Data Submission www.zfin.org.
- 28.Yamazaki T, Yoshimatsu Y, Morishita Y, Miyazono K, Watabe T (2009) COUP-TFII regulates the functions of Prox1 in lymphatic endothelial cells through direct interaction. Genes to Cells: 425–434. [DOI] [PubMed]
- 29. Pereira FA, Tsai MJ, Tsai SY (2000) COUP-TF orphan nuclear receptors in development and differentiation. Cell Mol Life Sci 57: 1388–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Christie TL, Carter A, Rollins EL, Childs SJ (2010) Syk and Zap-70 function redundantly to promote angioblast migration. Dev Biol 340: 22–29. [DOI] [PubMed] [Google Scholar]
- 31. Montemayor C, Montemayor O, Ridgeway A, Lin F, Wheeler D, et al. (2010) Genome-wide analysis of binding sites and direct target genes of the orphan nuclear receptor NR2F1/COUP-TFI. PLoS One 5: e8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Combined Supporting Information file.
(DOC)