Abstract
Background
Previous studies yielded controversial results about the alteration of lipid profiles in patients with subclinical hypothyroidism. We performed a meta-analysis to investigate the association between subclinical hypothyroidism and lipid profiles.
Material/Methods
We searched PubMed, Cochrane Library, and China National Knowledge Infrastructure articles published January 1990 through January 2014. Dissertation databases (PQDT and CDMD) were searched for additional unpublished articles. We included articles reporting the relationship between subclinical hypothyroidism and at least 1 parameter of lipid profiles, and calculated the overall weighted mean difference (WMD) with a random effects model. Meta-regression was used to explore the source of heterogeneity among studies, and the Egger test, Begg test, and the trim and fill method were used to assess potential publication bias.
Results
Sixteen observational studies were included in our analysis. Meta-analysis suggested that the serum total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and total triglyceride levels were significantly increased in patients with subclinical hypothyroidism compared with euthyroidism individuals; the WMD were 12.17 mg/dl, 7.01 mg/dl, and 13.19 mg/dl, respectively (P<0.001 for all). No significant difference was observed for serum high-density lipoprotein cholesterol (HDL-C). Match strategy was the main source of heterogeneity among studies in TC and LDL-C analysis. Potential publication bias was found in TC and LDL-C analysis by the Egger test or Begg test and was not confirmed by the trim and fill method.
Conclusions
Subclinical hypothyroidism may correlate with altered lipid profile. Previous studies had limitations in the control of potential confounding factors and further studies should consider those factors.
MeSH Keywords: Hypothyroidism, Lipid Metabolism, Meta-Analysis
Background
Subclinical hypothyroidism (SH), which refers to the status of mild elevation of thyroid-stimulating hormone (TSH) in patients with normal serum thyroxine levels, is the most common thyroid disorder, with a prevalence of 3.8–10% in adult populations [1–4]. The prevalence of SH rises with age, and varies between sexes and populations [5,6]. Although the relationships between SH and lipid profile have been reported in several studies, the results are conflicting. Cross-sectional studies on 7000 consecutive patients in Austria and 1423 females in Australia found no significant differences in serum total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG) [7,8]. On the other hand, many studies reported disparate results. Several studies found serum LDL-C levels increased in SH patients [9–14], some of them found higher serum TC levels in SH patients [9–12], and other studies found lower serum TC level [15,16]. Contradictory results were also reported in the alteration of serum HDL-C and TG levels under SH [13,15–18].
Abnormal lipid profile has been established as a risk factor of coronary heart disease (CHD). To prevent CHD in SH patients, it is important to investigate the association between SH and lipid profile. Since the results of lipid profiles alteration in SH patients were disparate in previous studies, we attempted to form a conclusion about the alteration of each parameter of lipid profiles under SH. Therefore, we reviewed previous studies and performed a meta-analysis to answer this question.
Material and Methods
Data sources and search strategy
We searched the PubMed, Cochrane Library, and China National Knowledge Infrastructure (CNKI) database for studies in humans that reported relationships between SH and lipid profiles or at least 1 parameter of lipid profiles (TC, LDL-C, HDL-C, and TG). Google Scholar was then used to search for studies that were not included in PubMed, CNKI, or Cochrane Library. We also searched PQDT (ProQuest Theses and Dissertations) and CDMD (Chinese Doctoral Dissertations and Master’s Theses full-text database) to find relevant unpublished data. Because the standardized diagnostic for SH and sensitive TSH assay were applied in the 1990s [19,20], we limited the publication date from January 1990 to January 2014. “Subclinical hypothyroidism”, “lipid profile”, “TC”, “LDL-C”, “HDL-C”, “TG”, and “cholesterol” were used as key words in the search. To avoid missing any potential articles, we also used relevant words as key words, such as “thyroid disease”, “thyroid disorder”, “thyroid dysfunction”, “hypothyroidism”, “dysthyroidism”, “thyroid hormone”, “TSH”, “thyrotropin”, and “dyslipidemia”.
Selection criteria
Two investigators reviewed the articles independently. To avoid repeated selection, if 2 or more articles that studied the same population were published within 2 years by the same research group, only the article with the largest sample size would be included. The included articles should provide full text, and describe their study populations, and diagnostic standard for SH, and measured at least 1 of 4 lipid profile parameters in SH and euthyroidism (EU) groups. The reference range of TSH was unclear, and several studies suggested different TSH upper limit cutoffs ranging from 2.5 to 5.0 μIU/L [21–25]. Since there were no clear standards, we did not set the cutoff value of TSH to define SH, and instead performed a meta-regression based on TSH cutoff values. Considering that most patients with increased TSH were subclinical but did not have overt hypothyroidism [26], we also included 3 studies which measured TSH without a thyroxine measurement [27–29], and performed a meta-regression to assess the effects; this strategy was used in a previous article [30].
Quality assessment and data extraction
We assessed the quality of articles by using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklists for cohort, case-control, or cross-sectional studies, depending on the design of studies [31]. Although those checklists were initially designed to improve the quality of observation studies, they have been used as quality assessment tools in previous studies [32,33]. All checklists contained 22 main items, and 2 investigators gave scores of 0 or 1 for each item for all included articles. Five of the main items had sub-items, and those sub-items would also be scored 0 or 1, and then averaged as the score of the main items. The score of the 22 main items would be summed as the STROBE scores to assess the articles. Disagreement on STROBE score was resolved by a group discussion of the investigators.
