Abstract
During development, tissues and organs must coordinate growth and patterning so they reach the right size and shape. During larval stages, a dramatic increase in size and cell number of Drosophila wing imaginal discs is controlled by the action of several signaling pathways. Complex cross-talk between these pathways also pattern these discs to specify different regions with different fates and growth potentials. We show that the Notch signaling pathway is both required and sufficient to inhibit the activity of Yorkie (Yki), the Salvador/Warts/Hippo (SWH) pathway terminal transcription activator, but only in the central regions of the wing disc, where the TEAD factor and Yki partner Scalloped (Sd) is expressed. We show that this cross-talk between the Notch and SWH pathways is mediated, at least in part, by the Notch target and Sd partner Vestigial (Vg). We propose that, by altering the ratios between Yki, Sd and Vg, Notch pathway activation restricts the effects of Yki mediated transcription, therefore contributing to define a zone of low proliferation in the central wing discs.
Introduction
Correct tissue development involves the successful co-ordination of growth and patterning mechanisms. One tissue that lends itself to the study of such co-ordination is the wing imaginal disc in Drosophila. The wing develops from groups of epithelial cells, which are specified during embryogenesis. When the embryo hatches, these wing imaginal discs consist of about 10 cells and then, through extensive proliferation during larval life, they reach several thousands cells by the time of metamorphosis. At the same time as they undergo this spectacular increase in cell number, they become fully patterned. Their proliferation, growth and patterning are regulated by the activity of several conserved signaling pathways, including Notch [1], and it is important to understand how these pathways co-operate to generate a wing of the right shape and size.
In wing imaginal discs, Notch controls tissue growth and cell proliferation through the regulation of a complex network of genes whose products act both cell autonomously (e.g. Myc, DIAP1, CycE) and cell non-autonomously (e.g. secreted ligands of the Wnt and Upd family) [2]–[5]. Strikingly, while Notch is required throughout the wing disc for tissue growth, the effects of its over-activation on cell proliferation are most evident at the periphery of the wing disc, outside of the central region or pouch [6], [7]. Activation of Notch involves two proteolytic cleavages, which release the intracellular part of the receptor (Nicd). Nicd then enters the nucleus, binds to the transcription factor Suppressor of Hairless (Su(H)) to turn on the transcription of target genes [8]. A major output of Notch activation is therefore the transcriptional up-regulation of responsive genes. The differential effects on growth in the wing appear to be the consequence of an additional set of genes, including scalloped and vestigial, which are upregulated by Notch in the central wing-pouch [9]–[12] where they prevent the expression of proliferation promoting genes [5].
At the same time as requiring Notch, the epithelial cells also depend on the Salvador/Warts/Hippo (SWH) pathway to regulate their proliferative potential. Defects in this pathway cause dramatic tissue overgrowth. At its core the SWH pathway contains a kinase-cassette, consisting of Warts and Hippo, whose activation results in the phosphorylation of the transcription co-activator Yorkie (Yki; YAP/TAZ in vertebrates). As a consequence Yki/YAP is excluded from the nucleus and is thus prevented from acting with its DNA-binding transcription factor partners. The latter include Scalloped/TEAD, Homothorax, p53 and, by binding with these, Yki/YAP promotes the expression of pro-survival and pro-proliferation genes [13]–[16]. One of the best characterized Yki targets is the gene expanded (ex) [15]. As ex encodes a cortical protein of the FERM family, which is itself implicated in activation of SWH pathway [17], the gene is part of a negative feedback loop regulating Yki activity.
One critical role of Notch in the wing disc is that it governs the expression of Sd and the Sd-binding partner Vestigial (Vg) to specify regions of the wing disc with distinct proliferative potential [3], [5], [10], . Since Sd is one of the partners of the SWH pathway effector Yki [20]–[22], this raises the possibility that, via its effects on sd and vg, Notch could integrate with SWH to control epithelial proliferation. Taking a genetic strategy, we have found that the Notch pathway down-regulates ex-lacZ, the reporter of SWH activity, cell autonomously in wing pouch cells. This inhibition either operates through Yki or downstream of Yki. We further show that downregulating the activity of the Notch target Vg enhances the expression of the two Yki targets expanded and thread/DIAP1. Thus, Vg mediates, at least in part, the repressive activity of Notch in the wing pouch on Yki targets. We propose that by modifying the ratio between Sd, Vg and Yki, Notch signaling prevents Yki from activating its targets in the wing pouch, thus helping to co-ordinate tissue growth with patterning.
Results and Discussion
Notch activity in the wing pouch inhibits ex-lacZ expression
In order to investigate the possibility of cross talk between the Notch and Sav/Warts/Hippo (SWH) pathways, we first compared the expression pattern of ex-lacZ, a reporter of Yki activity, which reveals the places where SWH activity is lowest and NRE-GFP, which gives a direct read out of Notch activity (Fig. 1A). In the wing pouch these reporters direct expression in patterns that are complementary. Thus, ex-lacZ expression is completely absent from the dorso-ventral boundary where Notch activity, reported by NRE-GFP, is at its highest (Fig. 1A). Conversely, in late stage discs, ex-lacZ expression is higher in pro-vein regions where Notch activity (NRE-GFP) is low. Because ex-lacZ gives a mirror image of SWH activity, these results suggest that both Notch and SWH pathways are active together in the D/V boundary and are largely inactive in the pro-veins.
