Abstract
In recent years, there has been a surge of interest in magnesium (Mg) and its alloys as biomaterials for orthopaedic applications, as they possess desirable mechanical properties, good biocompatibility, and biodegradability. Also shown to be osteoinductive, Mg-based materials could be particularly advantageous in functional tissue engineering to improve healing and serve as scaffolds for delivery of drugs, cells, and cytokines. In this paper, we will present two examples of Mg-based orthopaedic devices: an interference screw to accelerate ACL graft healing and a ring to aid in the healing of an injured ACL.
In vitro tests using a robotic/UFS testing system showed that both devices could restore function of the goat stifle joint. Under a 67-N anterior tibial load, both the ACL graft fixed with the Mg-based interference screw and the Mg-based ring-repaired ACL could restore anterior tibial translation (ATT) to within 2 mm and 5 mm, respectively, of the intact joint at 301, 601, and 901 of flexion. In-situ forces in the replacement graft and Mg-based ring-repaired ACL were also similar to those of the intact ACL. Further, early in vivo data using the Mg-based interference screw showed that after 12 weeks, it was non-toxic and the joint stability and graft function reached similar levels as published data. Following these positive results, we will move forward in incorporating bioactive molecules and ECM bioscaffolds to these Mg-based biomaterials to test their potential for functional tissue engineering of musculoskeletal and other tissues.
Keywords: Magnesium, Mg alloys, Biomaterials, Orthopaedic devices, Functional tissue engineering
1. Introduction
Biomaterials are used in orthopaedic surgery for bone substitutes, fixation and stabilization of fractured bones, ligament and tendon reconstruction procedures, total joint arthroplasties, and so on. Historically, non-degradable metals, namely stainless steel and titanium alloys, have been commonly used because they possess good mechanical strength, biocompatibility, and corrosion resistance (Geetha et al., 2009). However, with the advent of functional tissue engineering, bioresorbable materials have gained much attention and their usage has increased, as they could be replaced by the patient’s own tissue as well as be used for delivery of bioactive molecules to improve healing of various hard and soft tissues. These biomaterials are chosen based on the needed properties for the desired application, including specific mechanical properties, porosity, degradation profiles, biocompatibility, and adherence and incorporation into adjacent tissue (Butler et al., 2000).
Depending on the tissue to regenerate, there are now many options for biomaterials in orthopaedics (Navarro et al., 2008). For the repair or replacement of ligaments and tendons, fibrous collagen, extracellular matrix, silk, and synthetic polymer scaffolds have been explored (Ge et al., 2006). In addition, hydrogels have become an attractive option to fill irregularly-shaped voids and aid in the delivery of cells, growth factors, and other bioactive molecules (Drury and Mooney, 2003; Woo et al., 2011). For regeneration of the soft tissue-to-bone interface (e.g. ACL insertion to the femur), polymers have become a popular choice as they can be engineered to possess multi-phasic properties (Lu and Thomopoulos, 2013).
However, polymer materials may not be a suitable choice for some orthopaedic applications. When poly-L-lactic acid (PLLA) interference screws were used for graft fixation during ACL reconstruction, the devices often fractured during implantation due to their brittleness (Smith et al., 2003). Further, their rate of degradation varied greatly between patients and in most cases was very slow, with the screw remaining mostly intact even after 2 years (Johnston et al., 2011). Also, after degradation, the void left was not filled with regenerating bone. A 10-year follow-up study showed that an osseous cyst had formed after complete degradation of an interference screw (Walton and Cotton, 2007). Recently developed composite materials such as PLLA with tri-calcium phosphate (TCP) particles are designed to address the inadequate osteointegration, but results have been mixed as some studies reported improved results (Johnston et al., 2011) while others reported poor integration (Tecklenburg et al., 2006).
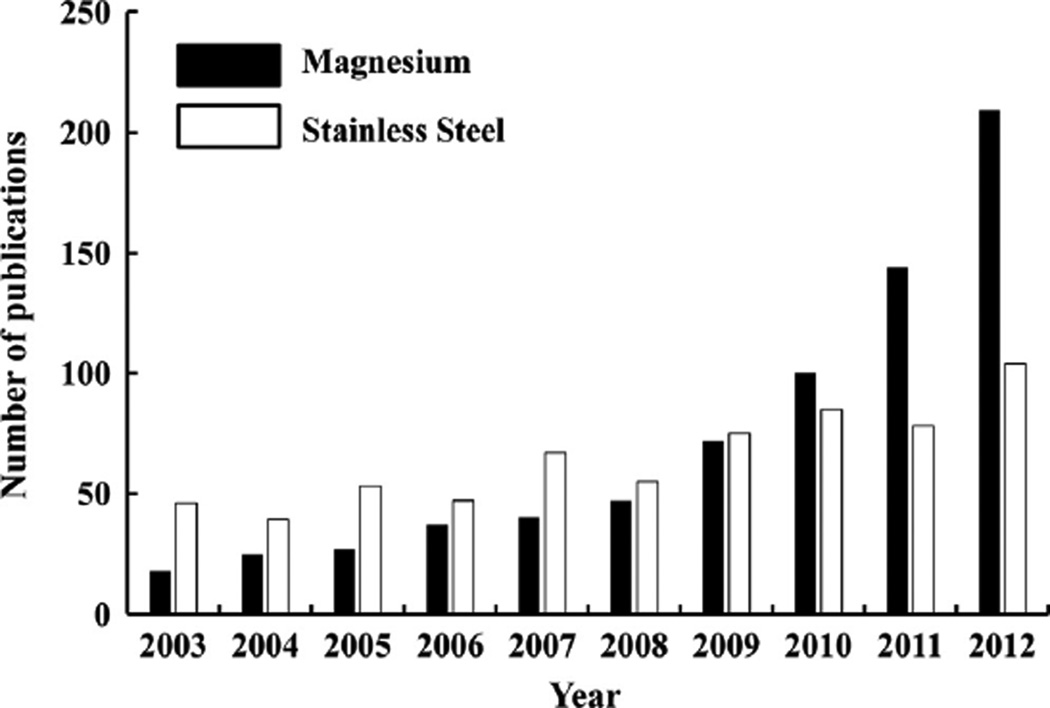
In this paper, we will present the development of a new class of biodegradable metallic materials for orthopaedic applications; namely, magnesium (Mg) and its alloys. These alloys have the mechanical properties required to meet loading requirements during and after implantation, but would not interfere with common imaging modalities for post-op care, such as MRI and CT. They could also be designed to degrade at a desired rate, thus allowing for regeneration of the surrounding soft or hard tissues. This controllable degradation is ideal for functional tissue engineering, as they could be designed to allow controlled drug release over time to enhance healing. The microstructure of Mg alloys could also be designed to be porous, which would allow for cellular infiltration and improve resorption and replacement by new bone. These aforementioned advantageous and desirable properties of Mg-based materials have led to increased interest in these materials for biomedical applications. In fact, a search on Web of Science using “magnesium” and “stainless steel” as search queries under the category “biomaterials” revealed that the number of publications on magnesium has exponentially increased in the past ten years compared to those on stainless steel, which only saw a modest increase (Fig. 1).