Data were extracted by 1 investigator and double-checked by another. The following information was extracted from each included article: author, study design, match strategy, year of publication, TSH cutoff value to define SH, characteristics of study populations (sample source, sample size, sex ratios, mean age, and race), mean value and standard deviation of serum TC, LDL-C, HDL-C, and TG levels in both SH and EU groups.
Data analysis
Units of lipid profile parameters in all included articles were measured in either mmol/L or mg/dL. We converted units of cholesterol (TC, LDL-C, and HDL-C) and TG from mmol/L into mg/dL by multiplying by 38.67 and 88.57, respectively [34,35]. For each parameter of lipid profiles, summary estimates of weighted mean difference (WMD) and 95% confidence interval (CI) were obtained with a random effects model. The variation across studies attributable to heterogeneity beyond chance was estimated with the I2 heterogeneity test [36], where a value greater than 50% may be considered substantial heterogeneity [37]. To determine the possible sources of heterogeneity, we performed meta-regression which included study design, match strategy, TSH cutoff value, whether thyroxine was measured or not, sample size, sex, mean age, and race as variates for each lipid profile parameter, and then performed stratified analysis based on the results of the meta-regression. Finally, we used the Egger test and the Begg test to assess potential publication bias [38,39]. If any possible bias was found, the trim and fill method would be used to identify those results [40]. For all tests, a two-tailed P value smaller than 0.05 was considered statistically significant. All statistical analyses were performed in STATA software, version 11.0 (Stata, College Station, Texas, USA).
Role of the funding source
This study was supported by the National Natural Science Foundation of China (No. 81102127) and Science Project of Shenzhen (No. 201202103). The funding source had no role in the study design, data collection, data analysis, or writing of the report.
Results
Study selection and quality of studies
We identified 2084 studies and excluded 1963 studies that were unrelated to the association between SH and lipid profile. Then, 73 studies were excluded due to lack of diagnostic code to define SH, and 31 studies were excluded due to special selection criteria for participants, or lack of data about sample sizes or lipid profiles. Of the 17 prospective studies which met our criteria, 2 studies [10,12] published by the same research group may have used repeated data, so we only included the one with the larger sample size [10]. During the selection process, we failed to make contact with the authors to obtain the necessary data for 1 article which was carried out in Colorado, USA [41]. Finally, 16 studies were included in our analysis (Figure 1). Those studies were carried out in 12 countries and included a total of 41 931 adults, among whom 4526 were identified as SH patients and 37405 as having a status of EU. All studies were published in English between 2000 and 2013. Thirteen studies were cross-sectional studies; another 3 were cohort studies which reported lipid profile as baseline of participants before L-T4 or thyroxine treatment. Four studies applied sex-age individual match strategy and 4 studies applied the frequency match strategy. One study included 1 patient with radioiodine therapy history [10], 1 study included 2 patients who underwent levothyroxine treatment [27], and other studies declared no thyroid medication history in SH patients. Fifteen studies measured all 4 parameters of lipid profile, and 1 study only measured TG and HDL-C levels [42]. The STROBE scores of the included articles varied from 13 to 20, with a median of 15.5. The TSH cutoff value varied from 3.5 to 6.7 μIU/L with a median of 4.5 μIU/L (Table 1).
Figure 1.
Selection process for eligible studies.
Table 1.
Characteristics of included studies.
| Study, year [reference] | Study design | Match strategy | SH/EU sample size, n | Source of samples | |
|---|---|---|---|---|---|
| Vierhapper et al., 2000 [7] | Cross-section | No match | 1055/4866 | Outpatients from Vienna, Austria | |
| Efstathiadou et al., 2001 [9] | Case-control | Individual match | 66/75 | Adults living in Ioannina, Greece | |
| Caraccio et al., 2002 [10] | Case-control | Individual match | 49/33 | Outpatients from Pisa, Italy | |
| Cikim et al., 2004 [27] | Cross-section | Frequency match | 25/23 | Adults from Istanbul, Turkey | |
| Hueston et al., 2004 [50] | Cross-section | No match | 215/8013 | Residents from USA (NHANES III) | |
| Luboshitzky et al., 2004 [51] | Cross-section | Frequency match | 44/19 | Outpatients from Haifa, Israel | |
| Walsh et al., 2005 [11] | Cross-section | No match | 119/1906;30/580 * | Adults from Busselton, Australia | |
