Abstract
Background
The anti-oxidative and anti-inflammatory activities of electro-acupuncture (EA), a traditional clinical method, are widely accepted, but its mechanisms are not yet well defined. In this study, we investigated the role of extracellular signal-regulated kinases1/2 (ERK1/2) pathways on electro-acupuncture – mediated up-regulation of heme oxygenase-1 (HO-1) in rabbit lungs injured by LPS-induced endotoxic shock.
Material/Methods
Seventy rabbits were randomly divided into 7 groups: group C, group M, group D, group SEAM, group EAM, group EAMPD, and group PD98059. Male New England white rabbits were given EA treatment on both sides once a day on days 1–5, and then received LPS to replicate the experimental model of injured lung induced by endotoxic shock. Then, they were killed by exsanguination at 6 h after LPS administration. The blood samples were collected for serum examination, and the lungs were removed for pathology examination, determination of wet-to-dry weight ratio, MDA content, SOD activity, serum tumor necrosis factor-α, determination of HO-1 protein and mRNA expression, and determination of ERK1/2 protein.
Results
The results revealed that after EA treatment, expression of HO-1and ERK1/2 was slightly increased compared to those in other groups, accompanied with less severe lung injury as indicated by lower index of lung injury score, lower wet-to-dry weight ratio, MDA content, and serum tumor necrosis factor-α levels, and greater SOD activity (p<0.05 for all). After pretreatment with ERK1/2 inhibitor PD98059, the effect of EA treatment and expression of HO-1 were suppressed (p<0.05 for all).
Conclusions
After electro-acupuncture stimulation at ST36 and BL13, severe lung injury during endotoxic shock was attenuated. The mechanism may be through up-regulation of HO-1, mediated by the signal transductions of ERK1/2 pathways. Thus, the regulation of ERK1/2 pathways via electro-acupuncture may be a therapeutic strategy for endotoxic shock.
MeSH Keywords: Acupuncture, Acute Lung Injury, Mitogen-Activated Protein Kinase 1
Background
Acute respiratory distress syndrome (ARDS), an indication of acute lung injury (ALI), is highly associated with endotoxic shock [1]. ALI and ARDS, which represent the severest form of lung injury associated with endotoxic shock, are the leading causes of morbidity and mortality in patients [2]. The first-line treatments for patients with endotoxic shock, such as antibiotics, source control, and resuscitation with intravenous fluids to optimize circulation and organ perfusion, are largely limited to persistent hypo-perfusion and end-organ dysfunctions [3]. With increasing evidence of its clinical efficacy, acupuncture is now a widely practiced treatment modality in complementary and integrative medicine [4]. It has been shown that acupuncture stimulation significantly attenuates the degree of lung injury in LPS-stimulated rats [5], but the mechanisms that underlie EA treatment for sepsis-induced lung dysfunction are poorly understood.
HO-1 is the inducible form of the first and rate-limiting enzyme of heme degradation, and it is a strong negative regulator in the development of oxidative stress and lung inflammatory responses during endotoxin-induced ALI [6]. Investigators have suggested that increased oxidative stress and end-organ damage is related to mortality during endotoxic shock, but the hypotension partly contributing to HO-1 protein and CO has no obvious relation [7]. Our previous studies also revealed that electro-acupuncture stimulation at ST36 and BL13 could attenuate lung injury during the endotoxic shock, and this effect was due to increased expression of HO-1 [8]. Recently, the effect of electro-acupuncture–mediated up-regulation of HO-1 in lungs of rabbits with endotoxic shock has received increased attention, although the mechanism has not been systematically studied.
The protective effects of HO-1 involve the modulation of numerous cellular targets, including the mitogen-activated protein kinases (MAPK) and different transcription factors [9]. MAPK act as a signaling pathway that serves to coordinate the cellular response with a variety of extracellular stimuli. MAPK have an important role in cellular processes, such as stress responses, apoptosis, and immune defense [10]. The MAPK activation modulates several gene and protein expressions, including HO-1 [11]. Extracellular signal-regulated kinase1/2(ERK1/2) is the most common member of the MAPK family [12]. Recently, studies showed that the LPS-induced inflammatory response was inhibited by expression of anti-inflammatory HO-1 via ERK; EA significantly increased the phosphorylation levels of ERK [13,14]. In particular, we demonstrated that the inhibition of the ERK1/2 pathway, the activation of which contributed to the induction and maintenance of HO-1, in endotoxic shock rats, was possibly involved in exacerbation of lung dysfunction during the endotoxic shock [15]. However, whether ERK1/2 pathways are involved in mediating the effect of EA on HO-1 in severe lung injury during endotoxic shock has not been fully demonstrated.
Our purpose was to study the molecular mechanisms of EA-mediated up-regulation of HO-1 in lungs of rabbits with endotoxic shock and to correlate it with ERK1/2 activation.
Material and Methods
Experimental animals
Approval was obtained from the Animal Use and Care Committee of Nan Kai Hospital, and the animals were managed in accordance with National Institutes of Health guidelines. Two-month-old male New Zealand white rabbits weighing 1.5–2.0 kg were studied. All treatment for the animals before the experiment was as we previously described [16]. All procedures were performed under adequate anesthesia/analgesia, and all efforts were made to minimize suffering.
Electro-acupuncture pretreatment
Electro-acupuncture was initiated 5 days before the experiment. Two pairs of stainless steel acupuncture needles were inserted into the rabbit equivalent of the ST36 acupoint, which is located between the tibia and fibula, approximately 5 mm lateral to the anterior tubercle of the tibia, and the BL13 acupoint, which is located between T3 and T4 of the spine, approximately 1.5 cm lateral to the midline, on both sides in groups EAM and EAMPD. Electro-acupuncture stimulation was delivered by HANS (Han’s acupoint nerve stimulator, model LH-202H, Neuroscience Research Institute, Peking University, Beijing, China) at a disperse-dense wave with either 2 or 100 Hz for 30 min every day [17]. Electro-acupuncture was also performed throughout the experimental procedure for 6 h on the day of the experiment [18]. Group SEAM received acupuncture at non-acupoints, which were not on any meridian and did not belong to any acupoint and were 0.5–1 cm lateral to BL13 and ST36 bilaterally, with the same frequency and intensity. Acupuncture points were identified by an experienced acupuncturist.