Fig. 1.
Histogram showing number of publications each year for the past ten years on research of magnesium and its alloys and stainless steel as biomaterials (retrieved from Web of Science using “magnesium” and “stainless steel” as a search query within the category “biomaterials”).
To provide the readers with a proper perspective, we will include a brief review of the advantageous properties of Mg, followed by the history of Mg in orthopaedic applications and the complications that initially limited its use. Then, we will discuss new and recent developments, including new alloys, surface treatments, and coatings, which have helped to overcome these complications. Examples of these new Mg-derived materials will be given together with the in vitro and in vivo testing methods used to evaluate them. Finally, we will explore the exciting promises of Mg alloys in orthopaedics, as well as new possibilities for its use in functional tissue engineering of ligaments and tendons, bone, and the soft tissue-to-bone interface.
2. Bioresorbable Mg and Mg alloys
Mg-based materials have significantly lower moduli than titanium-based materials (41–45 GPa vs. 110–117 GPa) (Hort et al., 2010). As a result, their mechanical properties are closer to those of cortical bone and could reduce the level of stress shielding. In terms of tensile strength, Mg-based materials are 3–16 times stronger than polymers (160–250 MPa vs. 16–69 MPa). They are also more ductile and have a higher ultimate strain that reaches up to 16%, which could reduce the risk of device fracture during implantation.
Mg-based materials can be engineered to degrade within a desired period of time. Recent studies demonstrated that various alloying elements (Zberg et al., 2009) and surface coating techniques (Liao et al., 2013) could control the degradation rate without significantly affecting the initial mechanical properties. By varying its Zn content, Zberg et al. could modulate the degradation rate of MgZnCa alloys while maintaining strength and modulus. Liao et al. showed that a surface treatment with phosphate on AZ31 alloy could control the degradation rates without affecting its mechanical properties. In addition to controlled degradation, Mg-based materials also do not significantly interfere with MRI compared to other metallic materials, thus allowing for accurate assessment of the device function and surgical outcome to be made during the post-operative periods.
Most importantly, Mg-based materials have been shown to be biocompatible in vivo, leading to good host response (Witte et al., 2005; Zhang et al., 2009). Further, they could promote bone formation compared to polymers, which helped the material to integrate well with the surrounding bone as well as to potentially allow full regeneration after degradation. These unique properties of Mg-based materials could overcome some of the disadvantages associated with the metallic and polymer materials.
3. History of Mg in orthopaedics
The initial use of biodegradable Mg implants in orthopaedics spanned the first half of the twentieth century (Witte, 2010). In 1900, the Austrian–German physician Erwin Payr introduced the use of Mg for joint arthroplastics to regain or preserve joint motion, for fracture fixation with wire (clerclage) and pegs as intramedullary rods (Witte, 2010). Inspired by Payr, Chlumsky also investigated the use of Mg sheets and successfully restored joint motion after bony separation of ankylotic joints in animals and humans, but rapid degradation of the sheets was noted.
A few years later, Lambotte from France investigated the use of Mg implants in children who had suffered from pseudoarthrosis, supracondylar fractures and transdiaphyseal humerus fractures and reported that all of the fractures healed without complications, except the formation of gas cavities around the implants (Witte, 2010). A number of other investigators also reported the use of Mg implants in animals and humans and found that they stimulated soft tissue production and promoted new bone growth while being completely resorbed over time (McBride, 1938; Zierold, 1924).
However, the most common complication was the rapid degradation of the Mg implants coupled with hydrogen gas formation. Even though the gas evolution was reported as not harmful to the surrounding tissue and did not cause any adverse clinical effect, such as infection, the inability to control the rate of degradation in vivo led to the abandonment of the use of Mg (Witte, 2010; Zierold, 1924). Surgeons had then gone on to use more corrosion-resistant metals such as stainless steel. For more details on the history of magnesium implants for orthopaedic applications, interested readers are referred to the review article by Witte published in 2010.
4. Recent advancement in Mg and its alloys
To combat the aforementioned challenges involving the use of Mg, new methods for alloying, coating, surface treatment, and processing have been developed. Metals like zinc (Zn), aluminum (Al), silver (Ag), yttrium (Y), zirconium (Zr), neodymium (Nd), and manganese (Mn) have been alloyed with Mg to achieve improved mechanical properties. An example would be Mg–Y alloys, which had increased mechanical properties compared to pure Mg (twofold increase in tensile strength) while maintaining the degradation rate (Chou et al., 2013). In addition, the microstructure of the Mg material could be designed to be porous in order to have its mechanical properties similar to those of cancellous bone, thus making it ideal to be used as a bone substitute (Wei et al., 2010).
Novel coatings and surface treatments can be applied to control the degradation of Mg and its alloys. A calcium phosphate (Ca-P) coating can be achieved through relatively simple chemical treatment and has been shown to slow down degradation of the AZ31 alloy by 2 orders of magnitude (Ishizaki et al., 2009). Further, polymers such as PLGA have been used to control degradation of Mg-based alloys, although challenges of durability still remain (Ostrowski et al., 2013). Physical vapor deposition of high-purity Mg, hydrofluoric acid treatment, and alkaline-heat treatment have also been shown to be effective to a varying degree (Gu et al., 2009; Salunke et al., 2011).
A number of experiments have been performed to screen for cytocompatibility and to assess degradation of these new materials. By culturing U2OS (osteoblast) cells with or without a Mg (99.95% purity) sample, it was found that there were no differences in cell viability or proliferation, nor mineralization (Yun et al., 2009). Further, a Mg-based alloy (AZ21; Al 2 wt% and Zn 1 wt %) could support stromal cells and promote their differentiation toward osteoblast-like phenotypes in vitro (Pietak et al., 2008).
Using a scaffold with a biomemetric, porous microstructure also proved to be positive for cellular activity, as MG63 cells seeded on a microporous/macroporous Mg–Ca-P scaffold showed significantly better cell proliferation and attachment as well as osteoblast differentiation than those seeded on a Ca-P cement scaffold (Wei et al., 2010). This was likely due to Mg’s role in bone mineralization and deposition and the continuous release on Ca and Mg ions from the scaffold. The fact that cells also migrated deep into the microporous scaffold could also be an advantageous feature for functional tissue engineering, as it could eliminate necrosis in the center of tissue-engineered constructs due to lack of nutrient and waste exchange.
Incorporating coatings to these alloys has been shown not only to slow degradation, but also elicit a beneficial effect on cells. When mouse fibrosarcoma cells (L929) were cultured on a Ca-P-coated Mg–Mn–Zn alloy, significant improvements in cell proliferation and biocompatibility were found (Xu et al., 2009). A layer-by-layer approach to coating PLLA and alginate could create a functionalized surface to allow chemical-crosslinking of proteins, such as fibronectin, to the surface (Kunjukunju et al., 2013). This approach allowed for vastly improved cell attachment (ten-fold increase in live cells compared to a bare Mg alloy), which was comparable to a titanium alloy (positive control). These in vitro experiments all demonstrated that Mg and its alloys have good degradation profiles and no cytotoxic effects.