| Erem, 2006 [18] | Cross-section | Individual match | 30/20 | Outpatients from Trabzon, Turkey | |
| Iqbal et al., 2006 [13] | Case-control | Frequency match | 84/145 | Adults from Tromsø, Norway | |
| Bell et al., 2007 [8] | Cross-section | No match | 81/1271 | Adults from Victoria, Australia | |
| Torun et al., 2009 [14] | Cross-section | Frequency match | 40/40 | Adults from Ankara, Turkey | |
| Lai et al., 2011 [42] | Cross-section | No match | 102/1283 | Adults from Shengyang, China | |
| Hernández et al., 2012 [16] | Cross-section | No match | 95/65 | Outpatients from Valencia, Spain | |
| Moriyama et al., 2012 [29] | Cross-section | No match | 137/2315 | Adults from Tokyo, Japan | |
| Shashikala, 2012 [28] | Cross-section | Individual match | 22/25 | Outpatients from Karnataka, India | |
| Palacios, 2013 [17] | Cross-section | No match | 2383/17306 | Adults from Pamplona, Spain | |
| Mean age (range or SD), y | Female, % | TSH cutoff, μIU/L | Thyroxine measured | Lipid profile parameter measured | STROBE score |
| – | – | 4.0 | Yes | TC, HDL-C, LDL-C, TG | 13 |
| 47.8 (12.4) | 90.1 | 4.5 | Yes | TC, HDL-C, LDL-C, TG | 17 |
| 33.8 (9.0) | 84.1 | 3.6 | Yes | TC, HDL-C, LDL-C, TG | 15 |
| 34.0 (8.8) | 93.8 | 4.2 | No | TC, HDL-C, LDL-C, TG | 14 |
| – | 51.8 | 6.7 | Yes | TC, HDL-C, LDL-C, TG | 20 |
| 51.6 (9.7) | 100.0 | 4.5 | Yes | TC, HDL-C, LDL-C, TG | 14 |
| 49.7 (16.8); 43.8 (17.4)* | 49.1; 47.0 * | 4.0 | Yes | TC, HDL-C, LDL-C, TG | 17 |
| 41.3 (13.1) | 78.0 | 5.0 | Yes | TC, HDL-C, LDL-C, TG | 13 |
| 61.3 (12.5) | 53.3 | 3.5 | Yes | TC, HDL-C, LDL-C, TG | 18 |
| – | 100.0 | 4.0 | Yes | TC, HDL-C, LDL-C, TG | 17 |
| 30.4 (3.7) | 100.0 | 5.0 | Yes | TC, HDL-C, LDL-C, TG | 15 |
| 45.1 (13.9) | – | 4.8 | Yes | HDL-C, TG | 14 |
| 56.7 (13.6) | 100.0 | 5.0 | Yes | TC, HDL-C, LDL-C, TG | 16 |
| 51.8 | 42.6 | 5.0 | No | TC, HDL-C, LDL-C, TG | 15 |
| 35.8 (12.7) | – | 6.0 | No | TC, HDL-C, LDL-C, TG | 17 |
| 57.1 (19.2) | 62.1 | 4.43 | Yes | TC, HDL-C, LDL-C, TG | 16 |
Only part of participants in Walsh et al. study measured serum HDL-C and LDL-C. The first data was information for TC and TG; the second data was information for HDL-C and LDL-C.
Subclinical hypothyroidism and TC
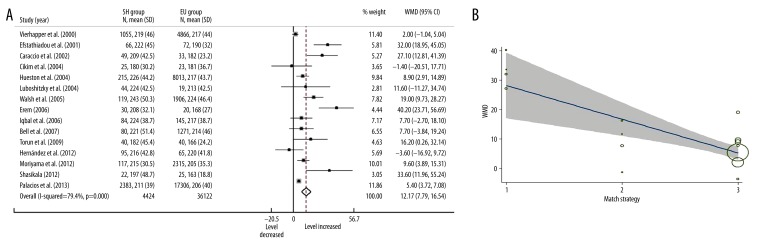
Fifteen included studies measured serum TC levels in 40 546 participants. Most studies suggested that there were higher serum TC levels in SH patients compared with EU participants and only 2 studies reported lower levels without statistical significance. In a random effects model, the overall serum TC level in SH patients was significantly higher than in EU participants (Figure 2A, WMD=12.17, 95% CI: 7.79–16.54, P<0.001).
Figure 2.
Forest plots for serum TC level in subclinical hypothyroidism, in where SH=subclinical hypothyroidism; EU=euthyroidism; WMD=weighted mean difference (A); and meta-regression bubble plot for effects of match strategy in TC analysis, in where size of the circles depend on the weight of the study, gray area showed the 95% confidence interval. 1=sex-age individual match; 2=frequency match; 3=no match (B).
Substantial heterogeneity was found between studies (I2=79.4%, P<0.001). Univariate meta-regression found heterogeneity was not associated with source of participants, mean age, sex ratio, TSH cutoff values, and whether thyroxine was measured. Match strategy was found associated with heterogeneity (Figure 2B, P=0.001), which reduced the overall heterogeneity I2 to 52.84%. In the multivariate analysis, the combination of match strategy (P=0.001) and source of participants (P=0.403) reduced the overall I2 to 46.5%. Grouped by match strategy and source of participants, the stratified analysis showed that individual match studies tended to report considerable alterations of TC levels in SH patients with a large confidence interval (WMD=32.58, 95% CI: 24.81–40.34); in contrast, studies with no match tended to provide conservative results with a much smaller confidence interval (WMD=6.72, 95% CI: 3.30–10.14).
Subclinical hypothyroidism and LDL-C
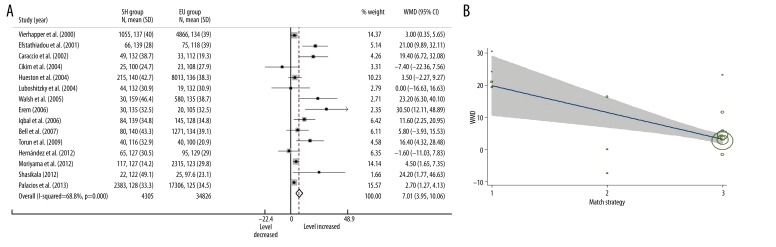
Fifteen included studies measured serum TC levels in 39 131 participants. Nine of these studies reported significantly higher LDL-C levels in SH patients when compared with EU participants. Only 1 article reported a slightly decreased LDL-C level in SH patients, which was not statistically significant. In a random effects model, the overall serum LDL-C level in SH patients was significantly higher than in EU participants (Figure 3A, WMD=7.01, 95% CI: 3.95–10.05, P<0.001).
Figure 3.
Forest plots for serum LDL-C level in subclinical hypothyroidism, in where SH=subclinical hypothyroidism; EU=euthyroidism; WMD=weighted mean difference (A); and meta-regression for effects of match strategy in LDL-C analysis, in where size of the circles depend on the weight of the study, gray area showed the 95% confidence interval. 1=sex-age individual match; 2=frequency match; 3=no match (B).
