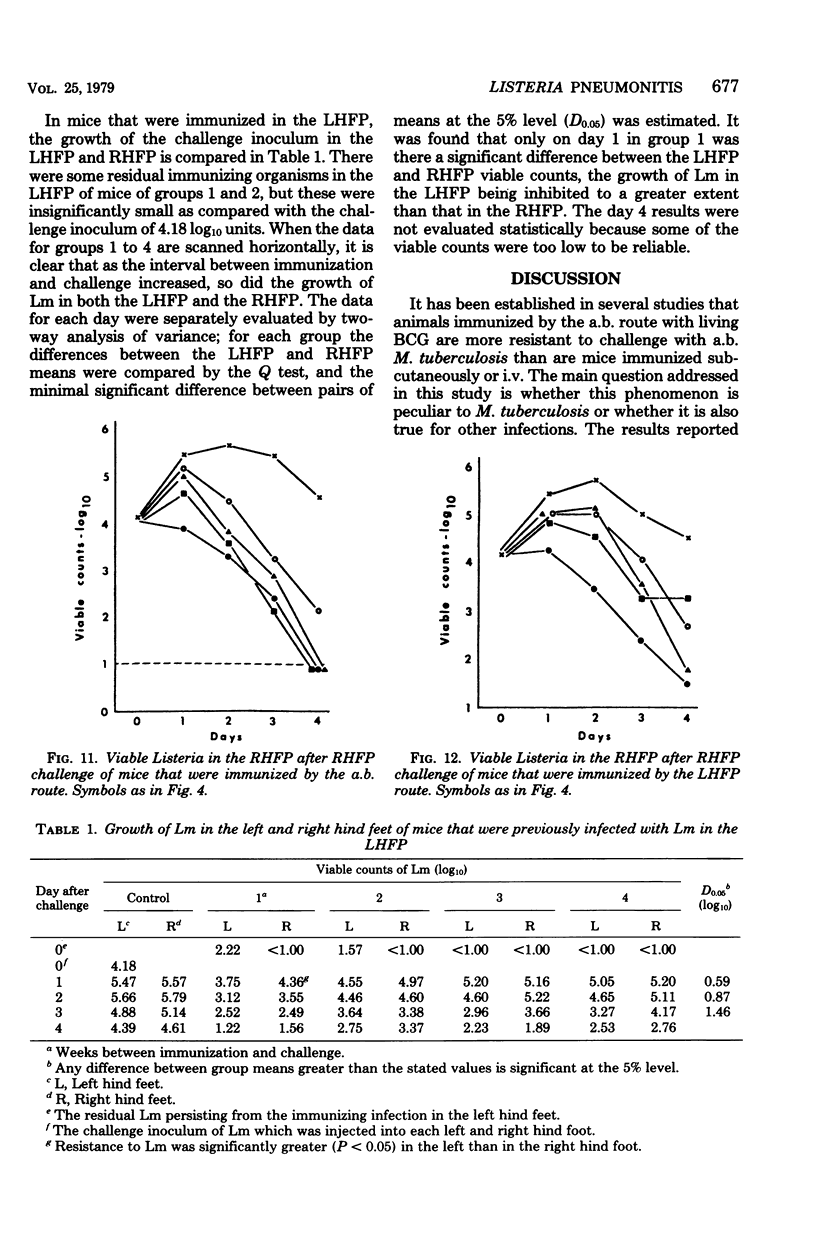
Abstract
Mice that are immunized with an airborne inoculum of BCG are more highly resistant to airborne challenge with Mycobacterium tuberculosis than are mice that are immunized by the subcutaneous or intravenous route. To discover whether this phenomenon is peculiar to tuberculosis, we studied the influence of the route of immunization upon pulmonary resistance in Listeria monocytogenes infection. Mice were immunized by the airborne, intravenous, or footpad route and were subsequently challenged by the same route at 1 to 4 weeks after immunization. Mice were highly and uniformly resistant to intravenous challenge, regardless of the route of immunization. The route of immunization bore no influence upon resistance to footpad infection, but resistance was appreciably better in mice challenged within 2 weeks of immunization than it was at later time points. In mice immunized by the footpad and intravenous routes, the pattern of resistance to airborne and footpad challenges was similar, in that there was substantially less immunity at 4 weeks than at 2 weeks after immunization. However, mice immunized by the airborne route were highly resistant to airborne challenge, regardless of the interval between immunization and reinfection. In this last respect, resistance of the lungs to reinfection was similar after Listeria and tuberculosis pneumonitis. It is suggested that a similar pattern of resistance may prevail in pneumonitis caused by other facultative intracellular parasites.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker R. L., Barclay W. R., Brehmer W., Goode G., List R. H., Ribi E., Tarmina D. F. Effectiveness of cell walls of Mycobacterium bovis strain BCG administered by various routes and in different adjuvants in protecting mice against airborne infection with Mycobacterium tuberculosis strain H37Rv. Am Rev Respir Dis. 1969 Feb;99(2):242–248. doi: 10.1164/arrd.1969.99.2.242. [DOI] [PubMed] [Google Scholar]
- Barclay W. R., Busey W. M., Dalgard D. W., Good R. C., Janicki B. W., Kasik J. E., Ribi E., Ulrich C. E., Wolinsky E. Protection of monkeys against airborne tuberculosis by aerosol vaccination with bacillus Calmette-Guerin. Am Rev Respir Dis. 1973 Mar;107(3):351–358. doi: 10.1164/arrd.1973.107.3.351. [DOI] [PubMed] [Google Scholar]