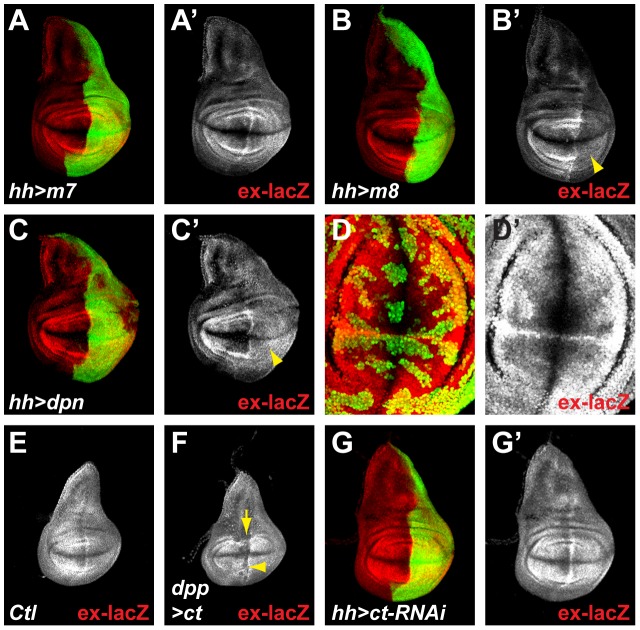
Figure 1. Notch signaling inhibits ex expression in the wing pouch.
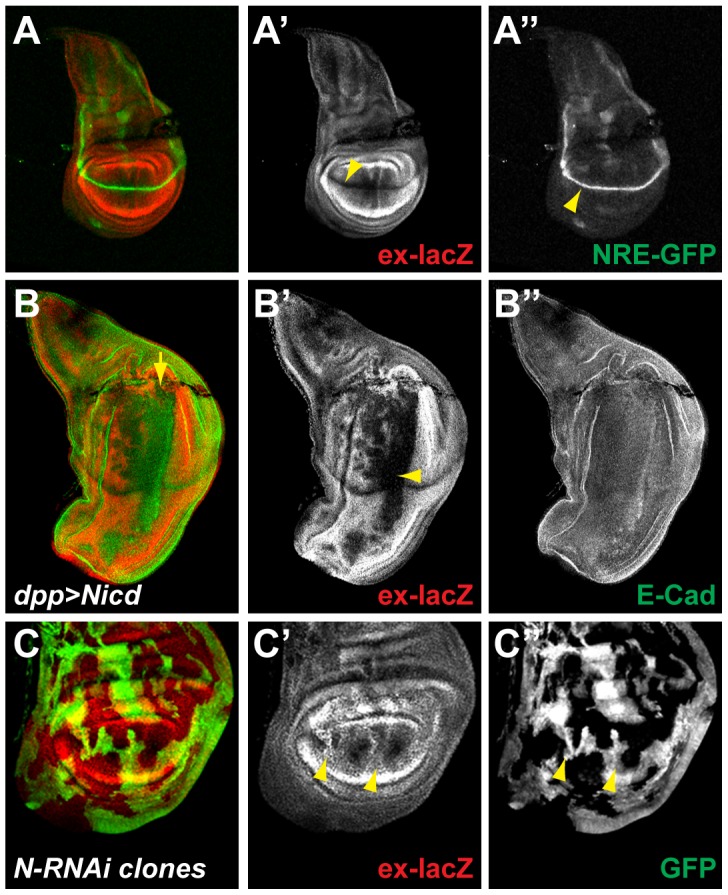
A. In third instar larval wing discs, the expression of the Yki target expanded monitored using the ex697-lacZ reporter line (ex-lacZ; red; A′) is strongest at the periphery and absent at the dorso-ventral boundary (D/V; yellow arrowhead). The activity of the Notch pathway monitored by the NRE-GFP reporter (green; A″) is highest at the D/V (yellow arrowhead). B. Over-expression of the active form of the Notch receptor (Nicd) in a stripe of cells along the antero-posterior (A/P) boundary of the wing disc using dpp-Gal4 (along the line indicated by the yellow arrow), leads to the repression of ex-lacZ (red; B′; yellow arrowhead), while E-Cadherin levels are unaffected (E-Cad; green; B″). C. Inhibition of the Notch pathway in randomly generated clones overexpressing an RNAi against Notch (N-RNAi; positively marked by GFP; green; D″), leads to the upregulation of ex-lacZ in the pouch (red; D′; yellow arrowheads).
We then investigated the consequences of modulating Notch activity on the expression of ex-lacZ as an indicator of its effects on SWH pathway. Expression of Nicd, the constitutively active form of the Notch receptor, promoted a strong down-regulation of ex-lacZ in the wing pouch. This effect was stronger in the region surrounding the D/V boundary and weaker towards the periphery. Little down-regulation occurred outside the pouch (Fig. 1B). Conversely, when Notch activity was impaired, through RNAi mediated knock-down in randomly generated overexpression clones, ex-lacZ levels were up-regulated. This effect was also only evident within the wing-pouch (Fig. 1C). Notch activity is therefore necessary and sufficient for the inhibition of ex-lacZ in the wing pouch (Fig. 1B&C), suggesting that it contributes to the normal down-regulation of ex-lacZ at the D/V boundary.
Notch acts at the level or downstream of Yki
In the wing pouch, ex-lacZ expression requires Yki. Therefore, to mediate the observed inhibition of ex-lacZ expression, Notch could either exert its actions upstream of Yki, by activating the SWH pathway, or downstream of Yki, by inhibiting Yki’s transcriptional activity. To determine which of these alternatives is correct, we assessed the consequences of Notch activity on ectopic Yki expression. When over-expressed in a stripe of cells along the A/P boundary, Yki was able to promote strong expression of ex-lacZ at the periphery of the wing pouch. Strikingly, the high levels of Yki were not able to force ex-lacZ expression at the D/V boundary where Notch activity is highest. These results suggest that the actions of Notch, ie ex-lacZ down-regulation, are epistatic to Yki (Fig. 2A). This was further verified when high levels of Yki were expressed together with high levels of Nicd. In this case, Nicd suppressed the ex-lacZ expression, demonstrating that it wins out over Yki in the wing pouch (Fig. 2B). However, at the periphery of the discs, Nicd was unable to modify the effects of Yki over-expression on ex-lacZ levels. Taken together these results suggest that Notch-mediated down-regulation of ex-lacZ occurs at the level or downstream of Yki.
Figure 2. Notch acts at the level or downstream of yki.