Experimental protocols
We used a rabbit model of endotoxic shock, similar to the experimental preparation that we have previously described [8]. Rabbits were fasted for 12 h but were allowed free access to water during the experiments. On the day of the experiment, the animals were anesthetized intravenously with 30 mg/kg of pentobarbital sodium. Anesthesia was maintained by additional doses as necessary throughout the experiment. The animals were kept supine and spontaneously breathing during the experiments. The right carotid artery was cannulated with a PE-50 catheter and connected to Hellige monitor instruments (Germany) to record mean arterial pressure (MAP) and heart rate. The right jugular vein was cannulated with PE-50 catheters for blood withdrawal and fluid resuscitation. All surgical procedures were performed under aseptic conditions. After instrumentation, the animals were allowed to stabilize for 15 min and baseline data was recorded.
Seventy rabbits were randomly divided into 7 groups: group C, group M, group D, group SEAM, group EAM, group EAMPD, and group PD98059. Electro-acupuncture treatment was performed on groups EAM and EAMPD, and group SEAM received acupuncture at a non-acupoint as described above, with no electro-acupuncture treatment on groups C, group D, group PD98059, and group M. Rabbits in group M, SEAM, EAM, EAMPD, and EAMP received 2 mL (5 mg/kg, intravenously) LPS (L2630, Sigma, USA) to replicate the experimental model of endotoxic shock, and those in group C, D, and PD98059 received 2 mL normal saline intravenously as a control [19]. After 0.5 h of administration of LPS or normal saline, groups EAMPD and PD98059 received PD98059 (0.3mg/kg) intravenously, while 10% DMSO was infused intravenously in group D instead [20]. The manipulations of the experimental groups are described in Table 1. The experimental model of endotoxic shock-induced lung injury was defined by the lowered MAP (<75% of the baseline) and oxygenation index (PaO2/FiO2 <300 mmHg).
Table 1.
Comparison of the manipulations of the experimental groups.
| Groups | LPS | EA | Non-acupoint-EA | PD98059 | DMSO |
|---|---|---|---|---|---|
| C | − | − | − | − | − |
| M | + | − | − | − | − |
| SEAM | + | − | + | − | − |
| EAM | + | + | − | − | − |
| EAMPD | + | + | − | + | + |
| D | − | − | − | − | + |
| PD | − | − | − | + | + |
+ Explains what the experimental group received; – explains the experimental manipulation not be provided.
Sampling and storage
The blood samples were collected 6 h after LPS or normal saline administration, and immediately centrifuged at 4000 rpm for 10 min at 4°C. The serum samples were then immediately frozen and stored at −80°C until analysis. The rabbits were killed and the lungs were removed and quickly perfused with phosphate-buffered saline to remove the blood. The left lung was used for measurement of lung wet/dry weight ratio, and the middle lobe of the right lung was fixed in 10% formalin for pathologic specimen preparation, and the remaining right lung was cut into small pieces, snap-frozen in liquid nitrogen, and stored at −80°C.
Morphology of lung tissues
The middle lobe of the right lung was fixed in 10% formalin and routinely processed into paraffin sections (4–5mm). Sections were used for hematoxylin and eosin staining. Six slices were selected from each group of rabbits, and 10 fields of each slice were visualized by microscopy (×200). Figure 1 shows the scores used to assess the degree of pathological change. The average values were taken as a semi-quantitative histological IQA of lung injury [21].
Figure 1.
Comparison of the lung IQA score among the 7 groups: “a” Significantly different compared with C group (P<0.05); “b” Significantly different compared with M group (P<0.05); “c” Significantly different compared with SEAM group (P<0.05); “d” Significantly different compared with EAM group (P<0.05). Data are presented as the mean ±SD, n=10 rabbits/group.
Serum tumor necrosis factor-α (TNF-α)
Serum levels of TNF-α were measured by a rabbit TNF-α ELISA kit (R&D, USA).
Lung wet/dry weight ratio, MDA content, and SOD activity
The left lung was harvested and weighed before and after drying for 24 h at 70°C. The water content of the lung tissue was calculated as the wet/dry weight ratio [22]. Lung MDA content and SOD activity were determined with test kits (Nanjing Jiancheng Bioengineering Institute, China) [23–25].
Western blot analysis
Tissue samples were homogenized in lysis buffer solution containing 13.2 mmol/L Tris-HCl, 5.5% glycerol, 0.44% SDS, and 10% β-mercaptoethanol. An equal amount of extracted soluble protein (50 μg) was fractionated by Tris-glycine-SDS-polyacrylamide gel (12%) electrophoresis. Proteins were transferred to a PVDF membrane (Bio-Rad) with a Bio-Rad transblot apparatus [26]. Blots were briefly washed with 1× Tris-buffered saline (TBS; 200mM Tris and 1.5MNaCl) and then incubated overnight at 25°C with rabbit anti-HO-1 antibody (ABcam, USA), rabbit anti-ERK antibody (ABcam, USA), and rabbit anti-pERK antibody (ABcam, USA). Beta-actin (Thermo Fisher Scientific) was used as a loading control. Blots were washed with TBS-0.05% Tween 20 and incubated for 2 h at 37°C with goat anti-rabbit IgG (Caltag Laboratories, San Francisco, CA). The antigen-antibody signal was visualized as previously described [27] and quantification was performed by densitometry (Molecular Analyst Image-analysis Software, Bio-Rad). The HO-1 and ERK1/2 protein concentrations were normalized by Beta-actin. The IOD ratio between HO-1 and Beta-actin was calculated and used as the expression of HO-1, while the IOD ratio between ERK1/2 and Beta-actin was calculated and used as the expression of ERK1/2.
Fluorescence quantitative (FQ) PCR
A fluorescence quantitative PCR method was used to determine the expression of HO-1mRNA. The primers used in this study were 5′-GTCTACGCCCCGCTCTACTTCCCG-3′ and 5′-TAGCCTCTTCCACCACCCTCTGCC-3′ for rabbit HO-1, 394 bp; 5′-AAACGAGACGAGATTGGCATGGCTTTA-3′; and 5′-GGGATGCTCGCTCCAACGACTGCT-3′ for rabbit β-actin, 143 bp. Total RNA was extracted from the lungs by using an animal RNA extraction kit (TIANGEN, China), and the RNA fraction was transcribed using a first-strand cDNA synthesis kit (MetaBios Inc., Canada). The PCR reaction system (20 μL) was 2× SuperReal PreMix 10 μL, upstream primer 0.3 μL, downstream primer 0.3 μL, cDNA template 0.3 μL, and RNase-free ddH2O. Reaction conditions were 95°C denaturation for 15 min, 95°C denaturation for 10 s, 60°C annealing extension for 30 s, and 40 cycles for previous operations. Fluorescence Quantitative PCR Detector (Bio-Rad, USA) with a fluorescence quantification kit (TIANGEN, China) was used to detect the CT and 2−ΔΔCT was adopted for the relative expression of the target gene [28].