- GANGADHARAM P. R., COHN M. L., DAVIS C. L., MIDDLEBROOK G. Infectivity and pathogenicity of Indian and British strains of tubercle bacilli studied by aerogenic infection of guinea pigs. Am Rev Respir Dis. 1963 Feb;87:200–205. doi: 10.1164/arrd.1963.87.2.200. [DOI] [PubMed] [Google Scholar]
- Gray D. F., Cheers C. The steady state in cellular immunity. II. Immunological complaisance in murine pertussis. Aust J Exp Biol Med Sci. 1967 Aug;45(4):417–426. doi: 10.1038/icb.1967.40. [DOI] [PubMed] [Google Scholar]
- LARSON C. L., WICHT W. C. Studies of resistance to experimental tuberculosis in mice vaccinated with living attenuated tubercle bacilli and challenged with virulent organisms. Am Rev Respir Dis. 1962 Jun;85:833–846. doi: 10.1164/arrd.1962.85.6.833. [DOI] [PubMed] [Google Scholar]
- Lefford M. J., Amell L., Warner S. Listeria pneumonitis: induction of immunity after airborne infection with Listeria monocytogenes. Infect Immun. 1978 Dec;22(3):746–751. doi: 10.1128/iai.22.3.746-751.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. Induction and expression of immunity after BCG immunization. Infect Immun. 1977 Dec;18(3):646–653. doi: 10.1128/iai.18.3.646-653.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIDDLEBROOK G. Immunological aspects of airborne infection: reactions to inhaled antigens. Bacteriol Rev. 1961 Sep;25:331–346. doi: 10.1128/br.25.3.331-346.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Nature of "memory" in T-cell-mediated antibacterial immunity: anamnestic production of mediator T cells. Infect Immun. 1975 Oct;12(4):754–760. doi: 10.1128/iai.12.4.754-760.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. T cell dependence of macrophage activation and mobilization during infection with Mycobacterium tuberculosis. Infect Immun. 1974 Jul;10(1):66–71. doi: 10.1128/iai.10.1.66-71.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rácz P., Tenner-Rácz K., Myrvik Q. N., Fainter L. K. Functional architecture of bronchial associated lymphoid tissue and lymphoepithelium in pulmonary cell-mediated reactions in the rabbit. J Reticuloendothel Soc. 1977 Jul;22(1):59–83. [PubMed] [Google Scholar]
- Truitt G. L., Mackaness G. B. Cell-mediated resistance to aerogenic infection of the lung. Am Rev Respir Dis. 1971 Dec;104(6):829–843. doi: 10.1164/arrd.1971.104.6.829. [DOI] [PubMed] [Google Scholar]


