Abstract
Background
The aim of this study was to investigate the influence of carotid artery stenting (CAS) on the cognition and quality of life of elderly patients with severe stenosis of the internal carotid artery.
Material/Methods
65 elderly patients with symptomatic severe stenosis of internal carotid artery were recruited into 2 groups: the pharmacotherapy group (n=29) and the CAS group (n=36). Before surgery and 1, 3, 6, and 12 months after surgery, Montreal cognitive assessment (MoCA) was used for the evaluation of cognition and WHOQOL-BREF was used for the assessment of quality of life.
Results
At 12 months after surgery, total MoCA score and WHOQOL-BREF score in the pharmacotherapy group was significantly reduced when compared with those before surgery (P<0.05). In the CAS group, the total MoCA score, scores of attention and delayed recall, and WHOQOL-BREF score increased significantly at different time points after surgery when compared with those before surgery (P<0.05). Moreover, in CAS group, the MoCA score and WHOQOL-BREF markedly increased gradually over time (P<0.05). Compared with the pharmacotherapy group, cognition and quality of life in the CAS group were improved dramatically during the follow-up period (P<0.05).
Conclusions
Severe stenosis of the internal carotid artery is a cause of cognition impairment, and CAS may improve cognition and quality of life.
MeSH Keywords: Angioplasty, Carotid Stenosis, Cognition Disorders, Quality of Life
Background
Carotid artery stenosis due to carotid atherosclerosis is not only an independent risk factor of ischemic cerebrovascular disease, but is also closely related to the cognition impairment [1]. Mild cognition impairment (MCI) is reversible [2], and treatment of carotid artery stenosis is likely to attenuate the MCI and control the development of dementia [3]. Currently, carotid artery stenosis due to carotid atherosclerosis is treated with drugs, carotid endarterectomy (CEA), and carotid artery stenting (CAS). Although CEA has been regarded a the criterion standard in the treatment of carotid artery stenosis [4], CAS has been widely applied in the treatment of carotid artery stenosis because it does not need general anesthesia, is minimally invasive, and has definite effectiveness during the progression of techniques and materials used for neurological interventions [5,6].
Currently, there is controversy on the influence of CAS on the cognition of patients with internal carotid artery stenosis [7–9], and in most studies, the cognition was compared before and after surgery and there were no control patients treated with drugs alone. In the present study, patients with internal carotid artery stenosis were divided into a CAS group and a pharmacotherapy group according to the therapeutic strategies, and were followed up. Patients treated with drugs alone served as a control group. Our study aimed to investigate the influence of CAS on the cognition and quality of life of elderly patients with severe internal carotid artery stenosis.
Material and Methods
Patients
From May 2008 to December 2012, a total of 36 inpatients received CAS due to severe internal carotid artery stenosis in the Department of Neurology. There were 20 males and 16 females, with a mean age of 72.1±4.4 years (range: 63–80 years). Of these patients, 14 patients presented transient ischemic attack (TIA) and 22 manifested minor stroke. In addition, concomitant hypertension was found in 28 patients, diabetes in 14, hyperlipidemia in 11, smoking in 16, drinking in 12, and coronary heart disease in 15.
In the control group, inpatients with severe symptomatic internal carotid artery stenosis had indications for CAS or CEA, but refused to receive CAS or CEA. Thus, they were treated with drugs alone. There were 29 patients in the control group, including 14 males and 15 females. The mean age was 73.1±5.1 years (range: 62–82 years). Of these patients, 11 patients manifested TIA and 18 showed minor stroke. In addition, hypertension was observed in 24 patients, diabetes in 12, hyperlipidemia in 9, smoking in 11, drinking in 10, and coronary heart disease in 13.
Inclusion criteria were: 1) symptomatic carotid stenosis and received digital subtraction angiography (DSA) of the whole brain; 2) DSA showed the internal carotid artery stenosis of greater than 70%, and patients could cooperate with physicians to perform the neuropsychological assessment; and 3) informed consent was obtained from patients and/or their relatives.
Exclusion criteria: 1) disturbance of consciousness; 2) patients had hemorrhagic cerebrovascular disease; 3) massive infarction of the cerebral cortex, basal ganglia, thalamus or brainstem; 4) concomitant intracranial arterial stenosis of >50% or intracranial arterial occlusion; 5) severe stenosis or occlusion of the contralateral carotid artery; 6) cerebral vascular malformations and/or aneurysms; 7) cranial CT or MRI showed intracranial space-occupying lesions; 8) diagnosed with psychotic disorders after evaluation of mental states (perception, thinking, behavior and emotion); (9) systemic severe diseases influencing the cognition and the evaluation of cognition.