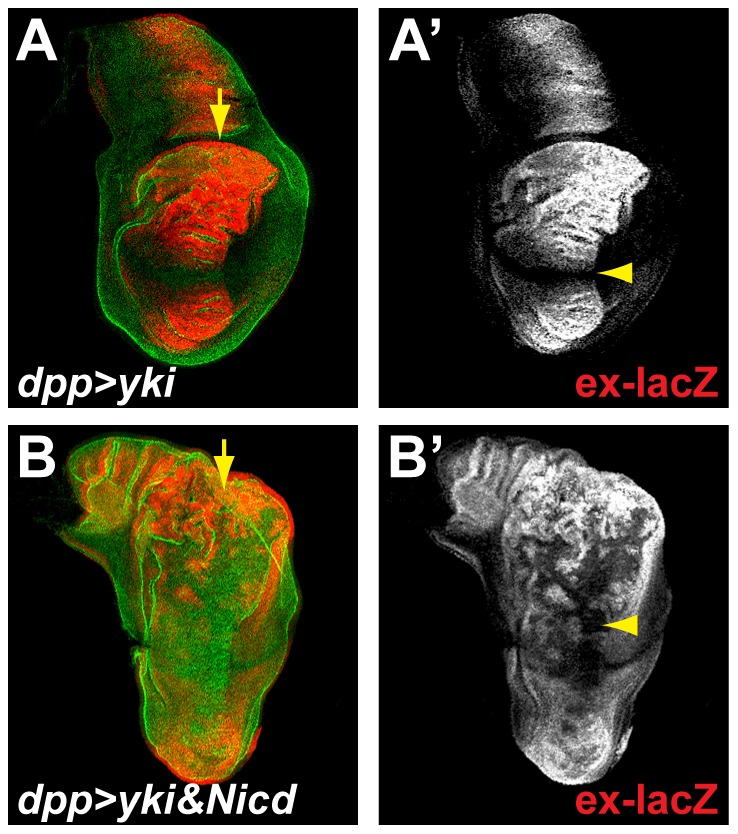
A. Over-expression of Yki along the A/P boundary of the wing disc using dpp-Gal4 (along the line indicated by the yellow arrow), leads to the strong increase in expression of ex-lacZ in the pouch (red; A′), except at the D/V where Notch activity is highest (yellow arrowhead). B. When co-expressed with Yki (using dpp-Gal4; yellow arrow), Nicd imposes an inhibition of ex-lacZ expression in the pouch (red; B′; yellow arrowhead) but not at the periphery. E-Cadherin staining (green, A; B) outlines all cells.
E(spl) and cut repressors are not required to pattern expanded expression
Since a major output of Notch pathway activity is the up-regulation of gene expression, we investigated whether any of the directly regulated Notch target-genes could be responsible for antagonizing Yki. Amongst the direct Notch targets identified in wing discs, several are predicted to encode transcriptional repressors. These include members of the HES family, E(spl)mβ, E(spl)m5, E(spl)m7, E(spl)m8 [23]–[27] and Deadpan (Dpn) [28], [29], as well as the homeodomain protein Cut. All of these proteins are normally expressed at high levels along the D/V boundary, in response to Notch activity, and hence are candidates to mediate the repression of ex-lacZ.
Over-expression of E(spl)mβ, E(spl)m5 or E(spl)m7 repressors had no effect on ex-lacZ expression (Fig. 3A and data not shown). In contrast, over-expression of either E(spl)m8 or of Dpn resulted in a robust down-regulation of ex-lacZ (Fig. 3B&C). The effect differed slightly from that from Nicd expression, in that ex-lacZ expression was not completely abolished and low levels persisted throughout the wing pouch domain (compare Fig. 1B with Fig. 3B&C). These results indicate that a subset of the HES bHLH proteins have the capability to repress ex-lacZ, and hence are candidates to antagonize Yki. Previous experiments have demonstrated that the E(spl)bHLH genes and dpn have overlapping functions, especially at the D/V boundary [28]–[30]. Therefore to determine whether these factors normally contribute to the repression of ex-lacZ, it was necessary to eliminate all of the E(spl)bHLH genes in combination with dpn. To achieve this we expressed a potent RNAi directed against dpn in MARCM clones that were homozygous mutant for a deficiency removing the entire E(spl) complex (details in Materials and Methods). No derepression of ex-lacZ was detectable in such clones, suggesting that none of the E(spl)bHLH/dpn genes can account for the repression of ex-lacZ at the D/V boundary or in the wing pouch (Fig. 3D). Therefore even though E(spl)m8 and Dpn expression is sufficient for ex-lacZ repression, they do not appear to be essential in the context of the wing pouch.
Figure 3. E(spl) and Cut repressors do not mediate the effects of Nicd on ex-lacZ expression.
A–C. When over-expressed in the posterior compartment using hh-Gal4 (marked by GFP; green), the individual HES factors have different effects on ex-lacZ (red; A′, B′, C′). While E(spl)m7 (and others see text; m7; A) has no effect on ex-lacZ, E(spl)m8 (m8; B) and Deadpan (dpn; C) induce a strong down-regulation of ex-lacZ (yellow arrowhead; B′, C′). D. MARCM clones, marked positively by GFP (green), which are homozygous mutant for all E(spl) genes (Df(3R)E(spl)[Grob32.2]) and express a strong RNAi against dpn show normal expression of ex-lacZ (red; D′). E–F. Early third instar larval wing discs showing ex-lacZ expression. Over-expression of Cut (ct) along the A/P boundary of the wing disc using dpp-Gal4 (along the line indicated by the yellow arrow) inhibits ex-lacZ expression (F; yellow arrowhead) compared to controls (E). G. Over-expressing an RNAi construct against ct in the posterior compartment using hh-Gal4 (marked by GFP; green) has no effect on ex-lacZ expression (red; G′).