Substantial heterogeneity was found between studies (I2=68.8%, P<0.001). Univariate meta-regression found that match strategy was associated with heterogeneity (Figure 3B, p=0.002) but other variates were not. The overall heterogeneity I2 was reduced to 38.6% after pull-in effects of match strategy. Multivariate analysis did not find any combination of variates that was associated with heterogeneity. Grouped by match strategy, the stratified analysis found individual match studies reported larger WMD and wider 95% CI (WMD=22.27, 95% CI: 15.07–29.48), compared with no match studies (WMD=6.37, 95% CI: 1.74–4.97). This result was similar to the findings of stratified analysis for TC.
Subclinical hypothyroidism and HDL-C
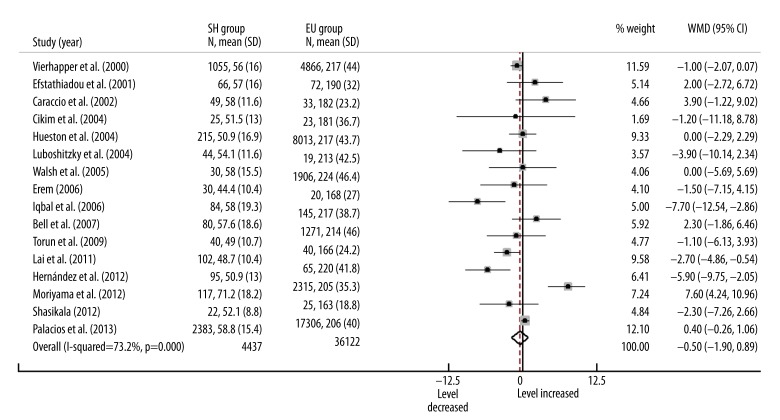
The included studies included a total 40 516 participants whose serum HDL-C levels were measured. Three of these studies reported significant lower HDL-C levels in SH patients and 1 reported significant higher level compared with EU participants. In a random effects model, the overall serum HDL-C level in SH patients was lower than in EU participants, but was not statistically significant (Figure 4, WMD=–0.50, 95% CI: –1.90–0.89, P=0.481). Substantial heterogeneity was found between studies (I2=73.2%, P<0.001). Meta-regression found no variates that were associated with heterogeneity.
Figure 4.
Forest plots for serum HDL-C level in subclinical hypothyroidism. SH=subclinical hypothyroidism; EU=euthyroidism; WMD=weighted mean difference.
Subclinical hypothyroidism and TG
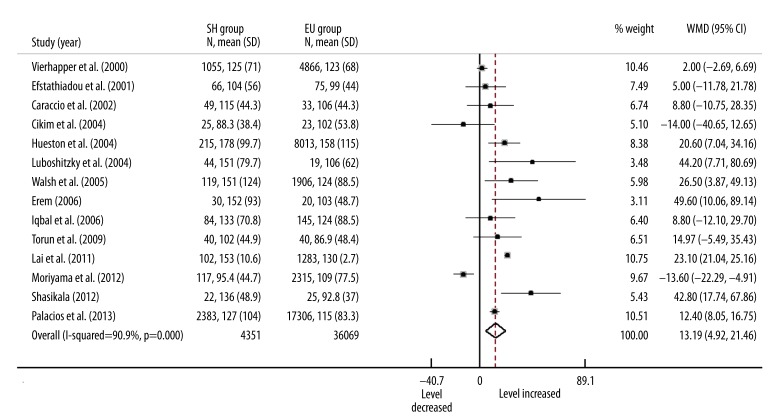
Sixteen studies measured serum TG levels. However, 2 studies used the median (and min, max) to describe TG levels [8,16]. To avoid potential statistical errors, we eliminated those studies in analysis of TG. A random effects model including other 14 studies with 40 420 participants found that the overall serum TG level in SH patients was significant higher than in EU participants (Figure 5, WMD=13.19, 95% CI: 4.92–21.46, P<0.001).
Figure 5.
Forest plots for serum TG level in subclinical hypothyroidism. SH=subclinical hypothyroidism; EU=euthyroidism; WMD=weighted mean difference.
A large heterogeneity was found between studies (I2=90.9%, p<0.001). Univariate meta-regression failed to find any variates associated with heterogeneity. In the multivariate analysis, the model included match strategy (P=0.296), source of participant (P=0.033), mean age (P=0.774), TSH cutoff value (P=0.039), and whether measured thyroxine (P=0.029) explained 75.7% heterogeneity, which reduced the I2 to 31.48%.
Publication bias
Egger test and Begg test found no evidence of publication bias for studies on HDL-C (P=0.562 for Egger test, P=0.753 for Begg test) and TG (P=0.343 for Egger test, P=0.101 for Begg test). For TC, although the Egger test indicated a potential publication bias (P=0.014), the Begg test (P=0.235) and the trim and fill method did not confirm this possibility. For LDL-C, both the Egger test and Begg test found publication bias (P=0.010 for Egger test, P=0.048 for Begg test). However, the trim and fill method found that the conclusions did not significantly change after adjusting for publication bias.