Statistical analysis
All data are presented as mean ±SD or median (interquartile range). Repeated-measures data was analyzed using repeated measures analysis of variance (ANOVA). Intergroup comparisons among the 9 groups were analyzed with one-way ANOVA followed by Tukey-Kramer post hoc multiple comparison. Statistical significance was set at P<0.05. SPSS version 11.5 statistical software (SPSS, Chicago, IL) was used for the data analysis.
Results
Comparison of the lung IQA score
The degree of rabbit lung parenchymal damage was determined under light microscopy. Few infiltrating neutrophils were observed and there was no evidence of hemorrhage or edema in the lungs of groups C and PD (Figure 2 (C and PD)). In groups M, SEAM and EAMPD, histologic examination showed typical pathologic traits of lung injury, such as diffuse edema and severe inflammatory cell (predominantly neutrophils) infiltration in the alveoli and interstitium of the lungs, hemorrhage, and thickened interlobular septa (Figure 2 (M, SEAM and EAMPD)), whereas that of group EAM showed minimal lung injury with a mild degree of neutrophil infiltration and thickening of the alveolar walls (Figure 2 (EAM)). The IQA score indicated no significant difference between the rabbits treated with PD98059 alone and that of sham-treat rabbits (P>0.05; Figure 1). Significantly higher IQA scores were observed in the group M rabbits compared with group C (P<0.05; Figure 1). Pretreatment with electro-acupuncture in group EAM attenuated the lung injury, reflected by decreased IQA scores compared with that of group M (P<0.05; Figure 1), whereas pretreatment with PD98059 in group EAMPD suppressed the efficacy of electro-acupuncture. As a result, the IQA scores were increased in group EAMPD compared to group EAM (P<0.05; Figure 1). The pretreatment with non-acupoint stimulation in group SEAM also did not decrease the levels of IQA scores compared with that of group M (all P>0.05; Figure 1).
Figure 2.
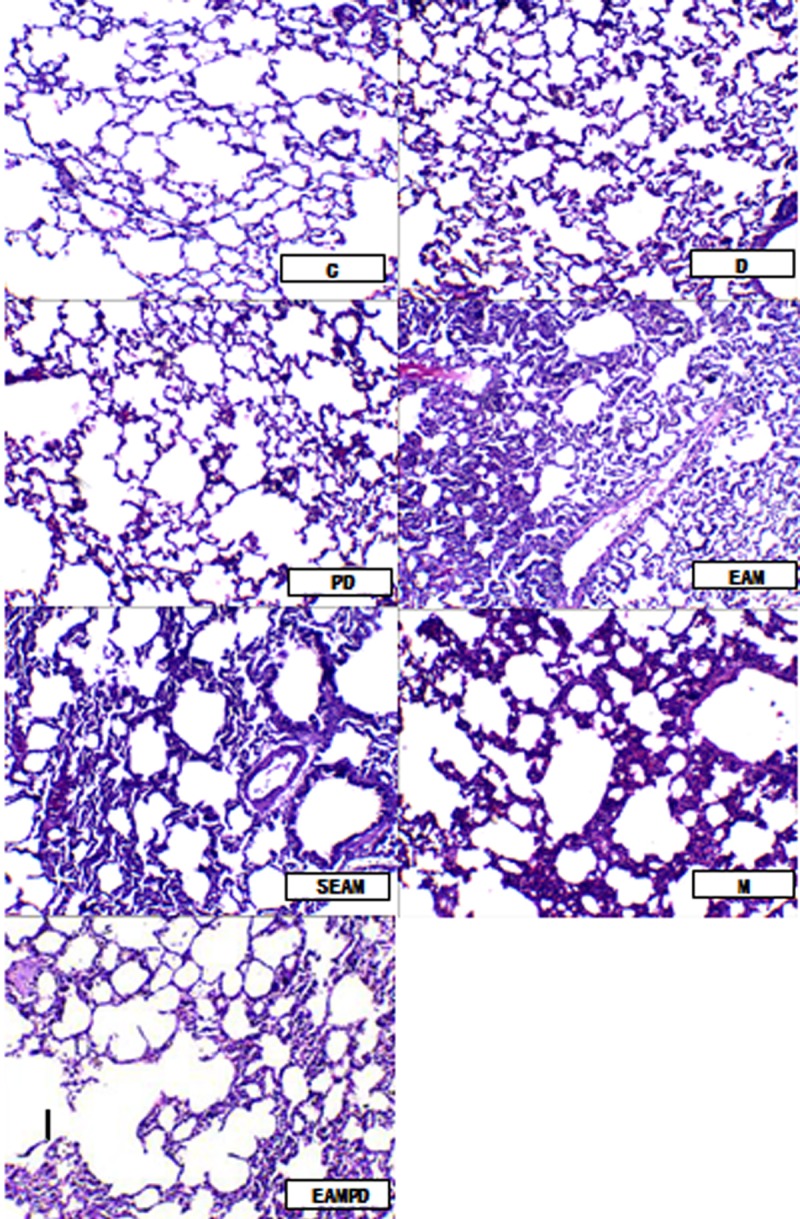
Microphotographs of morphological changes of lung tissues (×200): Few infiltrating neutrophils were observed and there was no evidence of hemorrhage or edema in the lungs of the control group rabbits (C and PD). In the models of the lung injury induced by endotoxic shock, whether given non-acupoint treatment or given PDTC, histologic examination showed typical pathologic traits of lung injury, such as diffuse edema and severe inflammatory cell (predominantly neutrophils) infiltration in alveoli and interstitium of the lung, hemorrhage, and thickened interlobular septa (M, SEAM and EAMPD), whereas the model with electro-acupuncture treatment showed minimal lung injury, with a mild degree of neutrophil infiltration and thickening of the alveolar walls (EAM).