All procedures were conducted in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration as revised in 1983. Carotid stenosis was diagnosed according to the criteria in the North American Symptomatic Carotid Endarterectomy Trial (NASCET) [10]. Stenosis degree = (1−diameter of the most stenotic vessels/diameter of proximal normal vessels) ×100%
Internal carotid artery stenting
Before surgery, detection of coagulation function, routine blood test, electrocardiography, chest X-ray, and heart ultrasonography were performed and patients were treated with oral aspirin at 100 mg/d and oral clopidogrel at 75 mg/d. Patients received no food or water for 6 h before surgery. Following focal anesthesia, Seldinger technique was used to puncture the right femoral artery, and an 8F vascular sheath was used, followed by insertion of an 8F catheter. Under the guidance of a wire, the catheter was inserted to the proximal part of the lesioned vessel. Heparin (5000 U) was intravenously injected for systemic heparinization. Under the guidance of a road map, a protective umbrella (Spider FX, EV3; Angioguard, Cordis; Accunet, Abbott Laboratories) was carefully inserted through the stenotic site, and a stent (Precise, Cordis; Acculink, Abbott Laboratories) was then inserted along the umbrella. After accurately locating the stent, the stent was released. The balloon (Viatrac, Abbott Laboratories) was selected according to the degree of stenosis, and then the umbrella was expanded, followed by retraction of the umbrella and performance of radiography. After surgery, the arterial sheath was indwelt for the natural neutralization of heparin. The electrocardiogram was monitored, and the sheath was removed 4 h later. On the day of surgery, aspirin and clopidogrel were administered, and the doses were identical to those before surgery. Six months later, aspirin treatment was stopped, and treatment with oral clopidogrel at 75 mg/d was continued. All the patients were treated with atorvastatin at 20 mg/d, and risk factors of stroke were also controlled after surgery.
Treatments in control group
After admission, patients were treated with oral aspirin at 100 mg/d and clopidogrel at 75 mg/d for 1 month. Then, aspirin treatment was stopped, and 75 mg/d clopidogrel treatment continued. All the patients were treated with atorvastatin (20–40 mg/d). The blood LDL-C was reduced to lower than 1.8 mmol/L (70 mg/dL) or by ≥50%, and risk factors of stroke were also controlled during the treatments.
Assessments
Assessment of cognition
Montreal cognitive assessment (MoCA) was used for the assessment of cognition [11]. The total score of the MoCA scale is 30 and the higher the score, the better the cognition. In the evaluation, a score of ≥26 was regarded normal, and an additional 1 point was added when the duration of education was ≤12 years. The assessment of cognition was performed by investigators who received standard training under the supervision of a neuropsychological expert. Cognition assessment was done on admission and at 1, 3, 6, and 12 months after surgery in the same room by the same investigator.
Assessment of depression and anxiety
The Hamilton Depression Rating Scale (HAMD) [12] was used to evaluate the degree of depression on a 5-point scale (0–4), and the total score range is 0 to 68. The higher the score, the more severe the depression is. A total score of <7 is regarded as normal, and a total score of 7–17 indicates possible depression. A total score of 18–24 can be used for the diagnosis of depression and a total score of >24 suggests severe depression. The Hamilton Anxiety Rating Scale (HAM-A) [13] was used for the evaluation of the degree of anxiety on a 5-point scale (0–4). The total score ranges from 0 to 56 and the higher the score, the more severe the anxiety is. A total score of <7 is regarded as absence of anxiety, a total score of 7–13 indicates possible mild anxiety, a total score of 14–20 indicates definite anxiety, and a total score of >20 indicates severe anxiety. The evaluation with HAMD and HAMA was performed in the same manner as in assessment with MoCA.
Evaluation of quality of life
The WHO Quality of Life-BREF (WHOQOL-BREF) (Chinese edition) was used to evaluate the quality of life. There are 4 domains (physiology domain, psychology domain, social relations domain, and environmental domain) and 24 items in this scale. The higher the score, the better the quality of life is. In each domain, the mean score of items is calculated and multiplied by 4. The resultant score is comparable to the score of WHOQOL-100. The total score is the sum of scores from each domain. The higher the score, the better the quality of life is.