Following in vitro experiments, live animal studies have also been conducted to evaluate the in vivo cytocompatibility and degradation of magnesium and its alloys for orthopaedic applications. These studies have demonstrated that Mg-based materials are cytocompatible and more importantly osteoinductive, with appropriate degradation profiles such that the scaffold is replaced over time by new bone tissue.
In a comparative study, 4 alloys, AZ31 (aluminum 3 wt% and zinc 1 wt%), AZ91 (Al 9 wt% and Zn 1 wt%), WE43 (Y 4 wt%, other rare earth metals 3 wt%, and Zr 0.4 wt%), and LAE442 (Li 4 wt%, Al 4 wt%, and rare earth metals 2 wt%) were implanted in the femora of guinea pigs for 6 and 18 weeks (Witte et al., 2005). The degradable polymer, poly-96L/4D–lactide was used as a control. In the Mg alloy groups, there were statistically significant increases in bone mass and mineral apposition rate at both 6 and 18 weeks, as well as more trabecular-like structures compared to the control. Thus, the osteoinductive capacity of Mg-based materials could be demonstrated.
Investigators have also alloyed or coated Mg with elements naturally found in the human body, e.g. calcium (Ca), zinc (Zn), manganese (Mn), fluorine (F), etc. to test their in vivo biocompatibility and osteoinductivity. Zhang et al. tested a Mg–Zn–Mn alloy (1 wt% Zn and 0.8 wt% Mn) in the femora of Sprague-Dawley rats and observed significant degradation (~50%) at 26weeks together with newly formed bone on the degrading surface without fibrous tissue (Zhang et al., 2009). In another study, a Mg–Ca alloy (0.8 wt % Ca) was coated with magnesium fluoride (MgF2) and implanted in the femora of New Zealand white rabbits (Thomann et al., 2010). Although the presence of the coating was not found to significantly affect the corrosion resistance of the implants, by 3 and 6 months, there was significant endosteal and periosteal bone growth on both coated and uncoated implants, with bone trabecule tightly adhered to the implant surfaces.
Modifying the porosity of the Mg scaffold was also explored in vivo. When a microporous/macroporous Mg–Ca-P scaffold was implanted into femoral bone defects in rabbits, new bone tissue had formed into the pores of the scaffold by 2 months of healing, and after 6 months, the majority of the scaffold had degraded. In fact, 81% of the bone defect had been replaced by new bone tissue by 6 months, compared with just 47% in a microporous/macroporous calcium phosphate cement (CPC) control. Additionally, numerous osteoblasts were visible in a newly-formed osteoid matrix, and the interface between the scaffold and bone was no longer visible (Wei et al., 2010). Thus, the combination of an Mg alloy with a porous structure may be advantageous as a biomimetric approach to functional tissue engineering of bone.
These in vivo studies clearly demonstrated that Mg-based materials could promote bone formation and appropriate degradation rates for replacement by new bone tissue, without cytotoxic effects. With these findings, biological agents should also be incorporated into these materials to further enhance healing. In addition to exploring these new materials for bone healing, the possibilities of using Mg for functional tissue engineering of ligaments and tendons, as well as their entheses can also be explored. This possibility is encouraged by recent findings showing that Mg may have beneficial effects on fibroblast adhesion to surfaces (Okawachi et al., 2012), as well as viability and cellular activity (Cao et al., 2013).
5. Examples of Mg-based devices for orthopaedic applications
To illustrate how that Mg devices could enhance musculoskeletal tissue regeneration, we have conducted two studies, the first being an interference screw for fixation of tissue autografts during anterior cruciate ligament (ACL) reconstruction. The choice of Mg-based interference screws was made because it would allow the effect of Mg on healing and remodeling of the hard and soft tissue interface to be tested. As Mg has been shown to promote bone regeneration, the use of Mg-based interference screws may have potential benefits in healing of the graft in the tunnel. The second study involves the application of a novel ring device for augmentation of ACL healing, as Mg’s good mechanical properties, controllable degradation, and positive effects on fibroblasts could lead to enhanced ACL healing.
The results obtained from these studies will serve as platforms for further investigations on how to incorporate Mg in tissue engineering strategies, such as drug, cell and cytokine delivery or combination with extracellular matrix bioscaffolds as well as to guide the potential use of Mg in regeneration of other tissues.
5.1. Study 1: Mg-based interference screw for ACL reconstruction
There are well over 100,000 cases of ACL tears annually in the United States (Beaty, 1999) and two thirds of these patients would immediately or eventually need surgical reconstruction to stabilize their knees to prevent further damage to other soft tissues that could lead to osteoarthritis. A patellar tendon or hamstring tendon autograft or an allograft is used to replace the injured ACL, and is often fixed within the femoral and tibial bone tunnels by means of interference screws.
Currently, interference screws are made of either a metallic material (usually a Ti-based alloy), or a polymer such as PLLA. While successful in restoring ACL function initially, these materials do not allow for complete regeneration of the surrounding bone (Walton and Cotton, 2007). Hence, our research center has chosen an alternative approach by developing interference screws made of Mg-based alloys to harness its biodegradability, biocompatibility and osteoinductive capacity. To do this, we first performed finite element analysis (FEA) to optimize the screw design (Flowers, 2012). We then performed ACL reconstruction on the goat stifle joint and fixed the autograft with a Mg-based interference screw. Both in vitro and then in vivo studies were conducted and the outcome in terms of graft function and joint stability were evaluated.