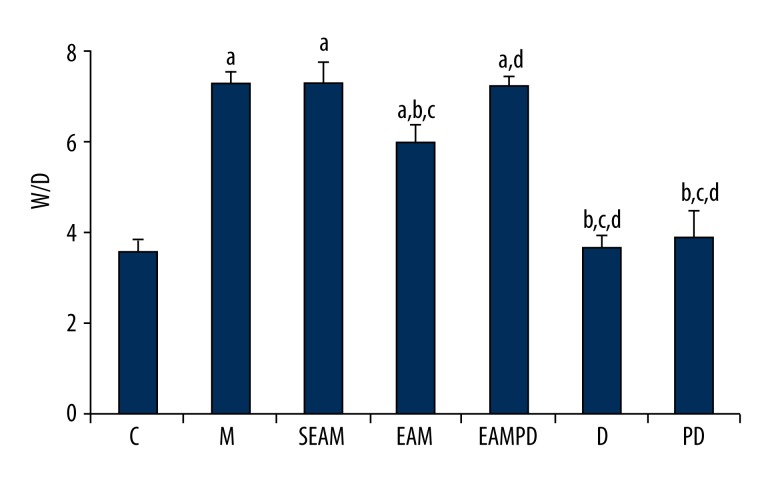
Comparison of wet/dry weight ratio
Wet/dry weight ratio also showed no significant difference between the rabbits treated with PD98059 alone and that of sham-treat rabbits (P>0.05; Figure 3). Significantly higher wet/dry weight ratios were observed in the group M rabbits compared with group C (P<0.05; Figure 3). Pretreatment with electro-acupuncture in group EAM attenuated the lung injury, reflected by decreased wet/dry weight ratio compared with that of group M (P<0.05; Figure 3), whereas pretreatment with PD98059 in group EAMPD suppressed the efficacy of electro-acupuncture. As a result, wet/dry weight ratio were increased in group EAMPD compared to group EAM (P<0.05; Figure 3). The pretreatment with non-acupoint stimulation in group SEAM also did not decrease the levels of wet/dry weight ratio compared with that of group M (all P>0.05; Figure 3).
Figure 3.
Comparison of lung wet/dry weight ratio (W/D): “a” Significantly different compared with C group (P<0.05); “b” Significantly different compared with M group (P<0.05); “c” Significantly different compared with SEAM group (P<0.05); “d” Significantly different compared with EAM group (P<0.05). Data are presented as the mean ±SD, n=10 rabbits/group.
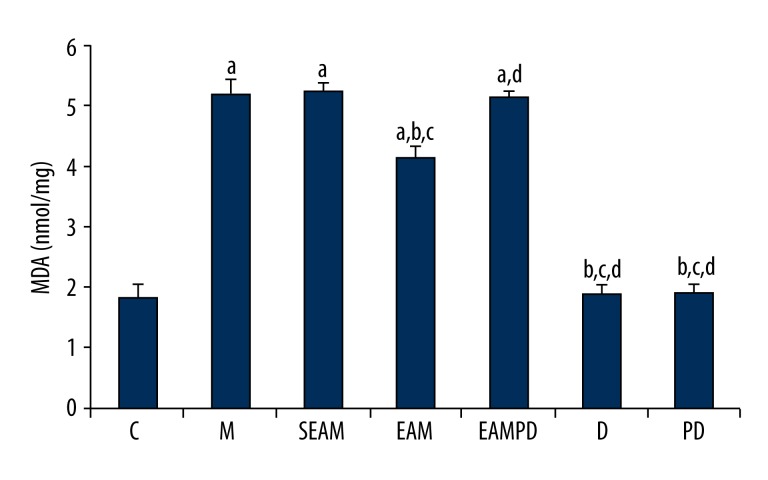
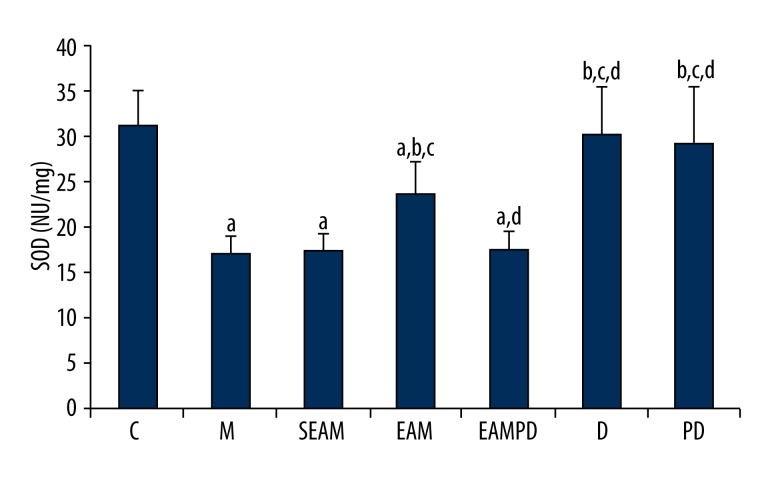
Comparison of SOD activity and MDA content
Consistent with the histologic findings, the comparisons of MDA and SOD activity among the 7 groups are shown in Figures 4 and 5. The results clearly show that the administration of LPS to normal rabbits could induce the oxidative stress, as reflected by increased MDA content and decreased SOD activity compared with group C (all P<0.05). Findings also show that electro-acupuncture could reduce the lung injury induced by LPS, as shown by lower MDA content and higher SOD activity in group EAM compared with group M (all P<0.05). In addition, non-acupoint stimulation in group SEAM did not induce a significant increase of SOD activity nor a significant decrease of MDA content compared with group M (all P>0.05). There were also no significant differences in MDA and SOD activities between group EAMPD and group M (all P>0.05).
Figure 4.
Comparison of malondialdehyde (MDA): “a” Significantly different compared with C group (P<0.05); “b” Significantly different compared with M group (P <0.05); “c” Significantly different compared with SEAM group (P<0.05); “d” Significantly different compared with EAM group (P<0.05). Data are presented as the mean ±SD, n=10 rabbits/group.
Figure 5.
Comparison of superoxide dismutase (SOD) Activity: “a” Significantly different compared with C group (P<0.05); “b” Significantly different compared with M group (P<0.05); “c” Significantly different compared with SEAM group (P<0.05); “d” Significantly different compared with EAM group (P<0.05). Data are presented as the mean ±SD, n=10 rabbits/group.