An alternative candidate was Cut, which encodes a transcriptional repressor and is expressed at the D/V boundary in response to Notch signaling [2], [10], [31], [32]. Similar to some of the HES genes, over-expression of Cut promoted a down-regulation of ex-lacZ (Fig. 3E&F). This was most clearly evident at early developmental stages because Cut induced a strong epithelial delamination at later stages, confounding the interpretation. However no up-regulation of ex-lacZ was detectable when Cut function was ablated, using RNAi, even though Cut levels where efficiently reduced (Fig. 3G & data not shown). Thus, as with the HES genes, Cut is capable of inhibiting ex-lacZ expression but does not appear to be essential for the regulation of ex under normal conditions in the wing pouch.
Vg mediates the effects of Notch on ex-lacZ
Recent studies have demonstrated that, in the absence of Yki, several SWH target genes are kept repressed by Sd, the DNA-binding partner of Yki. This so-called “default repression” requires Tgi, an evolutionarily conserved tondu domain containing protein, which acts as a potent co-repressor with Sd [33], [34]. There is no evidence that Drosophila tgi is a target of Notch in the wing disc, making it an unlikely candidate to mediate the inhibitory effects on Yki-mediated ex-lacZ expression. However, vg, which encodes another Sd binding-partner with a tondu domain [18], [19], is directly regulated by Notch in the wing pouch [9]–[11]. We therefore hypothesized that Vg could mediate the effects of Notch on Yki function and ex-lacZ down-regulation.
In agreement with the hypothesis, when Vg was over-expressed it strongly inhibited ex-lacZ expression in the pouch and promoted a modest overgrowth of the tissue (Fig. 4A). This overgrowth is somewhat puzzling since it appears that Yki activity, as monitored by ex-lacZ, is lowered in the presence of excess Vg. How over-expressed Vg triggers overgrowth remains poorly understood, but has been proposed to involve a cross-talk with the wg pathway [3], [10], [11]. More recently, it has been shown that the expansion of the pouch region is achieved by Vg activating transiently and non-autonomously Yki in cells not expressing Vg. These cells are then recruited to become wing pouch cells and turn on vg expression [35]. This model predicts a wave of Yki activation around Vg positive cells. Therefore, the overgrowth seen when Vg is over-expressed, could be due to a non-autonomous effect where more cells are recruited as pouch cells at the expense of more peripheral cells. Alternatively, Vg could promote proliferation of the pouch cells by an as yet unidentified mechanism, independent of Yki.
Figure 4. Vg mediates in part the effects of Nicd on ex-lacZ expression.
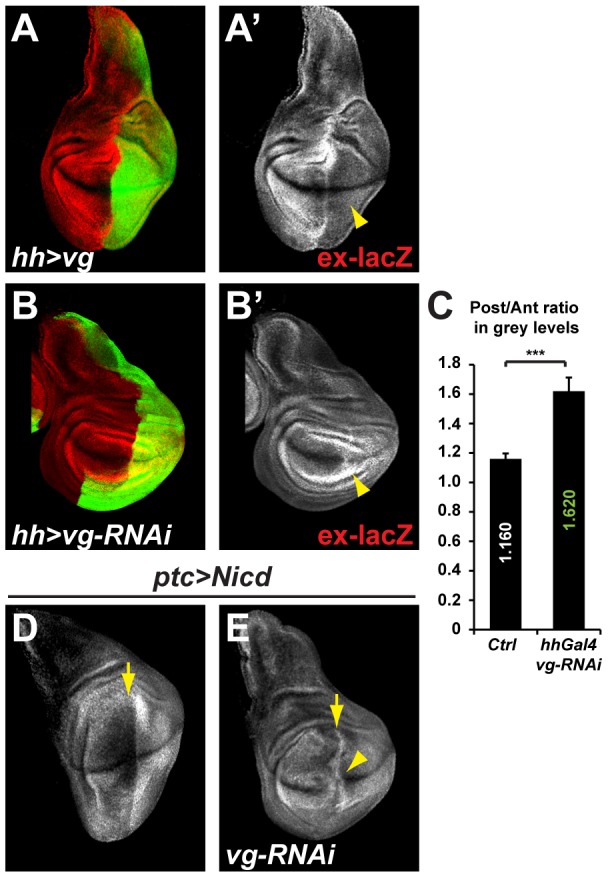
A–B. Effects of over-expressing the Notch target vestigial (vg; A) or RNAi constructs against vg (B) in the posterior compartment using hh-Gal4 (marked by GFP; green) on ex-lacZ expression (red; A′&B′). Vg over-expression and RNAi leads to the inhibition and up-regulation of ex-lacZ respectively (A′; B′; yellow arrowheads). C. Quantification of the average ex-lacZ intensity ratios between equivalent area picked in the posterior and anterior compartments in several discs. While the ratio is at 1.160 in control discs reflecting a slightly higher expression in the posterior compartment, this ratio rises to 1.620 in experimental discs showing that ex-lacZ expression is higher in vg depleted compartments. Standard error to the mean is shown; unpaired two-tailed student t-test was performed showing significance with p value = 0.0003 (***). D–E. While the overexpression of Nicd at the antero-posterior boundary (along the line indicated by the yellow arrow, using the ptc-Gal4 driver), leads to an inhibition of ex-lacZ (white; D), co-expressing an RNAi against vg suppresses this effect, and ex-lacZ expression is found throughout the ptc domain including the D/V boundary (white; E; yellow arrowhead).
Conversely to over-expressed Vg inhibiting ex-lacZ expression, lowering the levels of vg using RNA interference in the whole posterior compartment resulted in a significant up-regulation of ex-lacZ (Fig. 4B). Vg knock down has proven difficult to achieve in small populations of cells, due to their elimination from the wing pouch as documented previously [21], probably by cell competition. Thus, unlike the other factors tested, Vg is required for the repression of ex-lacZ in the wing pouch. We further show that, co-expressing with NICD a vg RNAi transgene in the patched domain, suppresses the NICD mediated ex-lacZ repression in the wing pouch (4C&D). Taken together, these results suggest that Vg mediates the repressive effects of Notch on expanded expression.