Detection of blood flow velocity of lesioned vessels
Transcranial Doppler (TCD) was employed to detect the blood flow velocity of the carotid artery. The Media Velocity (Vm) at the lesioned site was determined on admission and at 1, 3, 6, and 12 months after surgery.
Evaluation of stroke recurrence
The TIA and stroke were monitored after surgery, and cranial CT/MRI was performed to evaluate the recurrence rate of stroke.
Statistical analysis
Statistical analysis was performed with SPSS version 10.0. Quantitative data are expressed as mean ± standard deviation, and qualitative data as proportion (%). The comparisons of quantitative data were done with the chi-square test, and means were compared with t test between 2 groups and 1-way analysis of variance, followed by least-significant difference (LSD) test among groups. A value of P<0.05 was considered statistically significant.
Results
Comparisons of clinical information at baseline between two groups
On the basis of inclusion and exclusion criteria, a total of 65 patients were recruited into the present study and received regular follow-up. None were lost to follow-up. There were 36 patients in the CAS group and 29 patients in the pharmacotherapy group. There were no marked differences in gender, age, education level, degree of stenosis, hypertension, coronary heart disease, diabetes, abnormal lipid metabolism, smoking, drinking, body mass index, or location of lesioned vessels between the 2 group (P>0.05) (Table 1).
Table 1.
Clinical information of patients in two groups at baseline.
| Parameter | CAS group (N=36) | Control group (N=29) | Statistic values |
|---|---|---|---|
| Sex (male/female)-no. | 20/16 | 14/15 | x2=0.341; p=0.5591 |
| Age (mean ±SD) | 72.1±4.4 | 73.1±5.1 | t=0.814; p=0.4185 |
| Education (mean±SD) | 8.1±3.7 | 8.8±3.4 | t=0.786; p=0.4349 |
| Degree of stenosis (%) | 81.1±7.5 | 79.2±3.2 | t=−1.273; p=0.2078 |
| Hypertension (n,%) | 28 (77.8) | 24 (82.8) | x2=0.249; p=0.6178 |
| Coronary heart disease (n,%) | 15 (41.7) | 13 (44.8) | x2=0.065; p=0.7981 |
| Diabetes (n,%) | 14 (38.9) | 12 (41.4) | x2=0.042; p=0.8386 |
| Abnormal lipid metabolism (n,%) | 11 (30.6) | 9 (31.0) | x2=0.002; p=0.9668 |
| Smoking (n,%) | 16 (44.4) | 11 (37.9) | x2=0.281; p=0.5963 |
| Drinking (n,%) | 12 (33.3) | 10 (34.5) | x2=0.009; p=0.9224 |
| Body mass index (kg/m2) | 26.3±3.1 | 25.8±3.0 | t=−0.656; p=0.5144 |
| Location of lesioned vessels | |||
| Left | 20 (55.6) | 13 (44.8) | x2=0.740; p=0.3898 |
| Right | 16 (44.4) | 16 (55.2) | x2=0.740; p=0.3898 |
| Right-handed (n,%) | 36 (100.0) | 29 (100.0) | |
Post-operative findings in CAS patients
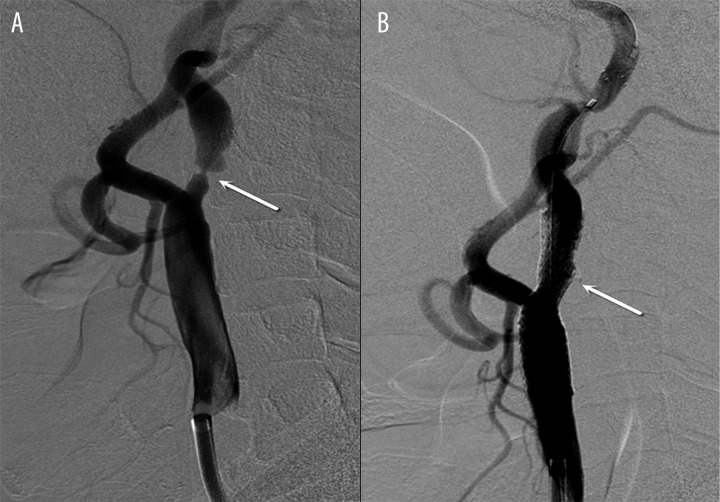
In the CAS group, the stent was accurately released (Figure 1A, 1B), with a success rate of 100%. The mean degree of stenosis was 81.1±7.5% (range: 70–98%) before surgery. After CAS, the mean degree of stenosis was 10.4±7.2% (range: 0–25%). The degree of stenosis was significantly reduced after surgery (P<0.01).