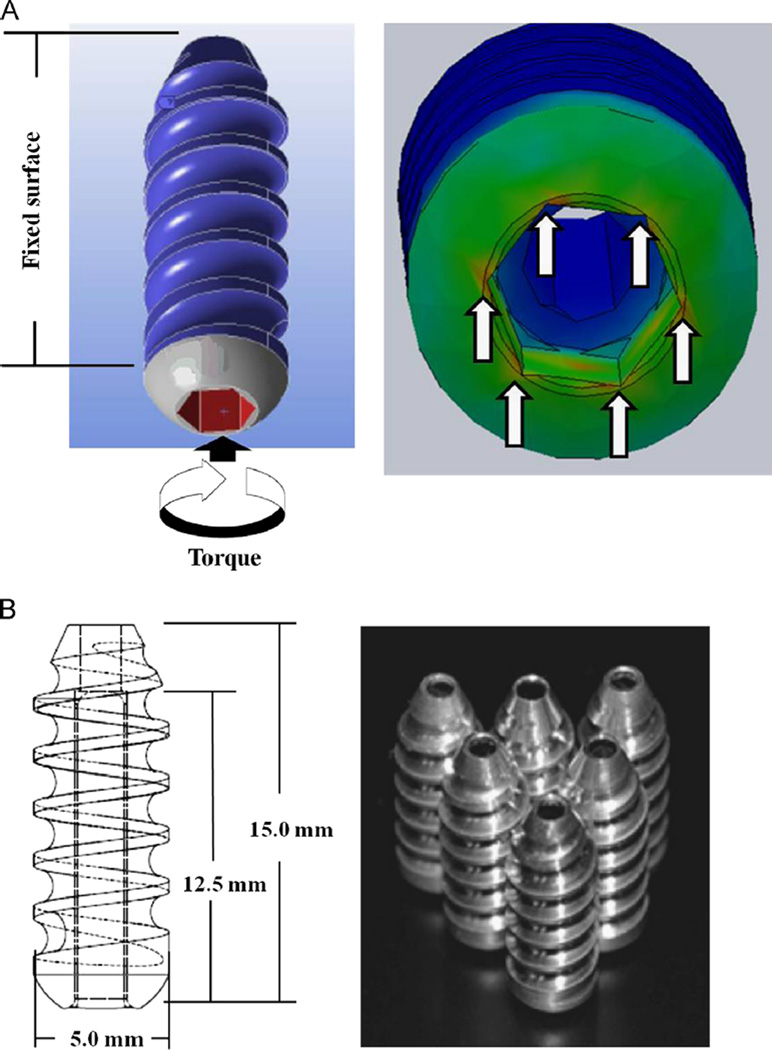
The initial design of the screw was based on that of a commercially available titanium alloy screw (Flowers, 2012). For the FEA, a CAD model of the interference screw was created and imported into FEA software (ANSYS, Canonsburg, PA) (Fig. 2A). The model was then validated using a previously published equation (Chapman et al., 1996) that determines the pull-out strength of a screw based on its thread design (pitch, depth, ultimate shear stress, and length). The pull-out force determined from the finite element model was matched to that determined by the equation for validation. Upon applying loading conditions simulating insertion torque, it was first observed that there were stress concentrations in the corners of the drive that would lead to stripping of the screw while being inserted. Through parametric optimization, we thus modified the design for the drive of the interference screw by increasing its width and drive depth to reduce stress levels at these corners.
Fig. 2.
(A) Finite element model of the first-generation Mg-based interference screw and (B) design and manufacturing of the new design.
The material used for the interference screw was an AZ31 alloy because it is stronger and more corrosion-resistant than pure Mg. The AZ31 Mg-based interference screws were machined and used to fix a bone-patellar tendon-bone (BPTB) graft in the femoral tunnel of 6 cadaveric stifle joints from skeletally mature goats (Fig. 2B) (Kim et al., 2013).
The kinematics and the graft function of the stifle joints were evaluated by the robotic/ universal force-moment sensor (UFS) testing system developed at our research center (Fox et al., 1998; Woo et al., 1999). This testing system is able to evaluate the function of diarthrodial joints in multiple DOF of motion and is an improvement over earlier linkage and other systems that could not repeat the bone positions and the path of motion accurately (Hollis et al., 1991; Smith et al., 1993). The robotic manipulator is capable of recording and reproducing motion within 0.1 mm in translation and 0.11 in rotation with six degree-of-freedom (DOF) motion. The UFS can record forces and moments along the three axes of a Cartesian coordinate system with a repeatability of 0.5 N for force and 0.05 N-m for torque. The system has been successfully used to measure joint translations and rotations in response to externally applied loads and in situ forces in ligaments and other soft tissues in and around the joint (Livesay et al., 1995; Woo et al., 2002).
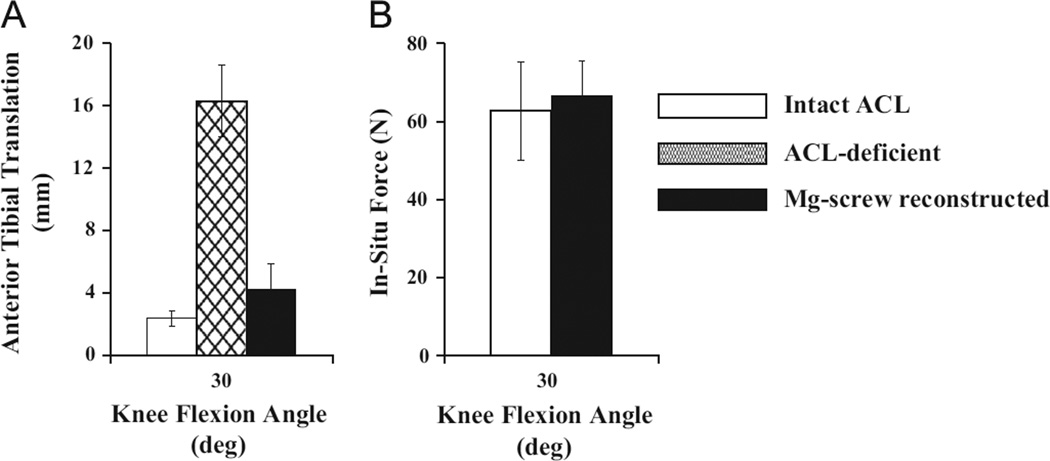
A 67-N anterior tibial load (ATL) was applied to the goat stifle joint at 30°, 60°, and 90° of joint flexion in three states: intact, ACL-deficient, and reconstructed (Papageorgiou et al., 2001; Woo et al., 2002). In the intact state, the anterior tibial translation (ATT) ranged between 1.9 mm and 2.4 mm at these three flexion angles (Fig. 3A). After transection of the ACL (ACL-deficient state), the ATT increased by more than 10 mm (between 13 mm and 16 mm). ACL reconstruction with the Mg-based interference screw fixation restored the ATT back to within 2 mm of the intact state. In terms of the in-situ forces in the graft, they were within 5 N of those in the intact ACL at all three flexion angles (51–67 N for the graft vs. 50–63 N for the ACL; Fig. 3B). These findings clearly showed that ACL reconstruction with the Mg-based interference screw fixation could restore the stifle joint stability and graft function (in-situ forces) following ACL reconstruction.
Fig. 3.
(A) Anterior tibial translation (ATT) in intact, ACL-deficient, and reconstructed goat stifle joints and (B) in-situ forces in intact ACLs and replacement grafts.
The structural properties of the graft were obtained by performing uniaxial tensile testing of the femur–graft–tibia complex (FGTC) after reconstruction. After removing all of the soft tissues around the joint, leaving just the femur, graft, and tibia, the FGTC specimen was mounted on a materials testing machine (Instron 4502, Norwood, MA) and loaded to failure. The resulting load– elongation curve was recorded and the stiffness and ultimate load were obtained (52±6 N/mm and 400±135 N, respectively) (Kim et al., 2013). These values were comparable to previous data from our research center using a titanium interference screw on a similar animal model (Musahl et al., 2003) and support the use of the Mg-based interference screw for fixation of the replacement graft in ACL reconstruction.