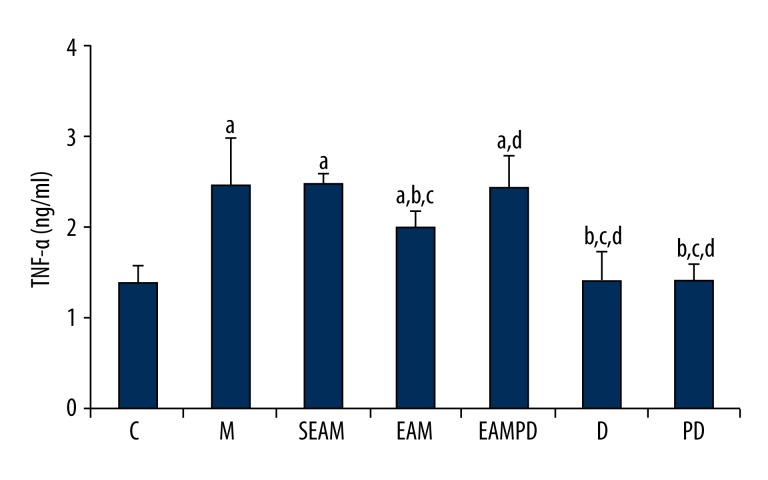
Comparison of serum TNF-α
According to our results, it is clear that the administration of LPS to the normal rabbits could also lead to lung inflammation, which is shown by an increased serum TNF-α compared with group C (all P>0.05; Figure 6). The results of serum TNF-α showed no significant difference between the rabbits treated with PD98059 alone and that of sham-treated rabbits (P>0.05; Figure 6). Significantly higher TNF-α was observed in the group M rabbits compared with group C (all P>0.05; Figure 6). Pretreatment with electro-acupuncture in group EAM decreased serum TNF-α compared with that of group M (P>0.05; Figure 6), whereas pretreatment with PD98059 in group EAMPD suppressed the efficacy of electro-acupuncture. As a result, serum TNF-α was increased in group EAMPD compared to group EAM (P>0.05; Figure 6). There was no significant difference in serum TNF-α between group SEAM and group M (all P>0.05; Figure 6).
Figure 6.
Comparison of serum tumor necrosis factor-α (TNF-α): “a” Significantly different compared with C group (P<0.05); “b” Significantly different compared with M group (P<0.05); “c” Significantly different compared with SEAM group (P<0.05); “d” Significantly different compared with EAM group (P<0.05). Data are presented as the mean ±SD, n=10 rabbits/group.
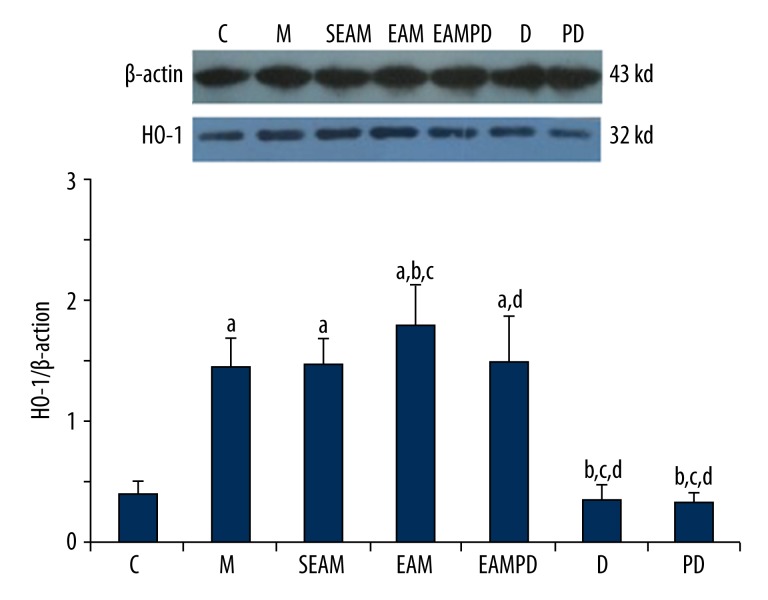
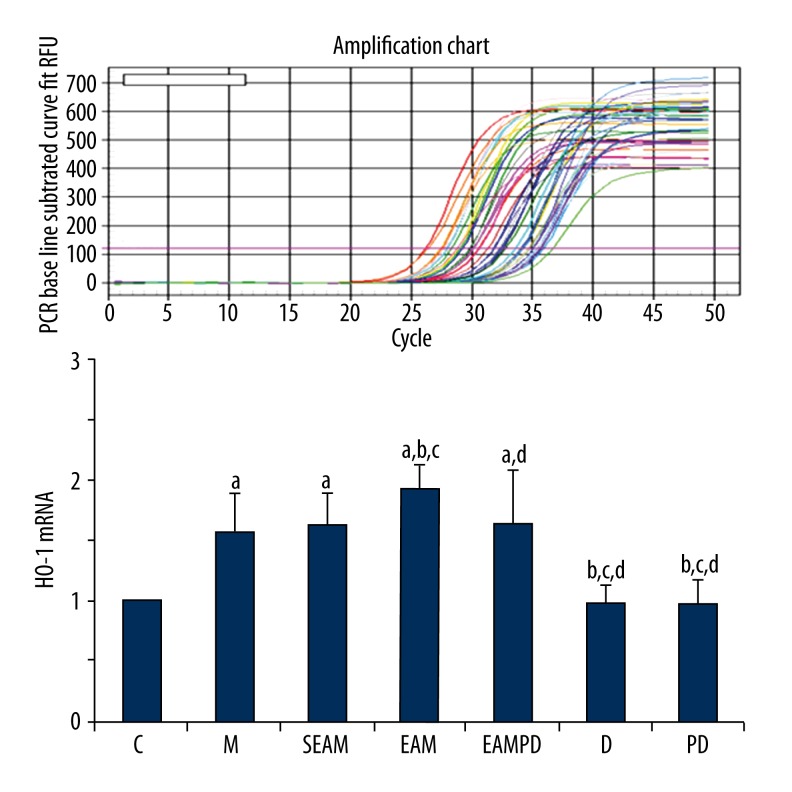
Comparison of HO-1 mRNA and HO-1 protein expression
HO-1 protein expression and HO-1 mRNA were investigated among the 7 groups, and the results are shown in Figures 7 and 8. The fluorescence quantitative PCR and Western blot analysis revealed that expression of HO-1mRNA and the level of HO-1 protein rapidly increased after administration of LPS compared with that of controls (all P<0.05), and electro-acupuncture could up-regulate the expression of the HO-1mRNA and increase the level of HO-1 protein in group EAM in contrast to that of group M (all P<0.05). However, PD98059 could eliminate the effect of electro-acupuncture on up-regulation of HO-1mRNA and HO-1 protein expression in group EAMPD (all P<0.05). Also, the expression of HO-1mRNA and HO-1 protein in group SEAM did not increase, in contrast to that of group M (all P>0.05).