Discussion
Altered lipid profiles, which include elevated levels of TC, TG, LDL-C, and/or a lower level of HDL-C, are thought to confer the risk of cardiovascular disease development [43,44]. Overt hypothyroidism is associated with an altered lipid profile [45,46]. However, the relationship between subclinical hypothyroidism and abnormal lipid profile is still unclear. Several cross-sectional studies with large sample sizes were carried out in different countries. The Colorado, USA study showed that SH patients had higher TC and LDL-C level compared with EU populations [41]. Another study in Austria did not find those differences [7]. An explanation for these conflicting results among these cross-sectional studies is poor control in potential confounding factors, such as sex, age, race, insulin resistance, smoking, and drinking behavior, which may be associated with lipid levels [47–49]. An analysis of National Health and Nutrition Examination Survey (NHANES III) samples found higher serum TC and TG levels in SH patients than in EU participants. However, this difference disappeared after adjusting for variables such as sex, age, and race [50]. To control for potential confounding factors, many studies applied the sex-age match method. Those studies reported higher TC and LDL-C levels in SH patients compared with EU participants [9,14,18,27,51].
In the article selection process of our study, we found many cohort studies reported lipid profile as baseline of SH and EU groups before L-thyroxine therapy [52–54]. We had to exclude those studies since their selection of participants, such as use the body mass index as part of the inclusion criteria or excluded patients with dyslipidemia, may have led to bias. We also had to exclude a cross-sectional study with a large sample size (the Colorado, USA study) due to lack of the standard deviation data [41]. Potential publication bias was found in our study. It is plausible that many small studies with insignificant results might have never been published, which could change the results of this study. Since we only searched articles published in English and Chinese, publication language may be another cause of bias. We found significant heterogeneity between studies, which could also result in publication bias [55].
For TC and LDL-C, many previous studies suggested SH patients had higher levels of these parameters compared with EU populations. The mechanisms are still unclear. Alteration to LDL-C receptor levels caused by slight decreases of thyroxine in SH may explain those results [56,57]. Some studies suggested that TSH, independent of thyroxine, may elevate serum TC and LDL-C levels [58,59]. Our study found increased levels of serum TC and LDL-C in SH patients compared with EU participants. This result was in accordance with the majority of conclusions of previous studies. For each included study, the trends of alterations in serum TC and LDL-C levels were consistent, and this is reasonable since low-density lipoprotein (LDL) contains 65% total cholesterol [60]. Only 2 studies reported an insignificant decrease in TC and LDL-C levels in SH patients [16,27]. One study explained this result as due to older patients and difference in TSH cutoff values [16]. However, we did not find that these 2 studies differed in study design, mean age, sex ratio, or TSH cutoff, compared with other studies. Substantial heterogeneities between studies were found in both TC and LDL-C analysis. Those heterogeneities were reduced after adjusting for match strategy. Sex-age individual match studies found more significant results than no-match studies did, which indicated that individual’s sex and age may be associated with serum TC and LDL-C levels; insignificant results from some studies may have been due to differences in the sex and age between EU and SH groups. Hence, we recommend that further studies should consider the effects of sex and age more carefully.
Higher serum TG levels were found in SH patients compared with EU participants in our study. Although this result was consistent with those of many previous studies, it should be treated with caution since there was large and unexplainable heterogeneity between studies. Convictive results on the association between SH and serum TG were not reported, as well as believable mechanisms. Previous studies found that L-T4 therapy could reduce serum TC or LDL-C levels, but in those studies serum TG levels remained consistent after L-T4 therapy [10,54]. This result indicated that thyroxine may have limited effects on TG. Since the association between SH and TG is still unclear, further studies based on large populations will be required. In the analysis we found several studies used median (and min max) values to describe their data on TG levels; this was reasonable since many studies found that serum TG levels did not match the normal distribution [61–63]. On the other hand, some studies that described their TG data by using the mean and standard deviation did not mention any tests for normality. Merging those data may therefore have caused error. To improved data quality, we recommend that further studies, especial those based on small sample sizes, perform normality tests for data on serum TG levels.
We found weak evidence for effects of SH on serum HDL-C level. Although many included studies reported significant alteration of HDL-C levels in SH patients, the overall WMD was insignificant. These results may contribute to the contrasting effects of thyroxine on enzymes related to HDL-C metabolism. Plasma cholesteryl ester transfer protein concentration decreased with slight reductions in serum thyroxine level in SH patients, which may result in higher HDL-C level [64]. On the other hand, the activities of hepatic lipase, lecithin cholesterol acyl transferase, and ATP-binding cassette transporter also decreased in SH patients, which may lead to lower HDL-C levels [64,65]. These 2 contrasting effects neutralized one another, and the HDL-C level may remain stable. Polymorphisms of genes and osteocalcin concentration may effect the metabolism of HDL-C [66,67], and those factors were not taken into account in most regular epidemiology studies. Another possible reason for the insignificant results was weak control for potential confounding factors in the included studies.
Our study had several limitations. First, all included studies in our analysis were observational studies. Most observational studies did not control potential confounding factors. In our analysis, only a few studies paired participants by sex and age, and even fewer studies adjusted for differences in insulin resistance and smoking and drinking behaviors. Hence, this meta-analysis of observational studies should be interpreted with caution [68]. Second, we could not extract information of the degree and duration of subclinical hypothyroidism in patients, which may have significantly affected their lipid profiles. To the best of our knowledge, existing technology cannot adequately measure the degree and duration of SH in patients. Third, we cannot explain the substantial heterogeneity between studies in HDL-C and TG analysis. The heterogeneity may be associated with potential confounding factors that were not mentioned in the studies. Further studies should utilize more rigorous inclusion criteria for participants.
Conclusions
Our analysis suggested that serum TC, LDL-C, and TG levels increased under subclinical hypothyroidism; however, weak evidence suggested subclinical hypothyroidism was associated with serum HDL-C levels. Higher serum TC, LDL-C, and TG levels increased the risk of CHD; therefore, the cardiovascular status of SH patients should be monitored carefully. In the analysis of TC and LDL-C, match strategy was the main source of heterogeneity. Previous studies had limitations regarding the control of potential confounding factors; further studies should take into account those factors.