Vg prevents Yki targets expression in the pouch
If the involvement of Vg downstream of Notch is a general mechanism for cross-talk between Notch and Yki, other targets of the Sd-Yki complex should be inhibited by Notch in a similar manner to ex-lacZ. However, apart from expanded, all other known Yki targets in the wing pouch, such as thread/DIAP1, diminutive/myc, and Cyclin E are also direct Notch targets [5], [20]–[22], [36]–[42]. Their final expression patterns are therefore a reflection of the balance between different transcriptional inputs, in particular Notch and Yki. Our model predicts that Notch could have a dual effect on the expression of genes: a positive direct effect through the NICD/Su(H) complex when bound in their promoters, but also a negative effect through the induction of Vg, which prevents the positive effect of Yki on Sd bound promoters.
In agreement with this model, thread/DIAP1 and diminutive/myc, two well established Yki targets in wing discs [21], [22], [38], which are normally refractory to Notch mediated activation in the centre of the pouch, become susceptible to Nicd when Vg or Sd levels are lowered through RNAi [5].
Focusing on DIAP1, we thought to separate the Notch and Yki direct inputs on transcription by isolating the Hippo pathway Responsive Elements (HREs) from any potential Notch Responsive Elements (NREs). IAP2B2C-lacZ is a previously described DIAP1-HRE driving lacZ reporter expression [21] that does not contain any NRE, at least based on our Su(H) ChIP data and bio-informatics prediction of Su(H) binding sites [5]. Our model predicts that this IAP2B2C-lacZ reporter should be inhibited by Vg.
In control wing discs, we confirm that IAP2B2C-lacZ is expressed at uniform low levels with a slight increase at the periphery of the pouch (Fig. 5A), where Vg protein levels have been shown to fade [10], [18]. The D/V boundary expression of DIAP1 is not reported by IAP2B2C-lacZ confirming that the NRE is absent in this reporter.
Figure 5. Vg represses th/DIAP1 expression in the wing pouch.
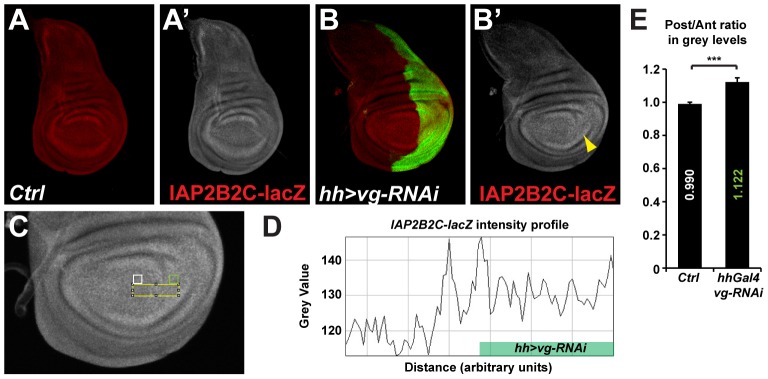
A–B. Expression of the SWH pathway specific th/DIAP1 reporter IAP2B2C-lacZ (red; A′&B′). While IAP2B2C-lacZ are uniform between the anterior and posterior of the pouch in control discs (A), a slight increase in the posterior compartment could be detected in discs where vg activity is lowered by RNAi only in the posterior compartment (B; marked by GFP; green; yellow arrowhead). C–E. Quantification of the posterior compartment increase of IAP2B2C-lacZ expression after vg RNAi knock-down. C. Higher magnification of the image in B′ showing IAP2B2C-lacZ expression in hh-Gal4 vg-RNAi expressing discs. D. Example of the profile of grey levels (reflecting IAP2B2C-lacZ levels), along the yellow box indicated in C. Levels are higher in the posterior compartment expressing the vg-RNAi than in anterior, except for effects at the boundary that are not understood. E. Quantification of the average IAP2B2C-lacZ intensity ratios between equivalent area picked in the posterior (green box in C) and anterior (white box in C) compartments in several discs. While the ratio is at 0.990 in control discs reflecting similar expression levels between the posterior and anterior compartments, this ratio rises to 1.122 in experimental discs showing that IAP2B2C-lacZ expression is 12% higher in the vg depleted compartment. Standard error to the mean is shown; unpaired two-tailed student t-test was performed showing significance with p value = 0.0006 (***).
We then lowered Vg levels using moderate RNAi knocked down in the whole posterior compartment using the hh-Gal4 driver (Fig. 5B; as mentioned before, since Vg is required for pouch identity, strong vg knock down results in delaminating cells that are difficult to interpret). In this experimental set-up, the posterior compartment is smaller than normal, and vg knock-down induced a 12% up-regulation of IAP2B2C-lacZ expression when compared to IAP2B2C-lacZ levels in the anterior control compartment (Fig. 5C–E), demonstrating that Vg has a negative effect on this reporter activity (there was no difference in IAP2B2C-lacZ expression between the anterior and posterior compartment in the pouch region of control discs; Fig. 5A&E). We note that IAP2B2C-lacZ expression was up-regulated in a small stripe of cells in the anterior compartment just at the boundary with vg depleted cells (Fig. 5D). This region was excluded from our quantifications, but suggests that the IAP2B2C-lacZ reporter fragment could be sensitive to a non-autonomous input acting around the boundary of cells with different Vg levels.
It appears therefore, that at least for the two Yki targets ex-lacZ and IAP2B2C-lacZ, Vg inhibits their expression in the wing pouch. Previous studies reported independent roles of Vg and Yki on the activation of their targets, and could appear to contradict this newly described inhibitory role of Vg on Yki targets. However, in these studies, the authors demonstrated that Vg and Yki do not require each other to promote wing pouch cell survival and to activate their respective targets (Pan Dev Cell 2008), which does not rule out any negative cross regulation, as shown in this report.