Figure 7.
Western blot analysis of HO-1 protein: β-actin served as loading control for HO-1 protein. “a” Significantly different compared with C group (P<0.05); “b” Significantly different compared with M group (P<0.05); “c” Significantly different compared with SEAM group (P<0.05); “d” Significantly different compared with EAM group (P<0.05). Data are presented as the mean ±SD, n=10 rabbits/group.
Figure 8.
Fluorescence quantitative PCR of HO-1mRNA: “a” Significantly different compared with C group (P<0.05); “b” Significantly different compared with M group (P<0.05); “c” Significantly different compared with SEAM group (P<0.05); “d” Significantly different compared with EAM group (P<0.05). Data are presented as the mean ±SD, n=10 rabbits/group.
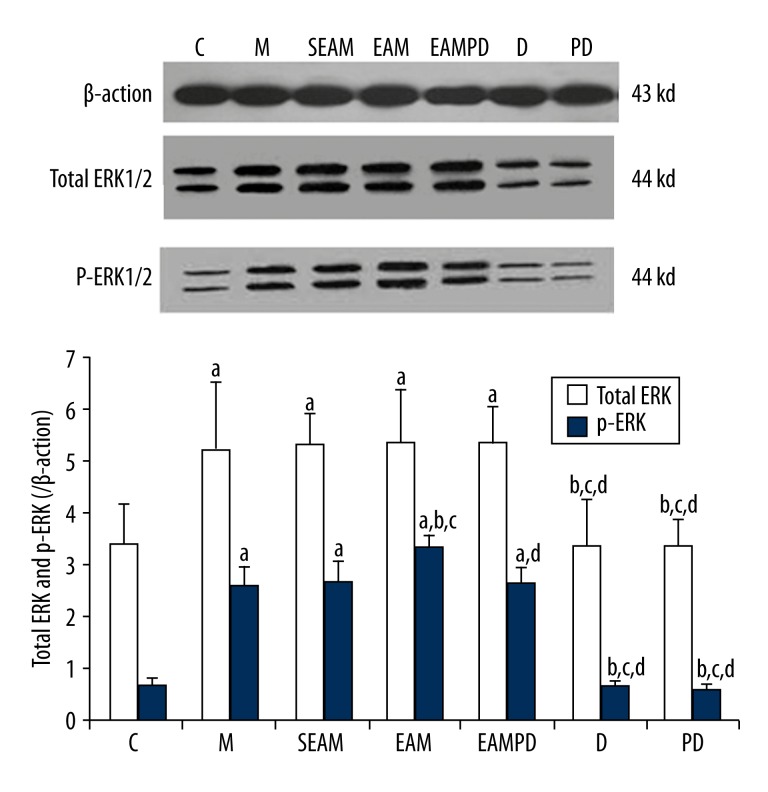
Comparison of total ERK1/2 protein and p-ERK1/2 protein expression
Total ERK1/2 protein and p-ERK1/2 protein in the rabbit lungs were analyzed by Western blot (Figure 9). The results show that protein expression of total ERK1/2 protein and p-ERK1/2 protein were up-regulated in the lung tissue of rabbits after administration of LPS compared with that of controls (all P<0.05). Electro-acupuncture could up-regulate the expression of p-ERK1/2 protein in group EAM, in contrast to that of group M (all P<0.05), whereas PD98059 could abolish the effect of electro-acupuncture on up-regulation of p-ERK1/2 protein expression in group EAMPD (all P<0.05). In addition, the expression of p-ERK1/2 protein did not increase in group SEAM compared with group M (all P>0.05). The expressions of total ERK1/2 protein were not significantly different in group M, SEAM, EAM, and EAMPD (all P>0.05).
Figure 9.
Comparison of total ERK1/2 protein and p-ERK1/2 protein: β-actin served as loading control for HO-1 protein. “a” Significantly different compared with C group (P<0.05); “b” Significantly different compared with M group (P<0.05); “c” Significantly different compared with SEAM group (P<0.05); “d” Significantly different compared with EAM group (P<0.05). Data are presented as the mean ±SD, n=10 rabbits/group.
Discussion
In the present study, we have shown that EA is capable of attenuating LPS-induced lung injury during endotoxic shock and significantly inhibiting LPS-induced up-regulation of MDA and TNF-α, as well as down-regulation of SOD activity. We have demonstrated that the ability of EA to inhibit LPS-induced inflammation and oxidative stress by increasing the expression of HO-1, triggering nuclear translocation of the ERK1/2 signaling pathway. These findings show a novel linkage between HO-1 and ERK1/2 signaling pathways in LPS-induced lung injury during endotoxic shock, which expands the understanding of the anti-inflammatory and anti-oxidative activities of EA, and outlines potential molecular mechanisms that explain the protective respiratory effects of EA.
HO-1 and the subsequent metabolites of heme catabolism appear to play vital roles in regulating important biological responses, including inflammation, oxidative stress, cell survival, and cell proliferation [29]. Our previous study showed that the induction of HO-1 by LPS can cause protection of lung, kidney, and liver during endotoxic shock in rats, and up-regulation of HO-1 may become a novel protective strategy for organ injury during endotoxic shock [7,22]. Another previous study also indicated that EA could attenuate lung injury by up-regulating HO-1 during endotoxic shock [18]. In this study, after EA stimulation, there is a further marked amelioration of lung injury scores, histologic examination, and lung wet/dry weight ratio, indicating that EA stimulation can attenuate the lung injury. Fluorescence quantitative PCR and Western blot analysis showed that HO-1 expression was significantly increased after EA stimulation, indicating that EA stimulation can attenuate lung injury by the up-regulation of HO-1 during endotoxic shock. In agreement with previous findings, these results suggest that EA stimulation (but not non-acupoint EA stimulation), exerts protective effects on lungs with endotoxic shock by increasing expression of HO-1.
The phosphorylation of ERK1/2, which are signaling molecules involved in upstream regulation of several transcription factors, has been observed in LPS-stimulated lung injury [30]. Recently, it has been found that EA activates phosphorylation of the ERK1/2 signaling pathway [31]. Our data support the findings of these previous studies. We found that EA powerfully increased the phosphorylation of ERK1/2 in LPS-stimulated lung injury.