Acknowledgements
The authors thank Dr. Bao Wei, Dr. Chen, Dong Meicheng, Hou Bin, Chen Zheng, Zhu Yumei, and the library staff of Peking Union Medical College for their assistance.
Footnotes
Conflict of interest statement
The authors declare that they have no conflicts of interest regarding this work and they have no financial and personal relationships with other people or organizations that could inappropriately influence their work.
Source of support: This study was supported by the National Natural Science Foundation of China (No. 81102127) and Science Project of Shenzhen (No. 201202103)
References
- 1.Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76–131. doi: 10.1210/er.2006-0043. [DOI] [PubMed] [Google Scholar]
- 2.Golden SH, Robinson KA, Saldanha I, et al. Prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94:1853–78. doi: 10.1210/jc.2008-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teng WP, Xin XP, Tong NW. Epidemiological investigation for Thyroid Disease in Ten cities of China. Presented in part at the 9th annual meeting of Chinese Society Endocrinology; Dalian, China. August 27–29, 2010. [Google Scholar]
- 4.Aksoy DY, Cinar N, Harmanci A, et al. Serum resistin and high sensitive CRP levels in patients with subclinical hypothyroidism before and after L-thyroxine therapy. Med Sci Monit. 2013;19:210–15. doi: 10.12659/MSM.883847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fade J, Franklyn J, Cross K, et al. Prevalence and follow-up of abnormal thyrotrophin (TSH) concentrations in the elderly in the United Kingdom. Clin Endocrinol. 1991;34:77–84. doi: 10.1111/j.1365-2265.1991.tb01739.x. [DOI] [PubMed] [Google Scholar]
- 6.Kanaya AM, Harris F, Volpato S, et al. Association between thyroid dysfunction and total cholesterol level in an older biracial population: the health, aging and body composition study. Arch Intern Med. 2002;162:773–79. doi: 10.1001/archinte.162.7.773. [DOI] [PubMed] [Google Scholar]
- 7.Vierhapper H, Nardi A, Grösser P, et al. Low-density lipoprotein cholesterol in subclinical hypothyroidism. Thyroid. 2009;10:981–84. doi: 10.1089/thy.2000.10.981. [DOI] [PubMed] [Google Scholar]
- 8.Bell RJ, Rivera-Woll L, Davison SL, et al. Well-being, health-related quality of life and cardiovascular disease risk profile in women with subclinical thyroid disease – a community– based study. Clin Endocrinol. 2007;66:548–56. doi: 10.1111/j.1365-2265.2007.02771.x. [DOI] [PubMed] [Google Scholar]
- 9.Efstathiadou Z, Bitsis S, Milionis HJ, et al. Lipid profile in subclinical hypothyroidism: is L-thyroxine substitution beneficial? Eur J Endocrinol. 2001;145:705–10. doi: 10.1530/eje.0.1450705. [DOI] [PubMed] [Google Scholar]
- 10.Caraccio N, Ferrannini E, Monzani F. Lipoprotein profile in subclinical hypothyroidism: response to levothyroxine replacement, a randomized placebo-controlled study. J Clin Endocrinol Metab. 2002;87:1533–38. doi: 10.1210/jcem.87.4.8378. [DOI] [PubMed] [Google Scholar]
- 11.Walsh JP, Bremner AP, Bulsara MK, et al. Thyroid dysfunction and serum lipids: a community – based study. Clin Endocrinol. 2005;63:670–75. doi: 10.1111/j.1365-2265.2005.02399.x. [DOI] [PubMed] [Google Scholar]
- 12.Monzani F, Caraccio N, Kozakowa M, et al. Effect of levothyroxine replacement on lipid profile and intima-media thickness in subclinical hypothyroidism: a double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2004;89:2099–106. doi: 10.1210/jc.2003-031669. [DOI] [PubMed] [Google Scholar]
- 13.Iqbal A, Jorde R, Figenschau Y. Serum lipid levels in relation to serum thyroid – stimulating hormone and the effect of thyroxine treatment on serum lipid levels in subjects with subclinical hypothyroidism: the Tromsø Study. J Intern Med. 2006;260:53–61. doi: 10.1111/j.1365-2796.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- 14.Torun AN, Kulaksizoglu S, Kulaksizoglu M, et al. Serum total antioxidant status and lipid peroxidation marker malondialdehyde levels in overt and subclinical hypothyroidism. Clin Endocrinol. 2009;70:469–74. doi: 10.1111/j.1365-2265.2008.03348.x. [DOI] [PubMed] [Google Scholar]
- 15.Hak AE, Pols HA, Visser TJ, et al. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med. 2000;132:270–78. doi: 10.7326/0003-4819-132-4-200002150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Hernández-Mijares A, Jover A, Bellod L, et al. Relation between lipoprotein subfractions and TSH levels in the cardiovascular risk among women with subclinical hypothyroidism. Clin Endocrinol. 2013;78(5):777–82. doi: 10.1111/cen.12064. [DOI] [PubMed] [Google Scholar]
- 17.Santos-Palacios S, Brugos-Larumbe A, Guillén-Grima F, et al. A cross-sectional study of the association between circulating TSH level and lipid profile in a large Spanish population. Clin Endocrinol. 2013;79(6):874–81. doi: 10.1111/cen.12216. [DOI] [PubMed] [Google Scholar]
- 18.Erem C. Blood coagulation, fibrinolytic activity and lipid profile in subclinical thyroid disease: subclinical hyperthyroidism increases plasma factor X activity. Clin Endocrinol. 2006;64:323–29. doi: 10.1111/j.1365-2265.2006.02464.x. [DOI] [PubMed] [Google Scholar]