A complex network between Notch, Yki, and Vg
Our analysis brings therefore new evidence of the central role of Vg in the complex network regulating wing disc growth [43], adding a new level of complexity in its interaction with the SWH pathway effector Yki.
Thus, Notch induced expression of Vg could give rise to an Sd-Vg repressive complex that prevents expression of Yki targets [44], [45]. In situations where SWH signaling is lowest, Yki levels may be sufficiently high to overcome this repression. This suggests that in the wing pouch, Notch and SWH would act co-operatively rather than antagonistically.
Outside of the pouch, at the wing disc periphery, sd and vg expressions are not promoted by Notch activity. Furthermore, other binding partners for Yki, such as Homothorax are expressed there and might substitute for Sd to control the expression of Yki targets in a way similar to what has been described in the Drosophila eye [46]. The differential expression of these transcription factors in the disc could explain why Notch only has an inhibitory effect on Yki targets in the wing pouch. Furthermore, it is also worth noting that Notch has very different effect outside of the pouch, where it promotes Yki stabilization non-autonomously via its regulation of ligands for the Jak/Stat pathway [47].
In summary, our evidence demonstrates that Notch activity can inhibit Yki under circumstances where Yki acts together with Sd. It does so by promoting the expression of Vg, a co-factor for Sd, counteracting the effects of Yki. This cross talk potentially extends to mammalian systems as the active form of NOTCH1, NICD1 promotes the up-regulation of VGLL3 (a human homologue of vg) in MCF-10A breast cancer derived cells [48]. Thus, similar mechanisms may also be important in mediating interactions between the NOTCH and SWH pathways in human diseases.
Because the end-point of SWH pathway activity is to prevent Yki function, the inhibitory effects of Notch on Yki could provide an explanation for those cellular contexts where the two pathways act co-operatively, as at the D/V boundary in the wing discs. Similar co-operative effects have been noted in the Drosophila follicle cells. However, in this case it is the SWH activity that is involved in promoting the expression of Notch targets [49], [50]. In other contexts, such as the mouse intestine, accumulation of Yap1, the mouse Yki homolog, and therefore inhibition of the SWH promotes Notch activity [51], [52]. These examples demonstrate that the interactions between Notch and the SWH are highly dependent on cellular context. Our results suggest that some of these differences may be explained by the nature of the target genes that are regulated and by which Yki co-operating transcription factors are present in the receiving cells.
Materials and Methods
Fly stocks
NRE-GFP (86Fb; {GreenRabbit-ins}) [53], P{lacW}ex697 (gift from Nic Tapon), and IAP2B2C-lacZ (gift from Duojia Pan) [21] were used to monitor the activity of the Notch and SWH pathways respectively.
UASt Nicd[79.2], UASt Nicd[MH3], UASt yki (gift from Nic Tapon), UASt E(spl)mb, UASt E(spl)m5, UASt E(spl)m7, UASt E(spl)m8, UASt dpn (gift from Harald Vaessin), UASt cut (gift from Joel Silber), UASt vg (gift from Joel Silber) transgenes carrying flies were crossed to flies carrying the wing discs drivers patched[559.1]-Gal4, dpp[disc]-Gal4, or hh-Gal4 (gift from Nic Tapon) at 25C to over-express the corresponding gene products either in a stripe of cells at the anterior-posterior boundary (ptc-Gal4 and dpp-Gal4), or in the whole posterior compartment (hh-Gal4).
The ct, dpn, Notch, and vg gene products were knocked-down using flies carrying the following UAS RNAi transgenes, P{TRiP.HMS00924}attP2, P{KK101812}VIE-260B, P{UAS-N.dsRNA.P}14E, P{GD1558}v16896 respectively and crossed with the hh-Gal4 driver at 30C.
MARCM clones of cells null mutant for all bHLH coding E(spl) genes and with a strong RNAi against dpn were generated crossing the two following lines:
- hsFLP122, UASt GFPn, tub-Gal4;; FRT82B tub-Gal80
- P{KK101812}VIE-206B; FRT82B Df(3R)E(spl)[Grob32.2] [gro+]
E(spl)[Grob32.2] is a deficiency covering the whole E(spl) complex with a breakpoint close to groucho. The chromosome carries a groucho [gro+] construct which fully rescues groucho function [25]. Progeny larvae from this cross were heat-shocked for 1h at 37C to induce MARCM clones positively marked with GFP. The larvae were subsequently maintained at 30C to ensure the most efficient RNAi effect on dpn.
Immunofluorescence
Immunostainings were performed according to standard protocols. Antibodies used were: mouse anti-b-Galactosidase (Developmental Studies Hybridoma Bank - DSHB 40-1a; 1/25), rat anti-ECadherin (DSHB DCAD2; 1/25), rabbit anti-GFP (Molecular Probes A6455; 1/2000).
Images were acquired on a Leica SP2 confocal microscope and analysed using Adobe Photoshop or the FIJI ImageJ package.
Acknowledgments
We thank Duojia Pan, Joel Silber, Nic Tapon, and Harald Vaessin, for sharing flies. We acknowledge the Bloomington and Vienna stock centers, and the Developmental Studies Hybridoma Bank for flies and antibodies.
