Abstract
Background
The aim of this study was to screen for retinopathy of prematurity (ROP) in southwestern China and understand the prevalence and risk factors of ROP, which may provide evidence useful in the prevention and treatment of ROP.
Material/Methods
1864 preterm infants (gestational age of <37 weeks and birth weight of ≤2500 g) underwent ROP screening from January 2009 to November 2012 in Southwest China. The medical information of infants during perinatal period was reviewed, and risk factors of ROP were determined. A total of 1614 infants were recruited for final analysis.
Results
Incidence of ROP was 12.8%. The first, second, third, and fourth stage of ROP was found in 64.6%, 29.6%, 3.4%, and 0.5% of infants, respectively. No fifth stage of ROP was observed. In addition, 7.7% of infants required surgical intervention. In our Department of Neonatology, the incidence of ROP was 20.0%, which was significantly higher than in non-hospitalized patients (9.9%). The incidence of ROP remained unchanged over the years. Independent risk factors of ROP included low birth weight (p=0.049), low gestational age (p=0.008), days of oxygen supplementation (p=0.008), and myocardial injury after birth (p=0.001).
Conclusions
The prevalence of ROP in preterm infants is relatively high in Southwest China, and low birth weight, low gestational age, days of oxygen supplementation, and myocardial injury after birth are independent risk factors for ROP.
MeSH Keywords: China, Prevalence, Retinopathy of Prematurity, Risk Factors
Background
Vision is crucial for the life of humans. Poor vision and blindness may cause difficulties in learning, living, and working and is a heavy burden for family and society. Although the number of blind children is low, the proportion of blind children is as high as 20% in blind persons after adjustment for disability-adjusted life years (DALYs) [1]. Thus, the control of blindness in children is considered a high priority by the World Health Organization’s (WHO’s) VISION 2020 – The Right to Sight program [2]. In about 6–8% of blind children, retinopathy of prematurity (ROP) is the major cause of blindness, and it is a main cause of blindness worldwide. ROP-induced vision impairment or blindness significantly impairs the quality of life of preterm infants [3]. In recent years, with the development of perinatal science and newborn technology, the survival rate of very low birth weight children is increasing significantly. ROP is considered an important cause of blindness in developed countries. In developing countries, increasing attention has also been paid to ROP [4]. Thus, it is imperative to investigate the epidemiology of ROP, aiming to reduce the incidence of ROP-induced vision injury and blindness.
In most countries, the prevalence of ROP has been surveyed. It was reported that the prevalence of ROP was 15.6% in the USA [5], 36.1% in Germany [6], 36.4% in Sweden [7], 29.2% in Singapore [8], 8.5% in Iran [9], and 18.2% in Brazil [10]. The significant difference in the prevalence of ROP among these countries suggests that the race, geographical region, country, degree of social and economic development, and medical level are factors influencing the incidence of ROP. In China, the prevalence of ROP was surveyed in Beijing [11], Shanghai [12], Guangzhou [13], and Shenzhen [14]. However, these cities are capitals and are located in the economically developed coastal areas. Thus, these data fail to represent the actual prevalence of ROP in China. As compared to economically developed areas such as Beijing and Guangzhou, western China is underdeveloped, and few studies have been conducted to investigate the prevalence of ROP in these areas [15]. In the present study, the prevalence of ROP was investigated in western China. Our findings may provide evidence for studies on ROP in western China, and present theoretical evidence for understanding the prevalence of ROP in China, which are beneficial for formulating government policy to assure prenatal and postnatal care and to increase the quality of life.
In terms of risk factors of ROP, studies on ROP focus on infants who are low birth weight and low gestational age [7,8,16], and the problems during gestation and after birth are less reported. Accordingly, to avoid risk factors, we investigated the risk factors of ROP other than low birth weight and low gestational age.
Material and Methods
General information
A total of 1864 preterm infants received retinal screening for ROP in the Department of Ophthalmology of Affiliated Children’s Hospital of Chongqing Medical University from January 2009 to November 2012. These infants were from western China. Preterm was defined as gestational age of <37 weeks in the Textbook of Pediatrics (Second Edition) [17]. We found that larger preterm infants with gestational age over 28 weeks or birth weight more than 1500 g were also diagnosed with severe ROP (12 cases of pre-threshold type 1 or threshold ROP in total), when analyzing all the data collected. Thus, preterm was defined as gestational age of <37 weeks and birth weight of ≤2500 g in this study. We recruited 1614 preterm infants into this study.
Inclusion criteria: 1) gestational age <37 weeks and birth weight ≤2500 g; 2) the parents or legal guardians agreed with screening for ROP; 3) refracting media had no turbidity and fundus image was clear; and 4) the medical information was complete and reliable.
Exclusion criteria: 1) gestational age ≥37 weeks or birth weight >2500 g; 2) the parents or legal guardians refused infant screening for ROP; 3) the refracting media were turbid due to congenital cataract, intraocular hemorrhage, or other diseases, and the fundus image was blurred; and 4) the medical information was incomplete (children with incomplete medical information were enrolled for calculation of overall prevalence, but not for analysis of ROP risk factors).
Ethics statement
The ethics committee of Children’s Hospital of Chongqing Medical University approved this study.
Time of screening
The initial screening was done at 4–6 weeks after birth or the corrected gestational age of 32 weeks.