Figure 1.
Internal carotid artery stenosis of 98% before surgery (A) and 8% after surgery (B). Arrow: stenotic site.
Of 5 patients receiving balloon dilatation and stenting, 3 developed a transient slowing of heart rate (40~50 beats/min). After intravenous treatment with atropine (1.0 mg) for 1–3 min, the heart rate returned to normal. Two patients developed reduction in blood pressure after stenting (70~90/50~60 mmHg), which returned to normal after intravenous injection of dopamine hydrochloride for 6–12 h. Other peri-operative complications were not observed in these patients.
Scores of MoCA, HAMD, HAMA, and WHOQOL-BREF at baseline and during follow-up period
At baseline, there were no marked differences in the scores of MoCA, HAMD, HAMA, and WHOQOL-BREF (P>0.05). In the pharmacotherapy group, the scores of MoCA and WHOQOL-BREF at month 12 was significantly reduced when compared with those at baseline (P<0.05). In CAS group, the total MoCA score, scores of attention and delayed recall, and WHOQOL-BREF score at months 1, 3, 6, and 12 increased significantly when compared with those at baseline (P<0.05), and the scores of MoCA and WHOQOL-BREF increased significantly over time (P<0.05). In the CAS group, the total MoCA score, scores of drawing clock, attention and delayed recall, and scores of HAMD, HAMA, and quality of life were significantly improved at 1, 3, 6, and 12 months after surgery when compared with pharmacotherapy at the same time points (P<0.05) (Table 2).
Table 2.
Scores of cognition, HAMD, HAMA and WHOQOL-BREF of patients in two groups at baseline and different time points after surgery
| Groups | MoCA score | Line connection test | Copy cube | Drawing clock | Naming | Attention | Sentence repeating | Verbal fluency | Abstraction | Delayed recall | Orientation | HAMD | HAMA | WHOQOL-BREF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control baseline | 24.59±2.51 | 0.66±0.48 | 0.79±0.41 | 2.21±0.73 | 2.62±0.49 | 3.97±1.02 | 1.83±0.38 | 0.79±0.41 | 1.86±0.35 | 3.52±0.74 | 5.62±0.62 | 14.72±4.67 | 14.34±4.78 | 55.76±8.65 |
| 1 month | 24.34±2.41 | 0.55±0.51 | 0.72±0.45 | 1.97±0.78 | 2.66±0.48 | 3.93±0.84 | 1.86±0.35 | 0.79±0.41 | 1.83±0.38 | 3.72±0.84 | 5.59±0.63 | 14.90±4.93 | 13.17±3.55 | 54.65±7.10 |
| 3 month | 24.93±2.34 | 0.69±0.47 | 0.69±0.47 | 2.17±0.71 | 2.62±0.49 | 4.03±0.91 | 1.90±0.31 | 0.83±0.38 | 1.86±0.35 | 3.79±0.82 | 5.62±0.62 | 14.66±4.58 | 13.66±3.93 | 54.49±6.30 |
| 6 month | 24.17±2.36 | 0.62±0.49 | 0.66±0.48 | 1.97±0.73 | 2.66±0.48 | 3.97±0.91 | 1.86±0.35 | 0.76±0.44 | 1.83±0.38 | 3.59±0.68 | 5.55±0.63 | 14.07±4.37 | 12.93±4.24 | 54.10±5.89 |
| 12 month | 23.34±2.51#,* | 0.52±0.51 | 0.66±0.50 | 1.93±0.70 | 2.66±0.48 | 3.62±0.82* | 1.79±0.41 | 0.79±0.41 | 1.76±0.44 | 3.41±0.73* | 5.48±0.63 | 13.83±4.03 | 12.14±3.69# | 53.53±5.96# |
| CAS baseline | 24.17±2.50 | 0.67±0.48 | 0.81±0.40 | 2.33±0.68 | 2.67±0.48 | 3.86±0.96 | 1.81±0.40 | 0.81±0.40 | 1.78±0.42 | 3.11±1.09 | 5.58±0.65 | 15.42±4.46 | 16.42±6.19 | 54.55±8.95 |
| 1 month | 25.58±2.26#,a | 0.75±0.44b | 0.86±0.35 | 2.53±0.51a | 2.72±0.45 | 4.31±1.04# | 1.86±0.35 | 0.86±0.35 | 1.81±0.40 | 3.56±0.88# | 5.58±0.65 | 11.94±4.13#,a | 10.22±3.73#,a | 56.26±8.45 |
| 3 month | 26.86±1.87#,*,a | 0.78±0.42 | 0.83±0.38 | 2.53±0.51a | 2.75±0.44 | 5.03±0.97#,a | 1.92±0.28 | 0.92±0.28 | 1.75±0.44 | 4.08±0.87# | 5.58±0.55 | 12.03±4.94#,a | 10.19±3.61#,a | 57.08±7.43#,b |