- 19.Stockigt J. Guidelines for diagnosis and monitoring of thyroid disease: nonthyroidal illness. Clin Chem. 1996;42:188–92. [PubMed] [Google Scholar]
- 20.Spencer CA, Takeuchi M, Kazarosyan M. Current status and performance goals for serum thyrotropin (TSH) assays. Clin Chem. 1996;42:140–45. [PubMed] [Google Scholar]
- 21.Stathatos N, Wartofsky L. Managing subclinical hypothyroidism in women. Womens Health Primary Care. 2002;5:239–46. [Google Scholar]
- 22.Ross DS. Serum thyroid-stimulating hormone measurement for assessment of thyroid function and disease. Endocrinol Metab Clin North Am. 2001;30:245–64. doi: 10.1016/s0889-8529(05)70186-9. [DOI] [PubMed] [Google Scholar]
- 23.Stephens P. The Endocrine Society: current issues in thyroid disease management. Endocr News. 2004;29:23–26. [Google Scholar]
- 24.Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease. JAMA. 2004;291:228–38. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 25.Helfand M. Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2004;140:128–41. doi: 10.7326/0003-4819-140-2-200401200-00015. [DOI] [PubMed] [Google Scholar]
- 26.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–99. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 27.Cikim AS, Oflaz H, Ozbey N, et al. Evaluation of endothelial function in subclinical hypothyroidism and subclinical hyperthyroidism. Thyroid. 2004;14:605–9. doi: 10.1089/1050725041692891. [DOI] [PubMed] [Google Scholar]
- 28.Shashikala S. Comparative study of lipid profile oxidant status renal and muscle damage in subclinical and overt hypothyroidism [dissertation] Karnataka, India: Rajiv Gandhi University; 2012. [Google Scholar]
- 29.Moriyama K, Takahashi E, Negami M, et al. Relationship between Thyroid Dysfunction and Serum Low-density Lipoprotein Cholesterol Levels in Subjects Undergoing Annual Health Examination in Japan. Ningen Dock. 2012;27:547–53. [Google Scholar]
- 30.Ochs N, Auer R, Bauer DC, et al. Meta-analysis Subclinical Thyroid Dysfunction and the Risk for Coronary Heart Disease and Mortality. Ann Intern Med. 2008;148:832–45. doi: 10.7326/0003-4819-148-11-200806030-00225. [DOI] [PubMed] [Google Scholar]
- 31.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Prev Med. 2007;45:247–51. doi: 10.1016/j.ypmed.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Olmos M, Antelo M, Vazquez H, et al. Systematic review and meta-analysis of observational studies on the prevalence of fractures in coeliac disease. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 4046:2008. doi: 10.1016/j.dld.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Van der Bilt I, Hasan D, Vandertop W, et al. Impact of cardiac complications on outcome after aneurysmal subarachnoid hemorrhage A meta-analysis. Neurology. 2009;72:635–42. doi: 10.1212/01.wnl.0000342471.07290.07. [DOI] [PubMed] [Google Scholar]
- 34.Yan AT, Yan RT, Tan M, et al. Contemporary management of dyslipidemia in high-risk patients: targets still not met. Am J Med. 2006;119:676–83. doi: 10.1016/j.amjmed.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Roeback JR, Hla KM, Chambless LE, et al. Effects of Chromium Supplementation on Serum High-Density Lipoprotein Cholesterol Levels in Men Taking Beta-BlockersA Randomized, Controlled Trial. Ann Intern Med. 1991;115:917–24. doi: 10.7326/0003-4819-115-12-917. [DOI] [PubMed] [Google Scholar]
- 36.Woodward M. / Boca Raton: Chapman and Hall; CRC Press; 1999. Epidemiology: study design and data analysis, 1sted. [Google Scholar]
- 37.Higgins JP, Green S, Collaboration C. Cochrane handbook for systematic reviews of interventions. Wiley Online Library; 2008. [Google Scholar]
- 38.Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 40.Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- 41.Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–34. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 42.Lai Y, Wang J, Jiang F, et al. The relationship between serum thyrotropin and components of metabolic syndrome. Endocr J. 2011;58:23–30. doi: 10.1507/endocrj.k10e-272. [DOI] [PubMed] [Google Scholar]
- 43.Després J-P, Golay A, Sjöström L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–34. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- 44.Chiasson J-L, Josse RG, Gomis R, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance. JAMA. 2003;290:486–94. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 45.Kuusi T, Taskinen MR, Nikkila EA. Lipoproteins, lipolytic enzymes, and hormonal status in hypothyroid women at different levels of substitution. J Clin Endocrinol Metab. 1988;66:51–56. doi: 10.1210/jcem-66-1-51. [DOI] [PubMed] [Google Scholar]
- 46.O’Brien T, Dinneen SF, O’Brien PC, et al. Hyperlipidemia in patients with primary and secondary hypothyroidism. Mayo Clin Proc. 1993;68(9):860–66. doi: 10.1016/s0025-6196(12)60694-6. [DOI] [PubMed] [Google Scholar]
- 47.Müller B, Zulewski H, Huber P, et al. Impaired action of thyroid hormone associated with smoking in women with hypothyroidism. N Engl J Med. 1995;333:964–69. doi: 10.1056/NEJM199510123331503. [DOI] [PubMed] [Google Scholar]