With these positive in vitro results, we demonstrated feasibility of the use of the Mg-based interference screw for fixation of the replacement graft in ACL reconstruction at time zero. We then proceeded to an in vivo study of ACL reconstruction on stifle joints of skeletally mature goats and evaluated them at 12 weeks of healing. Preliminary data (n=3) has shown the values for ATT were between 9 and 14 mm, and the in-situ forces in the healing BPTB autograft were between 20 and 40 N. These values were again comparable to previous results obtained using a similar animal model (Abramowitch et al., 2003).
After demonstrating its ability to restore the tissue function as well as osteoinductive capacity, we will incorporate delivery vehicles for drug, cells, or cytokines to the Mg-based interference screw to improve the healing and remodeling of the tunnel. One of the readily available methods is coating the surface of magnesium with a polymer. These polymer coatings on the Mg surface could also be functionalized to attach bioactive molecules to further enhance the healing response (Kunjukunju et al., 2013).
5.2. Study 2: a Mg-based ring for healing of an injured ACL
With the recent advances in functional tissue engineering, biological augmentation using stem cells (Agung et al., 2006), platelet-rich plasma (Murray et al., 2007), or ECM bioscaffolds (Fisher et al., 2012; Nguyen et al., 2013) has been successfully used to enhance the healing of a surgically transected ACL. However, these studies also found that the rate of healing was slow and mechanical augmentation would be required to maintain knee stability and facilitate the biological processes of ACL healing (Speziali et al., 2012). To this end, it was found that using sutures that directly connected the tibia and femur following ACL injury could restore knee stability and led to improvements in the healing of a transected ACL (Fisher et al., 2011; Fleming et al., 2008).
We have designed a novel Mg-based ring to bridge the gap between the two torn ends of the ACL in order to serve as an internal splint as an alternative for mechanical augmentation. The ring would degrade as the ACL healing progresses and bears more of the load. It is intended to be used alongside cells, cytokines, or bioscaffolds while releasing these biological agents on a timely basis in order to accelerate healing and remodeling.
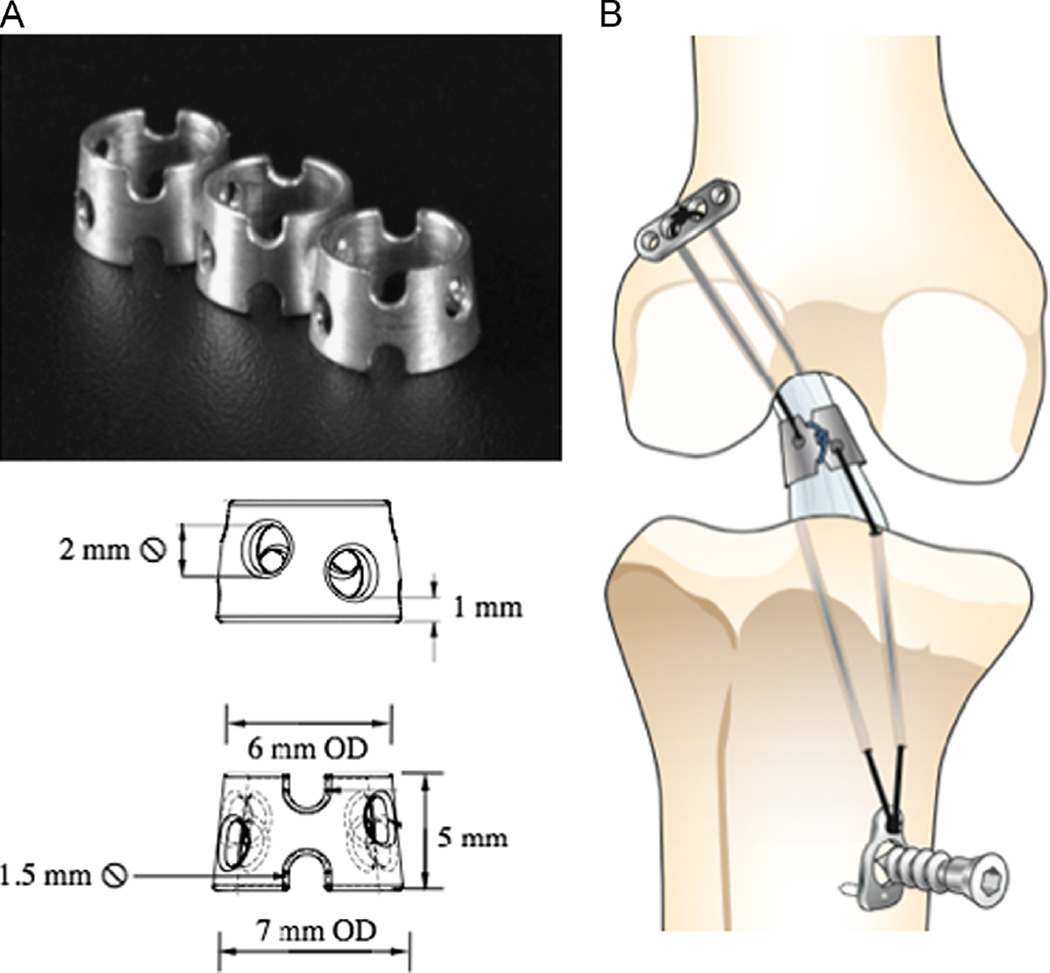
The ring was designed for the goat ACL based on the dimensions and anatomy of the midsubstance of the ACL, and was machined from an AZ31 alloy (Fig. 4A). A novel surgical implantation technique was also developed. It involved first drilling two sets of 1.5 mm diameter bone tunnels through the femur and tibia, passing anterior to the ACL’s femoral insertion and medial and lateral to its tibial insertion. Then, fixation sutures (Ethibond #2, Ethicon, Inc.) and repair sutures (PDS II #0, Ethicon, Inc.) were passed through each of the transected ends of the ACL. The fixation sutures were passed through the ring and opposite bone tunnels and fixed under tension, while the repair sutures were tied around the notches of the ring (Fig. 4B).
Fig. 4. AAA.
(A) Photograph and schematic of the Mg-based ring and (B) a schematic diagram of Mg-based ring repair of the ACL.
The function of the Mg-based ring-repaired ACL was evaluated in vitro using cadaveric goat stifle joints (n=8) (Farraro et al., 2013). Each joint was mounted on the robotic/UFS testing system as described previously in three joint states: (1) intact, (2) ACL-deficient, and following (3) ACL repair with the Mg-based ring. ATT and in-situ force in the ACL were measured for each state.
The ATT of the intact joint was measured to be 2.5±0.6, 2.7±0.9, and 1.8±1.0 mm at 30°, 60°, and 901 of knee flexion, respectively. After the ACL was transected, it increased to 15.2±2.3, 15.8±1.7, and 12.4±1.6 mm. With the application of the Mg-based ring, the ATT of the ACL-repaired stifle joint was reduced by 60–70% from the ACL-deficient state, and was within approximately 3 mm of that of the intact joint (5.0±1.0, 5.8±1.0, and 4.3±1.3 mm, respectively; P < 0.05). In terms of in-situ forces in the ACL, Mg-based ring repair was able to restore forces to levels of those of the intact ACL at all three flexion angles (60±8, 57±4, and 49±9 N vs. 61±7, 62±7, and 54±7 N; P > 0.05).