Numerous studies have demonstrated that severe lung injury during endotoxic shock can be caused by multiple factors and that long-lasting inflammatory responses play an important role in the progression of ALI and ARDS [32,33]. TNF-α, regarded as a potent pro-inflammatory mediator, can increase the production of other inflammatory cytokines, and encourage the expression of adhesion molecules, which strongly promote progression of LPS-induced lung injury [34]. EA has been found to inhibit overexpression of TNF-α, which plays a critical role in suppressing inflammation within lung injury [35]. In our study, we found that EA effectively inhibited LPS-induced overexpression of pro-inflammatory cytokine TNF-α, which is consistent with findings of previous reports. More importantly, EA powerfully inhibited overproduction of TNF-α accompanied by increasing the expression of HO-1 and p-ERK1/2 in LPS-stimulated lung injury. These results indicate that HO-1 and the ERK1/2 pathway are involved in the anti-inflammatory effects of EA.
Excessive production of reactive oxygen species, such as superoxide radical, have been proven to play crucial roles in the pathogenesis of endotoxin-induced ALI [36]. Our previous study demonstrated that down-regulation of the HO-1 protein by ZnPP-IX, a special inhibitor of HO-1, results in an increase of end-organ dysfunctions in endotoxic shock, while SOD activity was reduced and the MDA content was increased [22]. Another of our previous studies reported that the protective effects of electro-acupuncture is dependent on HO-1. The up-regulation of HO-1, in turn, results in a reduction of MDA and an increase of SOD activity [18]. Consistent with the previous study, our study found that up-regulation of HO-1 induced by EA significantly inhibited serum levels of MDA and increased the activities of SOD. After giving PD98059, accompanied with down-regulation of p-ERK1/2 and HO-1, this effect of EA against oxidative stress was significantly reduced, which shows that a critical mechanism of electro-acupuncture in protecting lungs with endotoxic shock against oxidative stress may be through the effect of the ERK1/2 pathway on up-regulation of HO-1.
PD98059, an inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase (MEK/ERK), affected the expression of the ERK1/2 pathway stimulated with LPS, which is a special inhibitor of the ERK1/2 pathway [37]. Wang et al. [38] reported that when the cells were pretreated for 30 min with PD98059 and then stimulated by LPS, the phosphorylation of ERK1/2 was significantly decreased. To clarify whether the ERK1/2 pathway induced the expression of HO-1 by electro-acupuncture in the lungs of rabbits with endotoxic shock, we used PD98059. To avoid the adverse effect of PD98059, PD98059 was given in a dose of 0.3 mg/kg in this study [20].
In summary, this study demonstrated that electro-acupuncture stimulation at acupoints ST36 and BL13, but not EA stimulation at non-acupoints, could up-regulate heme oxygenase-1 in the lungs of rabbits with endotoxic shock and attenuate lung injury during the endotoxic shock. The mechanism may be through up-regulation of the signal transductions of the ERK1/2 pathway. However, the pathophysiology of lung injury induced by endotoxic shock is a very complicated process, and the exact mechanisms underlying the regulatory role of the ERK1/2 pathway induced by electro-acupuncture on HO-1 expression need further investigation. A limitation of our study is that the dose of PD98059 is often unpredictable, so it is very hard to absolutely inhibit the ERK1/2 pathway, which affects the accuracy of our results. Although there is a long way to go before the exact mechanisms of electro-acupuncture on lung injury induced by endotoxic shock can be determined, we believe that the role of signaling pathways induced by electro-acupuncture on expression of HO-1 in the lungs of rabbits with endotoxic shock will draw increasing interest in the future.
Conclusions
Electro-acupuncture stimulation at ST36 and BL13, in contrast with the non-acupoint, can up-regulate heme oxygenase-1 in the lungs of rabbits with endotoxic shock and attenuate the lung injury during the endotoxic shock. The mechanism may be through up-regulation of the signal transductions of the ERK1/2 pathway.
Footnotes
Source of support: Financial support for this research work (equipment, drugs, etc) was provided by the Natural Science Foundation of Tianjin (11JCYBJC11000) and Tianjin Science & Technology Pillar Program (12ZCZDSY013300)
References
- 1.Tseng TL, Chen MF, Tsai MJ, et al. Oroxylin-A Rescues LPS-Induced Acute Lung Injury via Regulation of NF-κB Signaling Pathway in Rodents. PLoS One. 2012;7:e47403. doi: 10.1371/journal.pone.0047403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang RH, Yang LN, Shen XC, et al. Suppression of NF-κB pathway by crocetin contributes to attenuation of lipopolysaccharide-induced acute lung injury in mice. Eur J Pharmacol. 2012;674:391–96. doi: 10.1016/j.ejphar.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 3.Holst LB, Haase N, Wetterslev J, et al. Transfusion requirements in septic shock (TRISS) trial – comparing the effects and safety of liberal versus restrictive red blood cell transfusion in septic shock patients in the ICU: protocol for a randomised controlled trial. Trials. 2013;14:150. doi: 10.1186/1745-6215-14-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chon TY, Lee MC. Acupuncture. Mayo Clin Proc. 2013;10:1141–46. doi: 10.1016/j.mayocp.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Huang CL, Huang CJ, Tsai PS, et al. Acupuncture stimulation of ST-36 (Zusanli) significantly mitigates acute lung injury in lipopolysaccharide-stimulated rats. Acta Anaesthesiol Scand. 2006;50:722–30. doi: 10.1111/j.1399-6576.2006.01029.x. [DOI] [PubMed] [Google Scholar]
- 6.Camhi SL, Alam J, Wiegand GW, et al. Transcriptional activation of the HO-1 gene by lipopolysaccharide is mediated by 5′distal enhancers: role of reactive oxygen intermediates and AP-1. Am J Respir Cell Mol Biol. 1998;18:226–24. doi: 10.1165/ajrcmb.18.2.2910. [DOI] [PubMed] [Google Scholar]