Frequency of follow-up and end points
ROP was diagnosed and classified according to the criteria for ROP developed by the ROP International Conference in 1984 [18]: stage 1 or 2 ROP in zone II without plus disease, or stage 1 or 2 ROP in zone III: examination was done once weekly; pre-threshold ROP: the retinal examination was done once every 2–3 days; threshold ROP: laser or cryotherapy was done within 72 h; and stage 4 or 5 ROP: surgical intervention was required. The end points included the stabilization or scarcity of ROP or post-therapy.
For infants without ROP but with incomplete peripheral vascularization of the retina, follow-up was done once every 2 weeks until complete retinal vascularization was observed.
Instrument for screening
For screening, we used a digital wide-field ocular fundus imager (RetCamII; Massie Research Laboratories, USA) and binocular indirect ophthalmoscope (HEINE OMEGA180, Germany).
Methods for screening
Pupil dilation: One hour before examination, eye drops containing 0.5% tropicamide and 0.5% phenylephrine hydrochloride (Santen Pharmaceutical Co., Ltd., Japan) were used to dilate both pupils once every 5–10 min (a total of 2–3 times). At the same time, the lacrimal sac was pressed to avoid the discharge of eye drops via the nasolacrimal duct.
Fundus examination: an eye speculum was used to keep the eye open, followed by surface anesthesia with 0.4% hydrochloric oxybuprocaine eye drops (Santen Pharmaceutical Co., Ltd., Japan). Then, a fundus examination was done using an indirect ophthalmoscope, with a scleral device and +20.00D aspheric, and images were captured with the RetCamII system. Examination was done first in the right eye and then in the left eye, and attention was paid to the temporal retina. Once the ROP of both eyes was classified at different stages, results were recorded independently. Once ROP was present in 1 eye, ROP was recorded for calculation of prevalence of ROP. The eye with more severe ROP was recruited for staging of ROP. Once infants had received laser photocoagulation before admission and laser spots were observed, ROP was classified as threshold ROP. Screening was done in the presence of neonatologist physicians who closely monitored the conditions of infants. If necessary, heart rate, breathing, and oxygen saturation were monitored.
Collection of risk factors
The medical information was carefully reviewed in 459 preterm infants who received fundus examinations in the Department of Neonatology of Children’s Hospital. We recorded infant and maternal data. The infant data included: sex, gestational age, birth weight, neonatal pneumonia, respiratory distress syndrome (acute respiratory distress syndrome [ARDS]; apnea, respiratory failure, asphyxia), pulmonary hemorrhage, neonatal encephalopathy (intracranial hemorrhage, brain damage in preterm infants, hypoxic-ischemic encephalopathy [HIE]), sepsis, congenital heart disease, myocardial injury (the activity of creatine phosphokinase-Mb(CK-MB) is greater than 5 mg/L was defined as myocardial injury), coagulation disorders, anemia, hyperbilirubinemia, pathological jaundice, hypoalbuminemia, hypoglycemia, hyperglycemia, acid metabolic imbalance, oxygen supplement, days of oxygen supplement (days of mechanical ventilation + days of routine oxygen supplement), blood transfusion, volume of transfused blood, blood type, multiple deliveries, steroids, vasoactive substances, pulmonary surfactant, and erythropoietin. Maternal data collected included: maternal age, pattern of delivery (eutocia/cesarean section), gestational hypertension, premature rupture of membranes, amniotic fluid abnormalities, fetal distress, placenta previa, placental abruption, cholestasis of pregnancy, anemia in pregnancy, colds, and medications with dexamethasone, penicillin, or tocolytic agents.
Statistical analysis
Data were recorded in categories and filled in tables. Statistical analysis was done with SPSS version 17.0. Prevalence of ROP was tested with the chi-squared test. When 20% of theoretical frequencies were <5, P value was calculated with Fisher’s exact test. When a difference was noted among groups, comparisons between groups were further performed. The alpha level was 0.05/[n(n−1)/2+1], where n=number of groups [19].
Various risk factors were comprehensively analyzed on the basis of the presence of ROP. Univariate analysis was done with the chi-squared test or the t test. A value of P<0.05 was considered statistically significant. Multivariate analysis was done with logistic regression analysis. ROP served as a dependent variable (yes=1, no=0) and risk factors served as independent variables. A value of P<0.05 was considered statistically significant.
Results
Characteristics of subjects
From January 2009 to November 2012, a total of 1864 preterm infants received retinal examination for screening of ROP in the Department of Ophthalmology of Children’s Hospital of Chongqing Medical University. According to the inclusion criteria and exclusion criteria, 1614 were recruited, including 898 males (55.6%) and 716 females (44.4%) (M/F: 1.25:1). Among these infants, there were 459 hospitalized patients (28.4%) and 1155 subjects without hospitalization (71.6%). The demographics, gestational age, and birth weight are shown in Table 1.
Table 1.
Demographic data of the studied cases (n=1614).
| Data | n (%) | Gestational age (w) | Birth weight (g) | |
|---|---|---|---|---|
| Total | 1614 (100.0%) | 32.34±2.19 | 1773.7±389.9 | |
| Gender | M | 898 (55.6%) | 32.33±2.16 | 1804.3±382.4 |
| F | 716 (44.4%) | 32.35±2.23 | 1735.4±396.0 | |
| Source | Non-hospitalized | 1155 (71.6%) | 32.63±2.14 | 1842.0±382.7 |
| Hospitalized | 459 (28.4%) | 31.60±2.13*** | 1601.8±353.5* |
n – number;
P<0.001 vs. non-hospitalized patients.