These data demonstrated that the use of the Mg-based ring could restore joint function and provide the needed mechanical augmentation in the early stages of ACL healing. The ring could also load the repaired ACL during the healing process and therefore reduce the disuse atrophy of its insertion sites. Over time, the Mg-based ring would be resorbed, leaving only the healing ACL to remodel and strengthen.
In the future, this novel Mg-based ring will be used in combination with biological scaffolds in an in vivo animal study to examine the synergistic biological and mechanical approach (Fisher et al., 2012). It is hypothesized that with sufficient initial joint stability, the healing response via bioactive molecules could be enhanced and accelerated, resulting in improved ACL healing. We believe this combined functional tissue engineering approach, if successful, will lead to a paradigm shift on treatment for ligaments and other soft tissues following their injury.
6. Future developments
Thus far, our group and others have shown very encouraging results using new Mg-based alloys to make devices for orthopaedic applications (Farraro et al., 2013; Kim et al., 2013; Qin, 2013; Windhagen et al., 2013). In vitro studies have shown that when an Mg-based interference screw was used for graft fixation during ACL reconstruction, the stifle joint stability and graft function could be restored to the levels of a Ti screw (Kim et al., 2013). Also, an Mg-based ring-repaired ACL could bridge the gap between the two ends of an injured ACL to restore joint stability and function to near-normal levels (Farraro et al., 2013).
These encouraging results are needed to form the basis for in vivo animal studies in order to evaluate their efficacy as well as to learn more about their degradation and resorption processes. If these devices could restore the normal tissue function, especially at the enthesis, one can then design the Mg-based interferences screw as a delivery vehicle for drugs, cells, or cytokines so that the graft healing and remodeling at the tunnel could further be improved. Meanwhile, it would be attractive to employ bioscaffolds and hydrogels together with the Mg-based ring to accelerate the ACL healing while maintaining the integrity of its bony insertions.
The success of the Mg based interference screw has also led us to develop other devices, such as suture anchors for rotator cuff and acetabular labrum repair. As many as 300,000 surgeries to repair rotator cuff tears were performed annually in the US, and in each case, multiple suture anchors have been used (Colvin et al., 2012). Additionally, arthroscopic repair of torn acetabular labrum is on the rise and suture anchors are used to re-approximate the torn tissue. The majority of suture anchors for these applications are currently made of polymers, and it has been shown that these devices may cause osteolysis leading to osseous cyst formation (Dhawan et al., 2012). Excellent biocompatibility and osteoinductivity of Mg and its alloys may allow better bone healing. To develop these devices, the knowledge gained from the Mg-based interference screw will serve to facilitate the design of novel Mg-based suture anchors for in vitro and in vivo tests on animals.
Nevertheless, there are still a number of challenges that remain prior to widespread clinical use. Most notably is how to control the corrosion rates, which are highly dependent on geometry, composition, and location of the implant. To control degradation, we need to develop new alloys and manufacturing processes for devices to be applied for a wide range of clinical purposes. Along these lines, single crystal Mg (Shin et al., 2013) and new technologies of surface coatings using polymers (Tian and Liu, 2013) are on the horizon that would offer exciting possibilities. These new materials and surface coatings could be used to tailor the degradation to match healing such that the device can be replaced over time by new tissue.
Finally, an important challenge will be how this new class of devices will be able to fulfill the regulatory requirements, as the guidelines for FDA approval have not yet been fully established.
In conclusion, Mg and Mg-based alloys are very promising biomaterials for orthopaedics applications, because they combine the positive features of traditional, non-degradable metallic and degradable polymer materials that are used today. Their desirable mechanical properties, biocompatibility, and biodegradability are attractive features to be selected for implants. The possibilities of incorporating tissue engineering strategies, such as the addition of biological agents such as cytokines, growth factors, or stem cells to Mg-based devices for drug delivery could lead to new treatment options and improved outcomes for patients. Even though there is much work to be done, it is believed that Mg and its alloys are a new class of biomaterials that are poised to play a role in revolutionizing orthopaedic as well as other medical devices.
Acknowledgments
The financial support by the NSF Engineering Research Center (Grant #0812348) and the National Institute of Health training
programs: Biomechanics in Regenerative Medicine (BiRM, T32 EB000392), and the Cellular Approaches to Tissue Engineering and Regeneration (CATER, T32 EB001026) are gratefully acknowledged.
Footnotes
Conflict of interest statement
All authors confirm they have no financial or other conflicts of interest relevant to this manuscript.
References
- Abramowitch SD, Papageorgiou CD, Withrow JD, Gilbert TW, Woo SL-Y. The effect of initial graft tension on the biomechanical properties of a healing ACL replacement graft: a study in goats. J. Orthop. Res. 2003;21:708–715. doi: 10.1016/S0736-0266(02)00265-6. [DOI] [PubMed] [Google Scholar]
- Agung M, Ochi M, Yanada S, Adachi N, Izuta Y, Yamasaki T, Toda K. Mobilization of bone marrow-derived mesenchymal stem cells into the injured tissues after intraarticular injection and their contribution to tissue regeneration. Knee Surg., Sports Traumatol., Arthrosc. 2006;14:1307–1314. doi: 10.1007/s00167-006-0124-8. [DOI] [PubMed] [Google Scholar]
- Beaty JH. Knee and leg: soft tissue trauma. In: EA A, editor. OKU orthopaedic knowledge update. first ed. Vol. 442. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1999. p. xix. [Google Scholar]