Funding Statement
This work was supported by a project grant from the Wellcome Trust to AD and SJB (WT083576MA). AD is supported by an ATIP-Avenir grant. SJB is supported by a MRC programme grant (G0800034). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rafel N, Milán M (2008) Notch signalling coordinates tissue growth and wing fate specification in Drosophila. Dev Camb Engl 135: 3995–4001 10.1242/dev.027789 [DOI] [PubMed] [Google Scholar]
- 2. Neumann CJ, Cohen SM (1996) A hierarchy of cross-regulation involving Notch, wingless, vestigial and cut organizes the dorsal/ventral axis of the Drosophila wing. Dev Camb Engl 122: 3477–3485. [DOI] [PubMed] [Google Scholar]
- 3. Go MJ, Eastman DS, Artavanis-Tsakonas S (1998) Cell proliferation control by Notch signaling in Drosophila development. Dev Camb Engl 125: 2031–2040. [DOI] [PubMed] [Google Scholar]
- 4. Baonza A, Garcia-Bellido A (2000) Notch signaling directly controls cell proliferation in the Drosophila wing disc. Proc Natl Acad Sci U S A 97: 2609–2614 10.1073/pnas.040576497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Djiane A, Krejci A, Bernard F, Fexova S, Millen K, et al. (2013) Dissecting the mechanisms of Notch induced hyperplasia. EMBO J 32: 60–71 10.1038/emboj.2012.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giraldez AJ, Cohen SM (2003) Wingless and Notch signaling provide cell survival cues and control cell proliferation during wing development. Dev Camb Engl 130: 6533–6543 10.1242/dev.00904 [DOI] [PubMed] [Google Scholar]
- 7. Herranz H, Pérez L, Martín FA, Milán M (2008) A Wingless and Notch double-repression mechanism regulates G1-S transition in the Drosophila wing. EMBO J 27: 1633–1645 10.1038/emboj.2008.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bray SJ (2006) Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7: 678–689 10.1038/nrm2009 [DOI] [PubMed] [Google Scholar]
- 9. Williams JA, Paddock SW, Vorwerk K, Carroll SB (1994) Organization of wing formation and induction of a wing-patterning gene at the dorsal/ventral compartment boundary. Nature 368: 299–305 10.1038/368299a0 [DOI] [PubMed] [Google Scholar]
- 10. Kim J, Sebring A, Esch JJ, Kraus ME, Vorwerk K, et al. (1996) Integration of positional signals and regulation of wing formation and identity by Drosophila vestigial gene. Nature 382: 133–138 10.1038/382133a0 [DOI] [PubMed] [Google Scholar]
- 11. Klein T, Arias AM (1999) The vestigial gene product provides a molecular context for the interpretation of signals during the development of the wing in Drosophila. Dev Camb Engl 126: 913–925. [DOI] [PubMed] [Google Scholar]
- 12. Nagel AC, Wech I, Preiss A (2001) Scalloped and strawberry notch are target genes of Notch signaling in the context of wing margin formation in Drosophila. Mech Dev 109: 241–251. [DOI] [PubMed] [Google Scholar]
- 13. Pan D (2010) The hippo signaling pathway in development and cancer. Dev Cell 19: 491–505 10.1016/j.devcel.2010.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao B, Tumaneng K, Guan K-L (2011) The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol 13: 877–883 10.1038/ncb2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harvey K, Tapon N (2007) The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nat Rev Cancer 7: 182–191 10.1038/nrc2070 [DOI] [PubMed] [Google Scholar]
- 16. Yu F-X, Guan K-L (2013) The Hippo pathway: regulators and regulations. Genes Dev 27: 355–371 10.1101/gad.210773.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, et al. (2006) The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol 8: 27–36 10.1038/ncb1339 [DOI] [PubMed] [Google Scholar]
- 18. Halder G, Polaczyk P, Kraus ME, Hudson A, Kim J, et al. (1998) The Vestigial and Scalloped proteins act together to directly regulate wing-specific gene expression in Drosophila. Genes Dev 12: 3900–3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simmonds AJ, Liu X, Soanes KH, Krause HM, Irvine KD, et al. (1998) Molecular interactions between Vestigial and Scalloped promote wing formation in Drosophila. Genes Dev 12: 3815–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goulev Y, Fauny JD, Gonzalez-Marti B, Flagiello D, Silber J, et al. (2008) SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Curr Biol CB 18: 435–441 10.1016/j.cub.2008.02.034 [DOI] [PubMed] [Google Scholar]
- 21. Wu S, Liu Y, Zheng Y, Dong J, Pan D (2008) The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell 14: 388–398 10.1016/j.devcel.2008.01.007 [DOI] [PubMed] [Google Scholar]
- 22. Zhang L, Ren F, Zhang Q, Chen Y, Wang B, et al. (2008) The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell 14: 377–387 10.1016/j.devcel.2008.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jennings B, Preiss A, Delidakis C, Bray S (1994) The Notch signalling pathway is required for Enhancer of split bHLH protein expression during neurogenesis in the Drosophila embryo. Dev Camb Engl 120: 3537–3548. [DOI] [PubMed] [Google Scholar]
- 24. de Celis JF, de Celis J, Ligoxygakis P, Preiss A, Delidakis C, et al. (1996) Functional relationships between Notch, Su(H) and the bHLH genes of the E(spl) complex: the E(spl) genes mediate only a subset of Notch activities during imaginal development. Dev Camb Engl 122: 2719–2728. [DOI] [PubMed] [Google Scholar]
- 25. Heitzler P, Bourouis M, Ruel L, Carteret C, Simpson P (1996) Genes of the Enhancer of split and achaete-scute complexes are required for a regulatory loop between Notch and Delta during lateral signalling in Drosophila. Dev Camb Engl 122: 161–171. [DOI] [PubMed] [Google Scholar]
- 26. Bailey AM, Posakony JW (1995) Suppressor of hairless directly activates transcription of enhancer of split complex genes in response to Notch receptor activity. Genes Dev 9: 2609–2622. [DOI] [PubMed] [Google Scholar]
- 27. Lecourtois M, Schweisguth F (1995) The neurogenic suppressor of hairless DNA-binding protein mediates the transcriptional activation of the enhancer of split complex genes triggered by Notch signaling. Genes Dev 9: 2598–2608. [DOI] [PubMed] [Google Scholar]