| 6 month | 27.75±1.3#,*,a | 0.67±0.48 | 0.92±0.28a | 2.56±0.50a | 2.61±0.49 | 5.50±0.65#,a | 1.86±0.35 | 0.92±0.28b | 1.69±0.47 | 4.53±0.61#,a | 5.75±0.50 | 11.75±5.26#,b | 10.44±4.28#,b | 58.18±7.45#,a |
| 12 month | 28.44±1.16#,*,a | 0.78±0.42b | 0.78±0.46 | 2.56±0.50a | 2.61±0.49 | 5.81±0.40#,a | 1.83±0.38 | 0.94±0.23b | 1.75±0.44 | 4.86±0.35#,a | 5.78±0.48b | 11.50±5.68#,b | 11.14±5.11#,b | 59.02±6.6#,&,a |
p<0.05 vs. baseline;
p<0.05 vs. month 1 in the same group;
p<0.05 vs month 3 in the same group;
p<0.01;
p<0.05 vs. control group.
Blood flow velocity of stenotic vessels
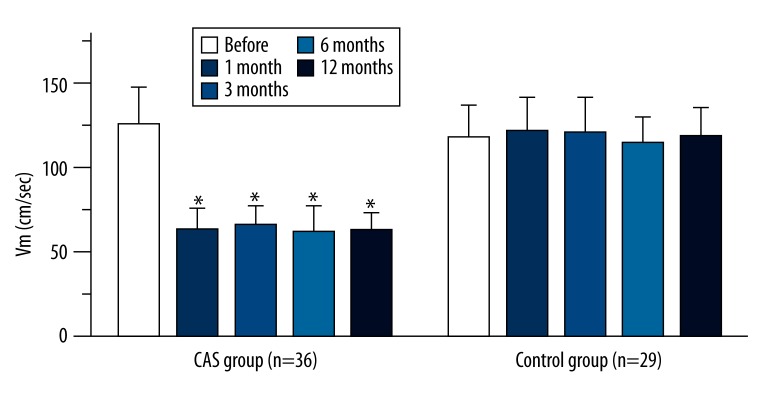
In both groups, blood flow signals were observed at the internal carotid arteries, and Vm of both groups are shown in Figure 2. Results showed the Vm of the internal carotid artery was at a high level before surgery, but reduced markedly after surgery and remained within the normal range. At all time points after surgery, the Vm was significantly different from that before surgery (P<0.01). At 1, 3, 6, and 12 months after surgery, the Vm remained unchanged in the CAS group (P>0.05). In the pharmacotherapy group, the Vm remained at a high level at all time points during the follow-up period (P>0.05).
Figure 2.
Vm (cm/sec) of internal carotid artery in the 2 groups (means ±SD). * P<0.01 vs. before SAA.
Recurrence of stroke
In the CAS group, there was no recurrence of stroke observed during the follow-up period. At 12 months after surgery, cranial MRI did not show asymptomatic cerebral infarction. In the control group, 7 (24.1%) patients had stroke recurrence (5 had symptomatic cerebral infarction and 2 had asymptomatic cerebral infarction), and the infarct site was consistent with the region supplied by the internal carotid artery.
Discussion
Carotid artery stenosis is not only an important cause of ischemic cerebrovascular diseases, but is also closely associated with cognition impairment [14]. When the ischemic or infarct site involves tissues related to cognition, patients may develop clinical manifestations of cognition impairment [15]. Especially, symptomatic carotid artery stenosis usually causes severe vascular cognition impairment, or even increases the risk for vascular dementia. However, this risk factor is preventable and treatable [1,16]. Thus, carotid artery stenosis plays an important role in the occurrence and development of vascular dementia [17]. Barnes et al. [18] found that carotid artery stenosis could increase the risk for mild cognition impairment and this relationship still existed after adjusting for other risk factors. Studies [19] have shown that severe carotid artery stenosis may cause chronic low perfusion, and the long-lasting insufficient perfusion may impair the energy metabolism in neurons, causing cognition impairment to various extents [20]. After the stenosis is improved, the perfusion increases, and the metabolism in cells is subsequently also improved [21]. Of these patients in our study, 16 patients (55.2%) in the pharmacotherapy group and 19 patients (52.8%) in the CAS group were diagnosed with mild cognition impairment. Thus, for patients with severe internal carotid artery stenosis, we should emphasize both the prevention of stroke and the cognition.