- Butler DL, Goldstein SA, Guilak F. Functional tissue engineering: the role of biomechanics. J. Biomech. Eng.-Trans. ASME. 2000;122:570–575. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- Cao JD, Martens P, Laws KJ, Boughton P, Ferry M. Quantitative in vitro assessment of Mg65Zn30Ca5 degradation and its effect on cell viability. J. Biomed. Mater. Res. B. 2013;101B:43–49. doi: 10.1002/jbm.b.32811. [DOI] [PubMed] [Google Scholar]
- Chapman JR, Harrington RM, Lee KM, Anderson PA, Tencer AF, Kowalski D. Factors affecting the pullout strength of cancellous bone screws. J. Biomech. Eng. 1996;118:391–398. doi: 10.1115/1.2796022. [DOI] [PubMed] [Google Scholar]
- Chou D-T, Hong D, Saha P, Ferrero J, Lee B, Tan Z, Dong Z, Kumta PN. In vitro and in vivo corrosion, cytocompatibility, and mechanical properties of biodegradable Mg-Y-Ca-Zr alloys as implant materials. Acta Biomater. 2013;9:8518–8533. doi: 10.1016/j.actbio.2013.06.025. [DOI] [PubMed] [Google Scholar]
- Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J. Bone Joint Surg. Am. 2012;94:227–233. doi: 10.2106/JBJS.J.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan A, Ghodadra N, Karas V, Salata MJ, Cole BJ. Complications of bioabsorbable suture anchors in the shoulder. Am. J. Sports Med. 2012;40:1424–1430. doi: 10.1177/0363546511417573. [DOI] [PubMed] [Google Scholar]
- Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- Farraro K, Sasaki N, Eason H, Kim K, Woo SL-Y. A magnesium-based ring for healing of an injured anterior cruciate ligament—design and in vitro robotic testing. Seattle, WA: Proceedings of the BMES Annual Meeting; 2013. [Google Scholar]
- Fisher MB, Jung HJ, McMahon PJ, Woo SL-Y. Suture augmentation following ACL injury to restore the function of the ACL, MCL, and medial meniscus in the goat stifle joint. J. Biomech. 2011;44:1530–1535. doi: 10.1016/j.jbiomech.2011.02.141. [DOI] [PubMed] [Google Scholar]
- Fisher MB, Liang R, Jung HJ, Kim KE, Zamarra G, Almarza AJ, McMahon PJ, Woo SL-Y. Potential of healing a transected anterior cruciate ligament with genetically modified extracellular matrix bioscaffolds in a goat model. Knee Surg., Sports Traumatol., Arthrosc.: Off. J. ESSKA. 2012;20:1357–1365. doi: 10.1007/s00167-011-1800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming BC, Carey JL, Spindler KP, Murray MM. Can suture repair of acl transection restore normal anteroposterior laxity of the knee? An ex vivo study. J. Orthop. Res. 2008;26:1500–1505. doi: 10.1002/jor.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers JR. Finite Element Analysis of a Magnesium Based ACL Interference Screw Drive to Improve Insertion Success (MS Thesis) Greensboro, NC: North Carolina A&T State University; 2012. [Google Scholar]
- Fox RJ, Harner CD, Sakane M, Carlin GJ, Woo SL-Y. Determination of the in situ forces in the human posterior cruciate ligament using robotic technology —a cadaveric study. Am. J. Sports Med. 1998;26:395–401. doi: 10.1177/03635465980260030901. [DOI] [PubMed] [Google Scholar]
- Ge ZG, Yang F, Goh JCH, Ramakrishna S, Lee EH. Biomaterials and scaffolds for ligament tissue engineering. J. Biomed. Mater. Res. A. 2006;77A:639–652. doi: 10.1002/jbm.a.30578. [DOI] [PubMed] [Google Scholar]
- Geetha M, Singh AK, Asokamani R, Gogia AK. Ti based biomaterials, the ultimate choice for orthopaedic implants—a review. Prog. Mater. Sci. 2009;54:397–425. [Google Scholar]
- Gu X, Zheng W, Cheng Y, Zheng Y. A study on alkaline heat treated Mg-Ca alloy for the control of the biocorrosion rate. Acta Biomater. 2009;5:2790–2799. doi: 10.1016/j.actbio.2009.01.048. [DOI] [PubMed] [Google Scholar]
- Hollis JM, Takai S, Adams DJ, Horibe S, Woo SL-Y. The effects of knee motion and external loading on the length of the anterior cruciate ligament (Acl)—a kinematic study. J. Biomech. Eng.-Trans. ASME. 1991;113:208–214. doi: 10.1115/1.2891236. [DOI] [PubMed] [Google Scholar]
- Hort N, Huang Y, Fechner D, Stormer M, Blawert C, Witte F, Vogt C, Drucker H, Willumeit R, Kainer KU, Feyerabend F. Magnesium alloys as implant materials—principles of property design for Mg-RE alloys. Acta Biomater. 2010;6:1714–1725. doi: 10.1016/j.actbio.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Shigematsu I, Saito N. Anticorrosive magnesium phosphate coating on AZ31 magnesium alloy. Surf. Coat. Technol. 2009;203:2288–2291. [Google Scholar]
- Johnston M, Morse A, Arrington J, Pliner M, Gasser S. Resorption and remodeling of hydroxyapatite-poly-L-lactic acid composite anterior cruciate ligament interference screws. Arthroscopy. 2011;27:1671–1678. doi: 10.1016/j.arthro.2011.06.036. [DOI] [PubMed] [Google Scholar]
- Kim KE, Sasaki N, Speziali A, Pickering AN, Farraro KF, Woo SL-Y. Proceedings of the International Symposium on Ligaments and Tendons-XIII. Italy: Arezzo; 2013. The development of a novel magnesium-based interference screw for acl reconstruction: a time-zero study in a goat model. [Google Scholar]
- Kunjukunju S, Roy A, Ramanathan M, Lee B, Candiello JE, Kumta PN. A layer-by-layer approach to natural polymer-derived bioactive coatings on magnesium alloys. Acta Biomater. 2013;9:8690–8703. doi: 10.1016/j.actbio.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Liao Y, Chen DS, Niu JL, Zhang J, Wang YP, Zhu ZJ, Yuan GY, He YH, Jiang Y. In vitro degradation and mechanical properties of polyporous CaHPO4-coated Mg-Nd-Zn-Zr alloy as potential tissue engineering scaffold. Mater. Lett. 2013;100:306–308. [Google Scholar]
- Livesay GA, Fujie H, Kashiwaguchi S, Morrow DA, Fu FH, Woo SL-Y. Determination of the in-situ forces and force distribution within the human anterior cruciate ligament. Ann. Biomed. Eng. 1995;23:467–474. doi: 10.1007/BF02584446. [DOI] [PubMed] [Google Scholar]
- Lu HH, Thomopoulos S. Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu. Rev. Biomed. Eng. 2013;15:201–226. doi: 10.1146/annurev-bioeng-071910-124656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride ED. Magnesium screw and nail transfixion in fractures. South. Med. J. 1938;31:508–514. [Google Scholar]
- Murray MM, Spindler KP, Abreu E, Muller JA, Nedder A, Kelly M, Frino J, Zurakowski D, Valenza M, Snyder BD, Connolly SA. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J. Orthop. Res. 2007;25:81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- Musahl V, Abramowitch SD, Gabriel MT, Debski RE, Hertel P, Fu FH, Woo SL-Y. Tensile properties of an anterior cruciate ligament graft after bone-patellar tendon-bone press-fit fixation. Knee Surg., Sports Traumatol., Arthrosc. 2003;11:68–74. doi: 10.1007/s00167-003-0354-y. [DOI] [PubMed] [Google Scholar]