Prevalence of ROP
According to the criteria for screening, 1614 subjects were included, of whom 206 were diagnosed with ROP. The overall prevalence of ROP was 12.8% (206/1614). Stage 1, 2, 3, and 4 ROP was found in 64.6% (133/206), 29.6% (61/206), 3.4% (7/206), and 0.49% (1/206) of subjects, respectively. No Stage 5 ROP was observed. In addition, 7.8% (16/206) of subjects required surgical intervention, including 4 patients with threshold ROP and 12 with pre-threshold type 1 ROP.
All the subjects were divided into total population (n=1614), non-hospitalized patients (n=1155) and hospitalized patients (n=459). Additionally, 245, 356, 467, and 546 subjects received diagnosis of ROP in 2009, 2010, 2011, and 2012, respectively.
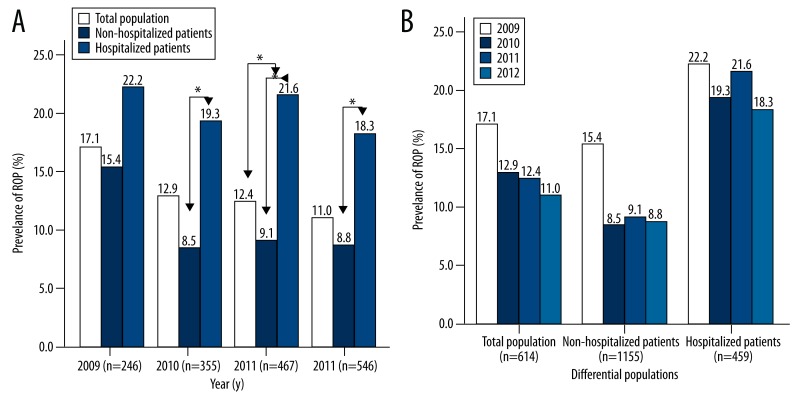
The change in the prevalence of ROP in different populations is shown in Figure 1A. The prevalence of ROP was the highest in hospitalized patients, followed by total population, and non-hospitalized patients had the lowest prevalence. This trend was obvious in 2010 (χ2=8.875, P=0.012), 2011 (χ2=13.04, P=0.001) and 2012 (χ2=8.955, P=0.011), but was not obvious in 2009. In 2009, the prevalence of ROP was not comparable among subjects of different populations (χ2=1.54, P=0.463).
Figure 1.
A) Prevalence of retinopathy of prematurity (ROP) in different years of different populations; (B) Prevalence of ROP of different populations in different years. All the subjects were divided into total population (n=1614), non-hospitalized patients (n=1155) and hospitalized patients (n=459). In addition, 245, 356, 467, and 546 subjects received diagnosis at 2009, 2010, 2011, and 2012, respectively. A value of P<0.0125 was considered statistically significant when comparisons were done between 2 groups (chi-squared test: P=0.05/[n(n−1)/2+1]; n=number of groups=3).
The prevalence of ROP in different years is shown in Figure 1B. The prevalence of ROP remained unchanged in different years (total population: χ2=5.884, P=0.117; non-hospitalized patients: χ2=7.44, P=0.059; hospitalized patients: χ2=0.676, P=0.879).
Correlation between gestational age and ROP
On the basis of complications, these preterm infants were divided into different gestational age groups. Then, the prevalence of ROP was calculated among these groups.
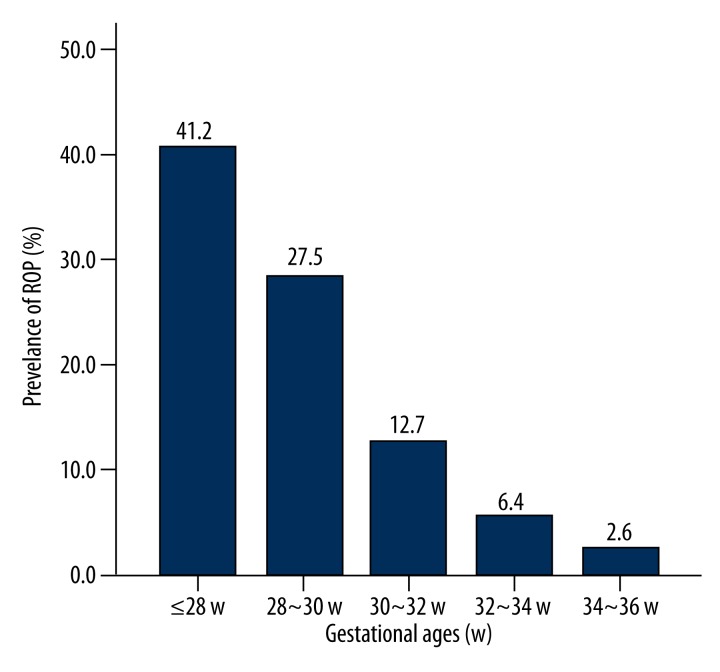
As shown in Figure 2 and Table 2, the prevalence of ROP was 41.2%, 27.5%, 12.7%, 6.4%, and 2.6% in subjects with gestational age of ≤28 w, 28–30 w, 30–32 w, 32–34 w, and 34–36 w, respectively. A significant difference was found among these groups (χ2=157.27, P<0.001): the lower the gestational age, the higher the prevalence of ROP was. As shown in Table 3, a significant difference was noted in the prevalence of ROP among subjects of different gestational ages.
Figure 2.