- 48.De Oliveira E, Silva ER, Foster D, McGee Harper M. Alcohol consumption raises HDL cholesterol levels by increasing the transport rate of apolipoproteins AI and A-II. Circulation. 2000;102(19):2347–52. doi: 10.1161/01.cir.102.19.2347. [DOI] [PubMed] [Google Scholar]
- 49.Bakker SJ, ter Maaten JC, Popp-Snijders C, et al. The relationship between thyrotropin and low density lipoprotein cholesterol is modified by insulin sensitivity in healthy euthyroid subjects. J Clin Endocrinol Metab. 2001;86:1206–11. doi: 10.1210/jcem.86.3.7324. [DOI] [PubMed] [Google Scholar]
- 50.Hueston WJ, Pearson WS. Subclinical hypothyroidism and the risk of hypercholesterolemia. Ann Fam Med. 2004;2:351–55. doi: 10.1370/afm.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luboshitzky R, Herer P. Cardiovascular risk factors in middle-aged women with subclinical hypothyroidism. Neuro Endocrinol Lett. 2004;25:264–68. [PubMed] [Google Scholar]
- 52.Caron P, Calazel C, Parra H, et al. Decreased HDL cholesterol in subclinical hypothyroidism: the effect of L-thyroxine therapy. Clin Endocrinol. 1990;33:519–23. doi: 10.1111/j.1365-2265.1990.tb03889.x. [DOI] [PubMed] [Google Scholar]
- 53.Arem R, Patsch W. Lipoprotein and apolipoprotein levels in subclinical hypothyroidism: effect of levothyroxine therapy. Arch Intern Med. 1990;150:2097–100. [PubMed] [Google Scholar]
- 54.Meier C, Staub J-J, Roth C-B, et al. TSH-controlled L-thyroxine therapy reduces cholesterol levels and clinical symptoms in subclinical hypothyroidism: a double blind, placebo-controlled trial (Basel Thyroid Study) J Clin Endocrinol Metab. 2001;86:4860–66. doi: 10.1210/jcem.86.10.7973. [DOI] [PubMed] [Google Scholar]
- 55.Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ. 2007;176:1091–96. doi: 10.1503/cmaj.060410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pearce EN. Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab. 2012;97:326–33. doi: 10.1210/jc.2011-2532. [DOI] [PubMed] [Google Scholar]
- 57.McDermott MT, Ridgway EC. Subclinical hypothyroidism is mild thyroid failure and should be treated. J Clin Endocrinol Metab. 2001;86:4585–90. doi: 10.1210/jcem.86.10.7959. [DOI] [PubMed] [Google Scholar]
- 58.Åsvold BO, Vatten LJ, Nilsen TI, et al. The association between TSH within the reference range and serum lipid concentrations in a population-based study. The HUNT Study. Eur J Endocrinol. 2007;156:181–86. doi: 10.1530/eje.1.02333. [DOI] [PubMed] [Google Scholar]
- 59.Xu C, Yang X, Liu W, et al. Thyroid stimulating hormone, independent of thyroid hormone, can elevate the serum total cholesterol level in patients with coronary heart disease: a cross-sectional design. Nutr Metab (Lond) 2012;9(1):44. doi: 10.1186/1743-7075-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sasaki S, Kawai K, Honjo Y, et al. [Thyroid hormones and lipid metabolism]. Nihon rinsho. Japanese Journal of Clinical Medicine. 2006;64:2323–29. [PubMed] [Google Scholar]
- 61.Kramer CK, Leitão CB, Pinto LC, et al. Clinical and laboratory profile of patients with type 2 diabetes with low glomerular filtration rate and normoalbuminuria. Diabetes Care. 2007;30:1998–2000. doi: 10.2337/dc07-0387. [DOI] [PubMed] [Google Scholar]
- 62.Criqui MH, Heiss G, Cohn R, et al. Plasma triglyceride level and mortality from coronary heart disease. N Engl J Med. 1993;328:1220–25. doi: 10.1056/NEJM199304293281702. [DOI] [PubMed] [Google Scholar]
- 63.Soto N, Bazaes RA, Peña V, et al. Insulin sensitivity and secretion are related to catch-up growth in small-for-gestational-age infants at age 1 year: results from a prospective cohort. J Clin Endocrinol Metab. 2003;88:3645–50. doi: 10.1210/jc.2002-030031. [DOI] [PubMed] [Google Scholar]
- 64.Tan K, Shiu S, Kung A. Plasma cholesteryl ester transfer protein activity in hyper-and hypothyroidism. J Clin Endocrinol Metab. 1998;83(1):140–43. doi: 10.1210/jcem.83.1.4491. [DOI] [PubMed] [Google Scholar]
- 65.Boone LR, Lagor WR, de la Llera Moya M, et al. Thyroid hormone enhances the ability of serum to accept cellular cholesterol via the ABCA1 transporter. Atherosclerosis. 2011;218:77–82. doi: 10.1016/j.atherosclerosis.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pac-Kożuchowska E, Krawiec P. Cholesterol ester transfer protein (CETP) gene polymorphism and selected parameters of lipid metabolism in children from families with history of cardiovascular system diseases. Med Sci Monit. 2013;19:818–25. doi: 10.12659/MSM.889550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rui X, Xu B, Su J, et al. Differential pattern for regulating insulin secretion, insulin resistance, and lipid metabolism by osteocalcin in male and female T2DM patients. Med Sci Monit. 2014;20:711–19. doi: 10.12659/MSM.890130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Altman DG. Systematic reviews in health care: Systematic reviews of evaluations of prognostic variables. BMJ. 2001;323:224. doi: 10.1136/bmj.323.7306.224. [DOI] [PMC free article] [PubMed] [Google Scholar]