- 7.Yu JB, Yao SL. Effect of heme oxygenase-endogenous carbon monoxide on mortality during septic shock in rats. Ir J Med Sci. 2009;178:491–96. doi: 10.1007/s11845-008-0260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu JB, Dong SA, Luo XQ, et al. Role of HO-1 in protective effect of electro-acupuncture against endotoxin shock-induced acute lung injury in rabbits. Exp Biol Med. 2013;238:705–12. doi: 10.1177/1535370213489487. [DOI] [PubMed] [Google Scholar]
- 9.Nils S, Matthias F, Christian IS, et al. Postconditioning with Inhaled Carbon Monoxide Counteracts Apoptosis and Neuroinflammation in the Ischemic Rat Retina. PLoS One. 2012;7:e46479. doi: 10.1371/journal.pone.0046479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Shepherd EG, Nelin LD. MAPK phosphatases – regulating the immune response. Nat Rev Immunol. 2007;7:202–12. doi: 10.1038/nri2035. [DOI] [PubMed] [Google Scholar]
- 11.Iles KE, Dickinson DA, Wigley AF. HNE increases HO-1 through activation of the ERK pathway in pulmonary epithelial cells. Free Radic Biol Med. 2005;39:355–64. doi: 10.1016/j.freeradbiomed.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–12. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 13.Li B, Lee DS, Choi HG, et al. Sauchinone Suppresses Pro-inflammatory Mediators by Inducing Heme Oxygenase-1 in RAW264.7 Macrophages. Biol Pharm Bul. 2011;34:1566–71. doi: 10.1248/bpb.34.1566. [DOI] [PubMed] [Google Scholar]
- 14.Xie G, Yang S, Chen A, et al. Electroacupuncture at Quchi and Zusanli treats cerebral ischemia-reperfusion injury through activation of ERK signaling. Exp Ther Med. 2013;5:1593–97. doi: 10.3892/etm.2013.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu LN, Yu JB, Gong LR, et al. Effect of ERK1/2signaling pathway on the expression of HO-1 in the lung during endotoxic shock in rats. Guo Ji Ma Zui Xue Yu Fu Su Za Zhi. 2013;34:216–19. [Google Scholar]
- 16.Yu JB, Yao SL. Protective effects of hemin pretreatment combined with ulinastatin on septic shock in rats. Chin Med J (Engl) 2008;121:49–55. [PubMed] [Google Scholar]
- 17.Ferreira AS, Lima JG, Ferreira TP, et al. Prophylactic effects of short-term acupuncture on Zusanli (ST36) in Wistar rats with lipopolysaccharide-induced acute lung injury. Zhong Xi Yi Jie He Xue Bao. 2009;7:969–75. doi: 10.3736/jcim20091011. [DOI] [PubMed] [Google Scholar]
- 18.Wang K, Wu H, Wang G, et al. The effects of electroacupuncture on TH1/TH2 cytokine mRNA expression and mitogenactivated protein kinase signaling pathways in the splenic T cells of traumatized rats. Anesth Analg. 2009;109:1666–73. doi: 10.1213/ANE.0b013e3181b5a234. [DOI] [PubMed] [Google Scholar]
- 19.Hsu CH, Hua Y, Jong GP, et al. Shock resuscitation with acupuncture: case report. Emerg Med J. 2006;23:e18. doi: 10.1136/emj.2004.023218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan JZ, Wu XQ, Elena A. The p38 Mitogen-Activated Protein kinase pathway is involved in the regulation of Heme Oxygenase-1 by acidic extracellular pH in aortic smooth muscle cells. Cell Biochem. 2008;105:1298–306. doi: 10.1002/jcb.21930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C, Wang HY, Liu ZW, et al. Effect of endogenous hydrogen sulfide on oxidative stress in oleic acid-induced acute lung injury in rats. Chin Med J (Engl) 2011;124:3476–80. [PubMed] [Google Scholar]
- 22.Yu JB, Zhou F, Yao SL, et al. Effect of heme oxygenase-1 on the kidney during septic shock in rats. Transl Res. 2009;153:283–87. doi: 10.1016/j.trsl.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–75. [PubMed] [Google Scholar]
- 24.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–58. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 25.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 26.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–54. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dennery PA, Sridhar KJ, Lee CS. Heme oxygenase-mediated resistance to oxygen toxicity in hamster fibroblasts. J Biol Chem. 1997;272:14937–42. doi: 10.1074/jbc.272.23.14937. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and 2(-Delta Delta C (T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 30.Wu TT, Chen TL, Loon WS, et al. Lipopolysaccharide stimulates syntheses of toll-like receptor 2 and surfactant protein-A in human alveolar epithelial A549 cells through upregulating phosphorylation of MEK1 and ERK1/2 and sequential activation of NF-κB. Cytokine. 2011;55:40–47. doi: 10.1016/j.cyto.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Wang K, Wu H, Wang G, et al. The effects of electroacupuncture on TH1/TH2 cytokine mRNA expression and mitogen-activated protein kinase signaling pathways in the splenic T cells of traumatized rats. Anesth Analg. 2009;109:1666–73. doi: 10.1213/ANE.0b013e3181b5a234. [DOI] [PubMed] [Google Scholar]
- 32.Wilson J, Higgins D, Hutting H, et al. Early propranolol treatment induces lung heme-oxygenase-1, attenuates metabolic dysfunction, and improves survival following experimental sepsis. Crit Care. 2013;17:R195. doi: 10.1186/cc12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji MH, Li GM, Jia M, et al. Valproic Acid attenuates lipopolysaccharide-induced acute lung injury in mice. Inflammation. 2013;36:1453–59. doi: 10.1007/s10753-013-9686-z. [DOI] [PubMed] [Google Scholar]
- 34.Zhao C, Sun J, Fang C, Tang F. 1,8-Cineol Attenuates LPS-Induced Acute Pulmonary Inflammation in Mice. Inflammation. 2014;37(2):566–72. doi: 10.1007/s10753-013-9770-4. [DOI] [PubMed] [Google Scholar]
- 35.Geng WY, Liu ZB, Song NN, et al. Effects of electroacupuncture at Zusanli (ST36) on inflammatory cytokines in a rat model of smoke-induced chronic obstructive pulmonary disease. J Integr Med. 2013;11:213–19. doi: 10.3736/jintegrmed2013024. [DOI] [PubMed] [Google Scholar]
- 36.Kristof AS, Goldberg P, Laubach V. Role of inducible nitric oxide synthase in endotoxin-induced acute lung injury. Am J Respir Crit Care Med. 1998;158:1883–89. doi: 10.1164/ajrccm.158.6.9802100. [DOI] [PubMed] [Google Scholar]
- 37.Cho YS, Kim CH, Ha TS, et al. Ginsenoside rg2 inhibits lipopolysaccharide-induced adhesion molecule expression in human umbilical vein endothelial cell. Korean J Physiol Pharmacol. 2013;17:133–37. doi: 10.4196/kjpp.2013.17.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Li Z, Zhang Y, et al. CX43 change in LPS preconditioning against apoptosis of mesenchymal stem cells induced by hypoxia and serum deprivation is associated with ERK signaling pathway. Mol Cell Biochem. 2013;380:267–75. doi: 10.1007/s11010-013-1683-x. [DOI] [PubMed] [Google Scholar]