- 28. San Juan BP, Andrade-Zapata I, Baonza A (2012) The bHLH factors Dpn and members of the E(spl) complex mediate the function of Notch signalling regulating cell proliferation during wing disc development. Biol Open 1: 667–676 10.1242/bio.20121172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Babaoğlan AB, Housden BE, Furriols M, Bray SJ (2013) Deadpan Contributes to the Robustness of the Notch Response. PLoS ONE 8: e75632 10.1371/journal.pone.0075632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zacharioudaki E, Magadi SS, Delidakis C (2012) bHLH-O proteins are crucial for Drosophila neuroblast self-renewal and mediate Notch-induced overproliferation. Dev Camb Engl 139: 1258–1269 10.1242/dev.071779 [DOI] [PubMed] [Google Scholar]
- 31. Micchelli CA, Rulifson EJ, Blair SS (1997) The function and regulation of cut expression on the wing margin of Drosophila: Notch, Wingless and a dominant negative role for Delta and Serrate. Dev Camb Engl 124: 1485–1495. [DOI] [PubMed] [Google Scholar]
- 32. de Celis JF, Garcia-Bellido A, Bray SJ (1996) Activation and function of Notch at the dorsal-ventral boundary of the wing imaginal disc. Dev Camb Engl 122: 359–369. [DOI] [PubMed] [Google Scholar]
- 33.Guo T, Lu Y, Li P, Yin M-X, Lv D, et al.. (2013) A novel partner of Scalloped regulates Hippo signaling via antagonizing Scalloped-Yorkie activity. Cell Res. doi:10.1038/cr.2013.120. [DOI] [PMC free article] [PubMed]
- 34. Koontz LM, Liu-Chittenden Y, Yin F, Zheng Y, Yu J, et al. (2013) The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression. Dev Cell 25: 388–401 10.1016/j.devcel.2013.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zecca M, Struhl G (2010) A feed-forward circuit linking wingless, fat-dachsous signaling, and the warts-hippo pathway to Drosophila wing growth. PLoS Biol 8: e1000386 10.1371/journal.pbio.1000386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harvey KF, Pfleger CM, Hariharan IK (2003) The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114: 457–467. [DOI] [PubMed] [Google Scholar]
- 37. Huang J, Wu S, Barrera J, Matthews K, Pan D (2005) The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 122: 421–434 10.1016/j.cell.2005.06.007 [DOI] [PubMed] [Google Scholar]
- 38. Neto-Silva RM, de Beco S, Johnston LA (2010) Evidence for a growth-stabilizing regulatory feedback mechanism between Myc and Yorkie, the Drosophila homolog of Yap. Dev Cell 19: 507–520 10.1016/j.devcel.2010.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tapon N, Harvey KF, Bell DW, Wahrer DCR, Schiripo TA, et al. (2002) salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 110: 467–478. [DOI] [PubMed] [Google Scholar]
- 40. Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G (2003) Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol 5: 914–920 10.1038/ncb1050 [DOI] [PubMed] [Google Scholar]
- 41. Wu S, Huang J, Dong J, Pan D (2003) hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114: 445–456. [DOI] [PubMed] [Google Scholar]
- 42. Ziosi M, Baena-López LA, Grifoni D, Froldi F, Pession A, et al. (2010) dMyc functions downstream of Yorkie to promote the supercompetitive behavior of hippo pathway mutant cells. PLoS Genet 6: e1001140 10.1371/journal.pgen.1001140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baena-Lopez LA, Nojima H, Vincent J-P (2012) Integration of morphogen signalling within the growth regulatory network. Curr Opin Cell Biol 24: 166–172 10.1016/j.ceb.2011.12.010 [DOI] [PubMed] [Google Scholar]
- 44. Guss KA, Nelson CE, Hudson A, Kraus ME, Carroll SB (2001) Control of a genetic regulatory network by a selector gene. Science 292: 1164–1167 10.1126/science.1058312 [DOI] [PubMed] [Google Scholar]
- 45. Halder G, Carroll SB (2001) Binding of the Vestigial co-factor switches the DNA-target selectivity of the Scalloped selector protein. Dev Camb Engl 128: 3295–3305. [DOI] [PubMed] [Google Scholar]
- 46. Peng HW, Slattery M, Mann RS (2009) Transcription factor choice in the Hippo signaling pathway: homothorax and yorkie regulation of the microRNA bantam in the progenitor domain of the Drosophila eye imaginal disc. Genes Dev 23: 2307–2319 10.1101/gad.1820009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Graves HK, Woodfield SE, Yang C-C, Halder G, Bergmann A (2012) Notch signaling activates Yorkie non-cell autonomously in Drosophila. PloS One 7: e37615 10.1371/journal.pone.0037615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mazzone M, Selfors LM, Albeck J, Overholtzer M, Sale S, et al. (2010) Dose-dependent induction of distinct phenotypic responses to Notch pathway activation in mammary epithelial cells. Proc Natl Acad Sci U S A 107: 5012–5017 10.1073/pnas.1000896107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Polesello C, Tapon N (2007) Salvador-warts-hippo signaling promotes Drosophila posterior follicle cell maturation downstream of notch. Curr Biol CB 17: 1864–1870 10.1016/j.cub.2007.09.049 [DOI] [PubMed] [Google Scholar]
- 50. Meignin C, Alvarez-Garcia I, Davis I, Palacios IM (2007) The salvador-warts-hippo pathway is required for epithelial proliferation and axis specification in Drosophila. Curr Biol CB 17: 1871–1878 10.1016/j.cub.2007.09.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, et al. (2007) YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol CB 17: 2054–2060 10.1016/j.cub.2007.10.039 [DOI] [PubMed] [Google Scholar]
- 52. Zhou D, Zhang Y, Wu H, Barry E, Yin Y, et al. (2011) Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci U S A 108: E1312–1320 10.1073/pnas.1110428108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Housden BE, Millen K, Bray SJ (2012) Drosophila Reporter Vectors Compatible with ΦC31 Integrase Transgenesis Techniques and Their Use to Generate New Notch Reporter Fly Lines. Genes Genomes Genet 2: 79–82 10.1534/g3.111.001321 [DOI] [PMC free article] [PubMed] [Google Scholar]