Prevalence of retinopathy of prematurity (ROP) in subjects of different gestational ages. The prevalence of ROP was 41.2%, 27.5%, 12.7%, 6.4%, and 2.6% in subjects with gestational age of ≤28 w, 28–30 w, 30–32 w, 32–34w, and 34–36 w, respectively.
Table 2.
Prevalence of ROP in subjects of different gestational ages.
| Gestational age (w) | No. of subjects | No. of ROP patients | Prevalence of ROP(%) |
|---|---|---|---|
| ≤28 w | 85 | 35 | 41.2 |
| 28–30 w | 258 | 71 | 27.5 |
| 30–32 w | 464 | 59 | 12.7 |
| 32–34 w | 532 | 34 | 6.4 |
| 34–36 w | 275 | 7 | 2.6 |
No. – number; ROP – retinopathy of prematurity.
Table 3.
Prevalence of ROP in subjects of different gestational ages.
| Gestational age (w) | χ2 | P |
|---|---|---|
| ≤28w/28–30w | 5.585 | <0.0045 |
| ≤28w/30–32w | 41.008 | <0.0045 |
| ≤28w/32–34w | 89.286 | <0.0045 |
| ≤28w/34–36w | 94.026 | <0.0045 |
| 28–30w/30–32w | 24.613 | <0.0045 |
| 28–30w/32–34w | 67.300 | <0.0045 |
| 28–30w/34–36w | 66.458 | <0.0045 |
| 30–32w/32–34w | 11.711 | <0.0045 |
| 30–32w/34–36w | 21.958 | <0.0045 |
| 32–34w/34–36w | 5.559 | <0.0045 |
(P=0.05/[n(n−1)/2+1]; n – number of groups=5; P=0.0045.
Correlation between birth weight and ROP
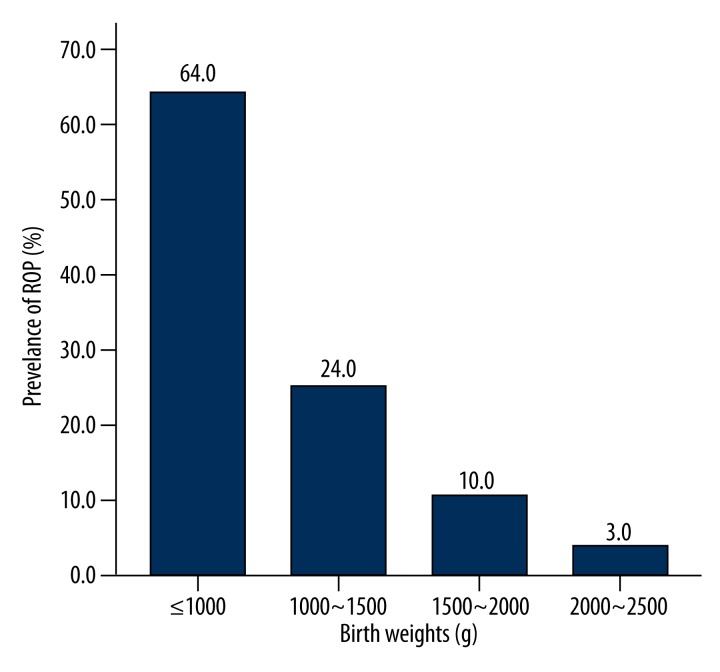
On the basis of birth weight, infants were divided into different birth weight groups. As shown in Figure 3 and Table 4, the prevalence of ROP was 64.0%, 24.0%, 10.0%, and 3.0% in preterm subjects with birth weight of ≤1000 g, 1000–1500 g, 1500–2000 g, and 2000–2500 g, respectively. A significant difference was noted in the prevalence of ROP among these groups (χ2=137.879, P<0.001): the lower the birth weight, the higher the prevalence of ROP was. As shown in Table 5, significant difference was noted in the prevalence of ROP among subjects of different birth weights.
Figure 3.
Prevalence of retinopathy of prematurity (ROP) in preterm infants with different birth weights. The prevalence of ROP was 64.0%, 24.0%, 10.0%, and 3.0% in preterm subjects with birth weight of ≤1000 g, 1000–1500 g, 1500–2000 g, and 2000–2500 g, respectively.
Table 4.
Prevalence of ROP in preterm infants with different birth weights.
| Birth weight (g) | No. of subjects | No. of ROP patients | Prevalence of ROP (%) |
|---|---|---|---|
| ≤1000 | 24 | 15 | 64.0 |
| 1000–1500 | 438 | 103 | 24.0 |
| 1500–2000 | 692 | 72 | 10.0 |
| 2000–2500 | 460 | 16 | 3.0 |
No. – number; ROP – retinopathy of prematurity.
Table 5.
Prevalence of ROP among subjects with different birth weights.
| Birth weight (g) | χ2 | P |
|---|---|---|
| ≤1000/1000–1500 | 18.183 | <0.0071 |
| ≤1000/1500–2000 | 54.194 | <0.0071 |
| ≤1000/2000–2500 | 122.887 | <0.0071 |
| 1000~1500/1500–2000 | 35.230 | <0.0071 |
| 1000~1500/2000–2500 | 78.365 | <0.0071 |
| 1500~2000/2000–2500 | 18.789 | <0.0071 |
(P=0.05/[n(n−1)/2+1]; n – number of groups=4; P=0.0071); ROP – retinopathy of prematurity.
Risk factors of ROP
ROP risk factors (neonatal and maternal factors) of were assigned values, followed by univariate analysis and logistic regression analysis (Tables 6 and 7).