With the development of the materials for interventional therapy and the neuroimaging techniques, especially the use of an embolic protection device in the treatment of cerebral infarction [22], CAS treatment of severe carotid artery stenosis is widely applied, and it has fewer complications [23]. Previous studies have shown that CAS can reduce the risk for stroke in patients with symptomatic or asymptomatic carotid artery stenosis. However, there is still controversy about the influence of CAS on cognition in patients with severe carotid artery stenosis. Some studies showed CAS may increase the neuropsychological score in these patients [24], but other studies reported that patients with severe carotid artery stenosis develop cognition impairment after CAS [9,25]. Thus, it is necessary to further investigate the influence of CAS on cognition in patients with severe carotid artery stenosis. In previous studies, the therapeutic efficacy was determined after comparison between manifestations before and after surgery, or patients receiving coronary stenting served as a control group. In these studies, there might be differences in clinical information at baseline. In our study, patients with severe carotid artery stenosis who received pharmacotherapy alone were recruited as a control group, which assured consistency in the clinical information at baseline. Our results showed that in the CAS group, clinical symptoms and cognition were improved after CAS. In addition, the MoCA score increased over time, suggesting the continuation of therapeutic effectiveness.
In the present study, our results showed CAS could improve cognition of patients with carotid artery stenosis, and the potential mechanisms include: (1) CAS improves cerebral perfusion, and some studies have shown that low perfusion is related to cognition impairment [26–28]. In the present study, vascular reconstruction was performed via stenting, which improves the blood supply to the brain and increases the cerebral perfusion. Monitoring of blood flow velocity by using TCD showed the hemodynamics of the lesioned vessels was also significantly improved, and this improvement remained over time. (2) CAS reduces the incidence of asymptomatic cerebral infarction; the stent may prevent the shedding of atheromatous plaques, which then reduces the risk for stroke due to the shedding of atheromatous plaques [29]. In addition, the protective umbrella further reduces the incidence of intraoperative embolization and decreases the incidence of cerebral infarction, which may prevent infarction-induced cognition impairment.
Patients usually develop negative emotional reactions after stroke, and anxiety and depression are the most common reactions in psychological stress. Approximately 36% of patients with stroke have symptoms of anxiety [30], and the incidence of depression is about 20–50% after stroke [31]. CAS is an invasive therapy and patients usually have little knowledge about precautions in this therapy as compared to other surgeries. As a “surgery”, CAS may cause stress in patients, and influence the emotion and psychology of patients to different extents. A previous study [32] has shown that the incidence of anxiety and depression is high before interventional therapy, the symptoms of depression and anxiety attenuated, and the incidence of anxiety and depression reduces after surgery. Our findings were consistent with previous reports. In the CAS group, the pre-operative incidence of depression and anxiety was 25.0% and 55.6%, respectively, which were significantly higher than those in the pharmacotherapy group (24.1% and 37.9%, respectively). However, after interventional therapy, the scores of HAMD and HAMA reduced significantly, and were markedly lower than those in the pharmacotherapy group. This suggests that negative emotions are improved after CAS. However, the scores of HAMD and HAMA remained unchanged in the pharmacotherapy group. This is ascribed to the elimination of stress, improvement of clinical symptoms, and low incidence of recurrent stroke, which improves the affective disorder.
With the transition of biological medicine into biological, psychological, and social medicine, a new series of parameters have been developed for the accommodation to this new medical mode in the evaluation of health using quality of life (QOL). Increased QOL has been used as an objective indicator for the drafting, implement, and evaluation of therapeutic efficacy, and as an ultimate goal in rehabilitation. Of scales used for evaluation of QOL, WHOQOL-BREF is the most often used. WHOQOL-BREF is derived from WHOQOL-100. In the WHOQOL-BREF, the 6 domains of WHOQOL-100 are reduced to 4 domains (physiology, psychology, social relationship, and environment). Studies have shown the favorable reliability and validity of WHOQOL-BREF [33–35]. In the present study, WHOQOL-BREF was used to evaluate the QOL of patients with severe internal carotid artery stenosis at different time points, aiming to evaluate the efficacy of interventional therapy and pharmacotherapy from the view of patients themselves. Our results showed that QOL was significantly improved after CSA.