Table 6.
Univariate analysis of risk factors of ROP.
| Risk factors | ROP patients | Non-ROP subjects | t | P | |
|---|---|---|---|---|---|
| Neonatal factors | Gestational age (w) | 30.3±2.2 | 32.0±2.0 | t=6.97 | <0.001 |
| Birth weight (g) | 1415±305 | 1660±350 | t=6.18 | <0.001 | |
| Total days of oxygen supplement (d)* | 27.2±21.8 | 14.3±12.2 | t=–5.46 | <0.001 | |
| Days of mechanical ventilation (d) | 3.6±8.8 | 1.4±3.5 | t=–3.75 | <0.001 | |
| Days of non-mechanical ventilation (d)** | 23.6±18. 4 | 12.9±11.3 | t=–5.35 | <0.001 | |
| Sex (M/F) | 50/42 | 212/155 | χ2=0.351 | 0.554 | |
| Neonatal pneumonia | 87 | 346 | χ2=0.011 | 0.915 | |
| ARDS, apnea, respiratory failure, asphyxia | 55 | 160 | χ2=7.739 | 0.005 | |
| Pulmonary hemorrhage | 6 | 21 | χ2=0.085 | 0.771 | |
| Encephalopathy of preterm infants | 78 | 255 | χ2=8.647 | 0.003 | |
| Sepsis | 11 | 34 | χ2=0.603 | 0.437 | |
| Congenital heart diseases | 47 | 163 | χ2=1.320 | 0.251 | |
| Myocardial injury | 55 | 254 | χ2=2.972 | 0.085 | |
| Coagulation dysfunction | 45 | 179 | χ2=0.001 | 0.981 | |
| Hyperbilirubinemia, pathological jaundice | 49 | 203 | χ2=0.125 | 0.724 | |
| Hypoproteinemia | 36 | 98 | χ2=5.496 | 0.019 | |
| Hypoglycemia | 9 | 39 | χ2=0.056 | 0.813 | |
| Hyperglycemia | 16 | 37 | χ2=3.848 | 0.050 | |
| Anemia | 78 | 255 | χ2=8.647 | 0.003 | |
| Acid-base imbalance | 47 | 167 | χ2=0.921 | 0.337 | |
| Oxygen supplement | 90 | 331 | χ2=5.648 | 0.017 | |
| Mechanical ventilation | 29 | 90 | χ2=1.876 | 0.171 | |
| Blood transfusion | 77 | 206 | χ2=23.642 | <0.001 | |
| Multiple deliveries | 28 | 123 | χ2=2.399 | 0.494 | |
| Vasoactive substances | 57 | 152 | χ2=12.513 | <0.001 | |
| Pulmonary surfactant | 22 | 49 | χ2=6.275 | 0.012 | |
| Erythropoietin | 15 | 49 | χ2=0.535 | 0.465 | |
| Maternal factors | Maternal age | 29.45±5.79 | 29.11±5.45 | t=–0.516 | 0.606 |
| Eutocia/Cesarean section | 43/49 | 158/209 | χ2=0.406 | 0.524 | |
| premature rupture of fetal membranes | 36 | 156 | χ2=0.001 | 0.977 | |
| Amniotic fluid abnormalities | 5 | 27 | χ2=0.419 | 0.517 | |
| Fetal distress | 10 | 45 | χ2=0.135 | 0.713 | |
| Placenta praevia | 3 | 32 | χ2=3.112 | 0.078 | |
| Placental abruption | 3 | 10 | χ2<0.001 | 1.000 | |
| Gestational hypertension | 7 | 34 | χ2=0.248 | 0.619 | |
| Gestational cholestasis | 5 | 87 | χ2=0.002 | 0.961 | |
| Gestational diabetes | 2 | 20 | χ2=1.086 | 0.297 | |
| Anemia in pregnancy | 5 | 24 | χ2=0.152 | 0.697 | |
| Cold | 25 | 110 | χ2=0.278 | 0.598 | |
| Dexamethasone | 20 | 101 | χ2=1.267 | 0.26 | |
| Penicillin | 1 | 14 | χ2=0.976 | 0.323 | |
| Tocolytic agents | 5 | 11 | χ2=0.676 | 0.411 |
Total days of oxygen supplementation = days of mechanical ventilation + days of non-mechanical ventilation;
Days of non-mechanical ventilation = total days of oxygen supplementation via nasal tube, mask or chamber.
ROP – retinopathy of prematurity; ARDS – Acute Respiratory Distress Syndrome; HIE – hypoxic-ischemic encephalopathy.
Table 7.
Logistic regression analysis of risk factors of ROP.
| Risk factor | OR | 95% CI | P |
|---|---|---|---|
| Gestational age | 0.82 | 0.707–0.950 | 0.008 |
| Birth weight | 0.999 | 0.998–1.000 | 0.049 |
| Total days of oxygen supplement | 1.02 | 1.005–1.035 | 0.008 |
| Myocardial injury | 2.327 | 1.383–3.917 | 0.001 |
ROP – retinopathy of prematurity; OR – odds ratio.
As shown in Table 6, among these risk factors, the neonatal factors of gestational age, birth weight, total days of oxygen supplementation, days of mechanical ventilation, ARDS, intracranial hemorrhage, hypoproteinemia, anemia, oxygen supplement, blood transfusion, vasoactive substances, and pulmonary surfactant were related to ROP (P<0.05). Other neonatal factors were not associated with ROP (P>0.05). Maternal factors including maternal age, pattern of delivery, gestational hypertension, premature rupture of membranes, amniotic fluid abnormalities, fetal distress, placenta previa, abruption, gestational cholestasis, pregnancy anemia, colds, and medications (dexamethasone, penicillin, or tocolytic agents) were not associated with ROP (P>0.05).
As shown in Table 7, among these risk factors, low gestational age, low birth weight, total days of oxygen supplementation, and myocardial injury were risk factors closely related to the occurrence of ROP (P<0.05). The other factors were not related to ROP.
Discussion
In the present study, severe ROP was usually found in preterm infants with gestational age of >28 weeks or birth weight of >1500 g (12 patients with threshold ROP or pre-threshold Stage 1 ROP). Once ROP was not identified in these patients, ROP might cause blindness. Thus, criteria from other countries are not applicable in China. In China, the Guidelines for Therapeutic Application of Oxygen and Prevention and Therapy of Retinal Lesions in Preterm Infants were developed in 2004 and used for screening of ROP [24]. On the basis of this definition and Chinese criteria developed in 2004, screening was done in subjects with gestational age of <37 weeks and birth weight of ≤2500 g. This study was conducted to validate the Chinese criteria and provide evidence useful in improving these criteria. The criteria for screening of ROP are developed according to the incidence of ROP and risk factors of ROP. Better criteria for screening can save medical resources, reduce medical risk, and reduce the incidence of mis-screening. Currently, there is no consensus on the criteria for screening of ROP. In the USA, screening is performed in infants with birth weight of <1500 g or gestational age of <28 weeks [20]; in the UK, screening is done in infants with birth weight of <1500 g and/or gestational age of <31 weeks[21]; in Canada, screening is conducted in infants with birth weight of <1500 g or gestational age <30 weeks [22]; and in Germany, screening is done in infants with birth weight of <1501 g and/or gestational age of <32 weeks [23].
Presence of a pre-threshold lesion is an indication for initial laser therapy and cryotherapy [24,25]. With the introduction of the “pre-threshold lesion” concept, studies found that laser therapy and/or cryotherapy conducted within 48 h can significantly improve the poor prognosis in infants with pre-threshold type 1 ROP, thus early intervention is recommended for patients with pre-threshold type 1 ROP [26,27]. In the present study, 6 eyes of 3 patients with threshold ROP and 24 eyes of 12 patients with pre-threshold type 1 ROP received laser therapy or cryotherapy. Other patients with ROP were closely followed up by fundus examination – retinal hemorrhage, macular traction, and retinal detachment were not found (i.e., only 7.3% [15/206] of ROP patients required surgical intervention), which was consistent with the above findings.
The prevalence of ROP varies among races, geographical areas, countries, survival rate of neonates, and level of perinatal care. In the present study, the overall prevalence of ROP was 12.8%, which was lower than that in Germany 36.1%) [6], Sweden (36.4%) [7], and Singapore (29.2%) [8], but comparable to that in developing countries, including Iran (8.5%) [9] and Brazil (18.2%) [10]. This may be attributed to the advanced technology in developed countries, and the survival rate of preterm infants with low gestational age and/or very low birth weight is high, which leads to a high incidence of ROP. However, in developing countries, the perinatal care is imperfect and the survival rate of preterm infants with very low birth weight is at a low level, which leads to a low incidence of ROP. In addition, we postulate that the late starting of retinal screening in developing counties, which may cause missed diagnosis of ROP. In the present study, the incidence of ROP in hospitalized infants was higher than that in non-hospitalized infants. This may be because hospitalized infants had shorter gestational age, lower birth weight, more severe diseases, and more concomitant complications than did non-hospitalized infants. In 2009, this difference was not apparent. It might be because fewer of these cases were collected in 2009 compared to other years. The sample size in our future studies should be expanded, in order to make the samples more equal. From 2009 to 2012, the incidence of ROP remained unchanged, which may be related to the short 4-year time span, thus the survival of preterm infants with very low birth weight remained relatively stable. In addition, the collection of subjects had good equality, and thus the prevalence of ROP remained stable.
Although the pathogenesis of ROP remains poorly understood, premature delivery and low birth weight are widely accepted as risk factors [7,8,16] negatively related to the occurrence of ROP. The lower the gestational age, the more immature the retinal development is and the wider the avascular regions are. Under this condition, the extra-uterine hyperoxic environment may cause damage to the vascular endothelial cells of retinal vessels, resulting in ROP [28]. Our findings fit this hypothesis.
In preterm infants, the development of organs is immature, and the immature retinal vessels are extremely sensitive to oxygen. Prolonged oxygen supplementation, high inspired oxygen concentration, and high arterial oxygen partial pressure may significantly increase the incidence and severity of ROP [29]. Some investigators propose that the occurrence of ROP is related to relative hypoxia: rapid discontinuation of oxygen supplementation after administration of oxygen at a high concentration may cause relative hypoxia, which promotes the occurrence, suggesting that the fluctuation of arterial oxygen partial pressure plays an important role in the pathogenesis of ROP [30]. Although the concentration of oxygen is determined according to Chinese Guidelines [26], but results of the present study indicate that total days of oxygen supplementation is an independent risk factor of ROP. This suggests that standardized oxygen supplementation, strict control of indications for and duration of oxygen supplementation and avoidance of repeated oxygen supplementation are important for preterm infants with low birth weight. Other investigators propose that patterns of oxygen supplementation are also closely related to ROP. There is evidence showing that the incidence of ROP in subjects receiving CPAP or mechanical ventilation is higher than that in subjects receiving oxygen supplement via nasal tube or mask [8]. In the present study, our results showed the patterns of oxygen supplementation had no influence on the incidence of ROP, and days of mechanical ventilation are not an independent risk factor of ROP. However, this needs further study in future investigations because the duration of oxygen supplementation was calculated in the form of days, which is relatively rough (oxygen supplementation for 1–24 h per day was used to define 1 day of oxygen supplementation). This also applies to mechanical ventilation and other patterns of oxygen supplementation, and may fail to accurately reflect the duration of oxygen supplementation.