Conclusions
Severe internal carotid artery stenosis may cause cognition impairment. For older patients with severe symptomatic internal carotid artery stenosis, CAS can improve cognition and QOL. In the present study, patients having indications for CAS or CEA, but refusing to receive surgical interventions, were recruited as the control group, which assures consistency in clinical information between the 2 groups and avoids the influence of other factors having effects on cognition. However, sample size was relatively small in our study, and further studies with larger sample sizes are required to confirm our findings.
Footnotes
Source of support: Departmental sources
References
- 1.Chmayssani M, Festa JR, Marshall RS. Chronic ischemia and neurocognition. Neuroimaging Clin N Am. 2007;17:313–24. viii. doi: 10.1016/j.nic.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Barnett HJ. Carotid disease and cognitive dysfunction. Ann Intern Med. 2004;140:303–4. doi: 10.7326/0003-4819-140-4-200402170-00013. [DOI] [PubMed] [Google Scholar]
- 3.De Rango P, Caso V, Leys D, et al. The role of carotid artery stenting and carotid endarterectomy in cognitive performance: a systematic review. Stroke. 2008;39:3116–27. doi: 10.1161/STROKEAHA.108.518357. [DOI] [PubMed] [Google Scholar]
- 4.Luebke T, Aleksic M, Brunkwall J. Meta-analysis of randomized trials comparing carotid endarterectomy and endovascular treatment. Eur J Vasc Endovasc Surg. 2007;34:470–79. doi: 10.1016/j.ejvs.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Mantese VA, Timaran CH, Chiu D, et al. The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST): stenting versus carotid endarterectomy for carotid disease. Stroke. 2010;41:S31–34. doi: 10.1161/STROKEAHA.110.595330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radu H, Bertog SC, Robertson G, et al. Long-term results after carotid stent implantation. J Interv Cardiol. 2013;26:613–22. doi: 10.1111/joic.12077. [DOI] [PubMed] [Google Scholar]
- 7.Zhou W, Hitchner E, Gillis K, et al. Prospective neurocognitive evaluation of patients undergoing carotid interventions. J Vasc Surg. 2012;56:1571–78. doi: 10.1016/j.jvs.2012.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ortega G, Alvarez B, Quintana M, et al. Cognitive improvement in patients with severe carotid artery stenosis after transcervical stenting with protective flow reversal. Cerebrovasc Dis. 2013;35:124–30. doi: 10.1159/000346102. [DOI] [PubMed] [Google Scholar]
- 9.Gaudet JG, Meyers PM, McKinsey JF, et al. Incidence of moderate to severe cognitive dysfunction in patients treated with carotid artery stenting. Neurosurgery. 2009;65:325–29. doi: 10.1227/01.NEU.0000349920.69637.78. discussion 29–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–53. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 11.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–99. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 14.Arntzen KA, Schirmer H, Johnsen SH, et al. Carotid atherosclerosis predicts lower cognitive test results: a 7-year follow-up study of 4,371 stroke-free subjects – the Tromso study. Cerebrovasc Dis. 2012;33:159–65. doi: 10.1159/000334182. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien JT. Vascular cognitive impairment. Am J Geriatr Psychiatry. 2006;14:724–33. doi: 10.1097/01.JGP.0000231780.44684.7e. [DOI] [PubMed] [Google Scholar]
- 16.Kim JE, Lee BR, Chun JE, et al. Cognitive dysfunction in 16 patients with carotid stenosis: detailed neuropsychological findings. J Clin Neurol. 2007;3:9–17. doi: 10.3988/jcn.2007.3.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright CB, Elkind MS, Rundek T, et al. Alcohol intake, carotid plaque, and cognition: the Northern Manhattan Study. Stroke. 2006;37:1160–64. doi: 10.1161/01.STR.0000217439.73041.b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes DE, Alexopoulos GS, Lopez OL, et al. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Arch Gen Psychiatry. 2006;63:273–79. doi: 10.1001/archpsyc.63.3.273. [DOI] [PubMed] [Google Scholar]
- 19.Hillis AE, Barker PB, Beauchamp NJ, et al. MR perfusion imaging reveals regions of hypoperfusion associated with aphasia and neglect. Neurology. 2000;55:782–88. doi: 10.1212/wnl.55.6.782. [DOI] [PubMed] [Google Scholar]
- 20.Ghogawala Z, Westerveld M, Amin-Hanjani S. Cognitive outcomes after carotid revascularization: the role of cerebral emboli and hypoperfusion. Neurosurgery. 2008;62:385–95. doi: 10.1227/01.neu.0000316005.88517.60. discussion 93–95. [DOI] [PubMed] [Google Scholar]
- 21.Saito H, Ogasawara K, Nishimoto H, et al. Postoperative changes in cerebral metabolites associated with cognitive improvement and impairment after carotid endarterectomy: a 3T proton MR spectroscopy study. Am J Neuroradiol. 2013;34:976–82. doi: 10.3174/ajnr.A3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stabile E, Salemme L, Sorropago G, et al. Proximal endovascular occlusion for carotid artery stenting: results from a prospective registry of 1,300 patients. J Am Coll Cardiol. 2010;55:1661–67. doi: 10.1016/j.jacc.2009.11.079. [DOI] [PubMed] [Google Scholar]
- 23.Dieter RS, Laird JR. Carotid artery stenting: update. Int J Cardiovasc Intervent. 2005;7:126–33. doi: 10.1080/14628840510039559. [DOI] [PubMed] [Google Scholar]
- 24.Raabe RD, Burr RB, Short R. One-year cognitive outcomes associated with carotid artery stent placement. J Vasc Interv Radiol. 2010;21:983–98. doi: 10.1016/j.jvir.2010.03.011. quiz 99. [DOI] [PubMed] [Google Scholar]
- 25.Chida K, Ogasawara K, Suga Y, et al. Postoperative cortical neural loss associated with cerebral hyperperfusion and cognitive impairment after carotid endarterectomy: 123I-iomazenil SPECT study. Stroke. 2009;40:448–53. doi: 10.1161/STROKEAHA.108.515775. [DOI] [PubMed] [Google Scholar]
- 26.Demarin V, Zavoreo I, Kes VB. Carotid artery disease and cognitive impairment. J Neurol Sci. 2012;322:107–11. doi: 10.1016/j.jns.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Balucani C, Viticchi G, Falsetti L, Silvestrini M. Cerebral hemodynamics and cognitive performance in bilateral asymptomatic carotid stenosis. Neurology. 2012;79:1788–95. doi: 10.1212/WNL.0b013e318270402e. [DOI] [PubMed] [Google Scholar]
- 28.Pereira FM, Ferreira ED, de Oliveira RM, Milani H. Time-course of neurodegeneration and memory impairment following the 4-vessel occlusion/internal carotid artery model of chronic cerebral hypoperfusion in middle-aged rats. Behav Brain Res. 2012;229:340–48. doi: 10.1016/j.bbr.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 29.Yan Y, Liang L, Chen T, et al. Treatment of symptomatic vertebrobasilar artery stenosis with stent-assistant angioplasty in the elderly. Rev Assoc Med Bras. 2012;58:422–26. [PubMed] [Google Scholar]
- 30.Bergersen H, Froslie KF, Stibrant Sunnerhagen K, Schanke AK. Anxiety, depression, and psychological well-being 2 to 5 years poststroke. J Stroke Cerebrovasc Dis. 2010;19:364–69. doi: 10.1016/j.jstrokecerebrovasdis.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Barker-Collo SL. Depression and anxiety 3 months post stroke: prevalence and correlates. Arch Clin Neuropsychol. 2007;22:519–31. doi: 10.1016/j.acn.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Development of the World Health Organization WHOQOL-BREF quality of life assessment. The WHOQOL Group. Psychol Med. 1998;28:551–58. doi: 10.1017/s0033291798006667. [DOI] [PubMed] [Google Scholar]
- 33.Stroobant N, Vingerhoets G. Depression, anxiety, and neuropsychological performance in coronary artery bypass graft patients: a follow-up study. Psychosomatics. 2008;49:326–31. doi: 10.1176/appi.psy.49.4.326. [DOI] [PubMed] [Google Scholar]
- 34.Saxena S, Carlson D, Billington R. The WHO quality of life assessment instrument (WHOQOL-Bref): the importance of its items for cross-cultural research. Qual Life Res. 2001;10:711–21. doi: 10.1023/a:1013867826835. [DOI] [PubMed] [Google Scholar]
- 35.Yao G, Chung CW, Yu CF, Wang JD. Development and verification of validity and reliability of the WHOQOL-BREF Taiwan version. J Formos Med Assoc. 2002;101:342–51. [PubMed] [Google Scholar]