To date, few studies have been conducted to investigate the correlation between myocardial injury and ROP. There is evidence showing that perinatal hypoxia can cause myocardial injury [31]. In the present study, our results showed myocardial injury was an independent risk factor of ROP. This may be related to the following factors: 1) The organs of preterm infants have immature development and the myocardial contraction and dilation are still imperfect, which may cause the susceptibility to myocardial injury. In addition, hypoxia and acidosis may directly cause damage to myocytes and energy metabolism disorder of myocytes, resulting in myocardial injury. 2) Preterm infants are susceptible to concomitant congenital heart diseases (e.g., patent ductus arteriosus [PDA]) [32]. The above factors may cause reduction in cardiac output and deteriorate hypoxia in tissues, including the retina, which further promotes the occurrence of ROP.
In the present study, univariate analysis showed myocardial injury was not a risk factor of ROP, but logistic regression analysis revealed it was a risk factor. Univariate analysis showed that encephalopathy in preterm infants was a risk factor of ROP, but logistic regression analysis showed it was not a risk factor. This is because multiple factors influence the occurrence of ROP in univariate analysis, which reflects not only the influence of a specific factor, but also the indirect influence of other factors. However, in multivariate analysis, the influence of other factors is excluded, which makes the results more reliable. There is evidence showing that preterm infants with low birth weight have high risk for hypoxic myocardial injury, and the incidence of myocardial injury in infants with low birth weight is significantly higher than that in infants with normal birth weight [33].
Our study has some limitations. The calculation of duration of oxygen supplementation was not precise. In our future study, the duration of oxygen supplementation will be calculated on the basis of hours (not days) of oxygen supplement, and the influence of mechanical ventilation with oxygen and other patterns of oxygen supplementation will be further studied. Although all the hospitalized infants were fed artificially, the feeding data of non-hospitalized infants were not collected. In the Methods section there is nothing mentioned about the feeding or growth recordings of these infants. Thus, the impacts of feeding methods on ROP incidence have not been investigated, which should be addressed in our future studies. In the analysis of risk factors, maternal factors (pregnancy-related diseases and medications during pregnancy) were retrospectively reviewed on the basis of medical records. However, the medical records may contain errors, and this needs to be investigated in prospective studies. The complete medical information of ROP patients has been recorded in detail, and our prospective study is ongoing to investigate the relationship between ROP and development of visual function in children and to explore the influence of ROP on eye development and visual function in infants of various age groups.
Conclusions
The prevalence of ROP in preterm infants is relatively high in southwestern China. Low birth weight, low gestational age, days of oxygen supplementation, and myocardial injury after birth are independent risk factors of ROP.
Footnotes
Source of support: This work was funded by Science and Technology Council of Chongqing (CSTC, 2010AC5140)
References
- 1.Sun BC. Low vision in clinical practice. Huaxia Publishing House. 1999 [Google Scholar]
- 2.Gilbert C, Foster A. Childhood blindness in the context of VISION 2020 – the right to sight. Bull World Health Organ. 2001;79:227–32. [PMC free article] [PubMed] [Google Scholar]
- 3.Courtright P, Hutchinson AK, Lewallen S. Visual impairment in children in middle- and lower-income countries. Arch Dis Child. 2011;96:1129–34. doi: 10.1136/archdischild-2011-300093. [DOI] [PubMed] [Google Scholar]
- 4.Forbes BJ, Khazaeni LM. Evaluation and Management of a Premature Infant’s Eyes. Pediatr Case Rev. 2003;3:105–10. doi: 10.1097/01.PCA.0000063465.08289.80. [DOI] [PubMed] [Google Scholar]
- 5.Lad EM, Hernandez-Boussard T, Morton JM, Moshfeghi DM. Incidence of retinopathy of prematurity in the United States: 1997 through 2005. Am J Ophthalmol. 2009;148:451–58. doi: 10.1016/j.ajo.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Seiberth V, Linderkamp O. Risk factors in retinopathy of prematurity. A multivariate statistical analysis. Ophthalmologica. 2000;214:131–35. doi: 10.1159/000027482. [DOI] [PubMed] [Google Scholar]
- 7.Larsson E, Carle-Petrelius B, Cernerud G, et al. Incidence of ROP in two consecutive Swedish population based studies. Br J Ophthalmol. 2002;86:1122–26. doi: 10.1136/bjo.86.10.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah VA, Yeo CL, Ling YL, Ho LY. Incidence, risk factors of retinopathy of prematurity among very low birth weight infants in Singapore. Ann Acad Med Singapore. 2005;34:169–78. [PubMed] [Google Scholar]
- 9.Saeidi R, Hashemzadeh A, Ahmadi S, Rahmani S. Prevalence and predisposing factors of retinopathy of prematurity in very low-birth-weight infants discharged from NICU. Iran J Pediatr. 2009;19:59–63. [Google Scholar]
- 10.Fortes Filho JB, Eckert GU, Procianoy L, et al. Incidence and risk factors for retinopathy of prematurity in very low and in extremely low birth weight infants in a unit-based approach in southern Brazil. Eye (Lond) 2009;23:25–30. doi: 10.1038/sj.eye.6702924. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Li XX, Yin H, et al. Risk factors for retinopathy of prematurity in six neonatal intensive care units in Beijing, China. Br J Ophthalmol. 2008;92:326–30. doi: 10.1136/bjo.2007.131813. [DOI] [PubMed] [Google Scholar]
- 12.Chang Q, Jiang R, Luo XG, et al. Annual result of retinopathy of prematurity screening in Shanghai area. Chin J Ocul Fundus Dis (Chin) 2008;24:35–37. [Google Scholar]
- 13.Yang XH, Guo R, Yin DM, Zhang YL. Clinical research on incidence and risk factors of retinopathy of prematurity. Journal of Practical Medicine. 2010;26:1536–39. [Google Scholar]
- 14.Zhang GM, Zeng J, Huang LN. Screening results of retinopathy of prematurity in preterm infants in 3 hospital s in Shenzhen. Chinese Journal of Ocular Fundus Diseases. 2008;24:38–40. [Google Scholar]
- 15.Chu ZJ, Wang YS. [Incidence of retinopathy of prematurity in mainland of China over the last 20 years]. Zhonghua Yan Ke Za Zhi. 2012;48:179–83. [PubMed] [Google Scholar]
- 16.Darlow BA, Hutchinson JL, Henderson-Smart DJ, et al. Prenatal risk factors for severe retinopathy of prematurity among very preterm infants of the Australian and New Zealand Neonatal Network. Pediatrics. 2005;115:990–96. doi: 10.1542/peds.2004-1309. [DOI] [PubMed] [Google Scholar]
- 17.Xue XD. Pediatrics. People’s Medical Publishing House. 2012 [Google Scholar]
- 18.An international classification of retinopathy of prematurity. Prepared by an international committee. Br J Ophthalmol. 1984;68:690–97. doi: 10.1136/bjo.68.10.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun ZQ. Medical Statistics. People’s Medical Publishing House. 2004 [Google Scholar]
- 20.Lichtenstein S, Buckley E, Ellis G, et al. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2006;117:572–76. doi: 10.1542/peds.2005-2749. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson AR, Haines L, Head K, Fielder AR. UK retinopathy of prematurity guideline. Early Hum Dev. 2008;84:71–74. doi: 10.1016/j.earlhumdev.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Jefferies A. Retinopathy of prematurity: Recommendations for screening. Paediatr Child Health. 2010;15:667–74. doi: 10.1093/pch/15.10.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jandeck C, Kellner U, Lorenz B, Seiberth V. [Guidelines for ophthalmological screening of premature infants in Germany]. Klin Monbl Augenheilkd. 2008;225:123–30. doi: 10.1055/s-2008-1027168. [DOI] [PubMed] [Google Scholar]
- 24.Infants EfGoPaToRiP. [Guidelines for therapeutic use of oxygen and prevention and treatment of retinopathy in premature infants]. Zhonghua Er Ke Za Zhi. 2007;45:672–73. [PubMed] [Google Scholar]
- 25.Group CfRoPC. Multicenter trial of cryotherapy for retinopathy of prematurity. One-year outcome – structure and function. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol. 1990;108:1408–16. [PubMed] [Google Scholar]
- 26.Early Treatment For Retinopathy Of Prematurity Cooperative G. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–94. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 27.Huang LN, Zhang GM, Wu BQ. Retinal lesions in premature infants. Guangdong Science and Technology Publishing House. 2007 [Google Scholar]
- 28.Hartnett ME. Studies on the pathogenesis of avascular retina and neovascularization into the vitreous in peripheral severe retinopathy of prematurity (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2010;108:96–119. [PMC free article] [PubMed] [Google Scholar]
- 29.Gallo J, Jacobson L, Broberger U. Perinatal factors associated with retinopathy of prematurity. Acta Paediatrica. 1993;82:829–34. doi: 10.1111/j.1651-2227.1993.tb17621.x. [DOI] [PubMed] [Google Scholar]
- 30.York JR, Landers S, Kirby RS, et al. Arterial oxygen fluctuation and retinopathy of prematurity in very-low-birth-weight infants. J Perinatol. 2004;24:82–87. doi: 10.1038/sj.jp.7211040. [DOI] [PubMed] [Google Scholar]
- 31.Szymankiewicz M, Matuszczak-Wleklak M, Vidyasagar D, Gadzinowski J. Retrospective diagnosis of hypoxic myocardial injury in premature newborns. J Perinat Med. 2006;34:220–25. doi: 10.1515/JPM.2006.040. [DOI] [PubMed] [Google Scholar]
- 32.Agarwal R, Deorari AK, Paul VK. Patent ductus arteriosus in preterm neonates. Indian J Pediatr. 2008;75:277–80. doi: 10.1007/s12098-008-0059-9. [DOI] [PubMed] [Google Scholar]
- 33.Li CY, Liu YX, Li L, et al. A clinical study on the myocardial injury in HIE and its relationship with birth status. Chinese Pediatrics of Integrated Traditional and Western Medicine. 2010;02:471–72. [Google Scholar]