- Navarro M, Michiardi A, Castano O, Planell JA. Biomaterials in orthopaedics. J. R. Soc. Interface. 2008;5:1137–1158. doi: 10.1098/rsif.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DT, Geel J, Schulze M, Raschke MJ, Woo SL-Y, van Dijk CN, Blankevoort L. Healing of the goat anterior cruciate ligament after a new suture repair technique and bioscaffold treatment. Tissue Eng. Part A. 2013;19:2292–2299. doi: 10.1089/ten.tea.2012.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okawachi H, Ayukawa Y, Atsuta I, Furuhashi A, Sakaguchi M, Yamane K, Koyano K. Effect of titanium surface calcium and magnesium on adhesive activity of epithelial-like cells and fibroblasts. Biointerphases. 2012;7 doi: 10.1007/s13758-012-0027-9. [DOI] [PubMed] [Google Scholar]
- Ostrowski NJ, Lee B, Roy A, Ramanathan M, Kumta PN. Biodegradable poly (lactide-co-glycolide) coatings on magnesium alloys for orthopaedic applications. J. Mater. Sci.: Mater. Med. 2013;24:85–96. doi: 10.1007/s10856-012-4773-5. [DOI] [PubMed] [Google Scholar]
- Papageorgiou CD, Ma CB, Abramowitch SD, Clineff TD, Woo SL-Y. A multidisciplinary study of the healing of an intraarticular anterior cruciate ligament graft in a goat model. Am. J. Sports Med. 2001;29:620–626. doi: 10.1177/03635465010290051501. [DOI] [PubMed] [Google Scholar]
- Pietak A, Mahoney P, Dias GJ, Staiger MP. Bone-like matrix formation on magnesium and magnesium alloys. J. Mater. Sci.—Mater. Med. 2008;19:407–415. doi: 10.1007/s10856-007-3172-9. [DOI] [PubMed] [Google Scholar]
- Qin L. Personal Communication with S.L-Y. Woo. 2013. Clinical trials of novel Mg-based orthopaedic devices. [Google Scholar]
- Salunke P, Shanov V, Witte F. High purity biodegradable magnesium coating for implant application. Mater. Sci. Eng. B—Adv. 2011;176:1711–1717. [Google Scholar]
- Shin KS, Jung HC, Bian MZ, Nam ND, Kim NJ. Characterization of biodegradable magnesium single crystals with various crystallographic orientations. Eur. Cells Mater. 2013;26:4. [Google Scholar]
- Smith BA, Livesay GA, Woo SL-Y. Biology and biomechanics of the anterior cruciate ligament. Clin. Sports Med. 1993;12:637–670. [PubMed] [Google Scholar]
- Smith CA, Tennent TD, Pearson SE, Beach WR. Fracture of Bilok interference screws on insertion during anterior cruciate ligament reconstruction. Arthroscopy. 2003;19:E115–E117. doi: 10.1016/j.arthro.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Speziali A, Farraro KF, Kim KE, Woo SL-Y. Biological and mechanical augmentation for healing of ligaments and tendons. GIOT. 2012;38:26–30. [Google Scholar]
- Tecklenburg K, Burkart P, Hoser C, Rieger M, Fink C. Prospective evaluation of patellar tendon graft fixation in anterior cruciate ligament reconstruction comparing composite bioabsorbable and allograft interference screws. Arthroscopy. 2006;22:993–999. doi: 10.1016/j.arthro.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Thomann M, Krause C, Angrisani N, Bormann D, Hassel T, Windhagen H, Meyer-Lindenberg A. Influence of a magnesium-fluoride coating of magnesium-based implants (MgCa0.8) on degradation in a rabbit model. J. Biomed. Mater. Res. A. 2010;93:1609–1619. doi: 10.1002/jbm.a.32639. [DOI] [PubMed] [Google Scholar]
- Tian P, Liu XY. Anticorrosion and cytocompatibility of biodegradable polylactide/MgO composite coating on AZ31 alloy. Proceedings of the 5th Symposium on Biodegradable Metals. 2013;26, p:48. [Google Scholar]
- Walton M, Cotton NJ. Long-term in vivo degradation of poly-L-lactide (PLLA) in bone. J. Biomater. Appl. 2007;21:395–411. doi: 10.1177/0885328206065125. [DOI] [PubMed] [Google Scholar]
- Wei J, Jia JF, Wu F, Wei SC, Zhou HJ, Zhang HB, Shin JW, Liu CS. Hierarchically microporous/macroporous scaffold of magnesium-calcium phosphate for bone tissue regeneration. Biomaterials. 2010;31:1260–1269. doi: 10.1016/j.biomaterials.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Windhagen H, Radtke K, Weizbauer A, Diekmann J, Noll Y, Kreimeyer U, Schavan R, Stukenborg-Colsman C, Waizy H. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: short term results of the first prospective, randomized, controlled clinical pilot study. Biomed. Eng. Online. 2013;12 doi: 10.1186/1475-925X-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte F. The history of biodegradable magnesium implants: a review. Acta Biomater. 2010;6:1680–1692. doi: 10.1016/j.actbio.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Witte F, Kaese V, Haferkamp H, Switzer E, Meyer-Lindenberg A, Wirth CJ, Windhagen H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials. 2005;26:3557–3563. doi: 10.1016/j.biomaterials.2004.09.049. [DOI] [PubMed] [Google Scholar]
- Woo SL-Y, Alejandro JA, Karaoglu S, Liang R, Fisher MB. Functional tissue engineering of ligament and tendon injuries. In: Atala A, Lanza R, Thomson JA, Nerem R, editors. Principles of Rengenerative Medicine. second ed. Amsterdam; Boston: Elsevier, Academic Press; 2011. pp. 997–1021. [Google Scholar]
- Woo SL-Y, Debski RE, Withrow JD, Janaushek MA. Biomechanics of knee ligaments. Am. J. Sports Med. 1999;27:533–543. doi: 10.1177/03635465990270042301. [DOI] [PubMed] [Google Scholar]
- Woo SL-Y, Kanamori A, Zeminski J, Yagi M, Papageorgiou C, Fu FH. The effectiveness of reconstruction of the anterior cruciate ligament with hamstrings and patellar tendon—a cadaveric study comparing anterior tibial and rotational loads. J. Bone Joint Surg. Am. 2002;84A:907–914. doi: 10.2106/00004623-200206000-00003. [DOI] [PubMed] [Google Scholar]
- Xu LP, Pan F, Yu GN, Yang L, Zhang EL, Yang K. In vitro and in vivo evaluation of the surface bioactivity of a calcium phosphate coated magnesium alloy. Biomaterials. 2009;30:1512–1523. doi: 10.1016/j.biomaterials.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Yun Y, Dong ZY, Yang DE, Schulz MJ, Shanov VN, Yarmolenko S, Xu ZG, Kumta P, Sfeir C. Biodegradable Mg corrosion and osteoblast cell culture studies. Mater. Sci. Eng. C—Mater. 2009;29:1814–1821. [Google Scholar]
- Zberg B, Uggowitzer PJ, Loffler JF. MgZnCa glasses without clinically observable hydrogen evolution for biodegradable implants. Nat. Mater. 2009;8:887–891. doi: 10.1038/nmat2542. [DOI] [PubMed] [Google Scholar]
- Zhang EL, Xu LP, Yu GN, Pan F, Yang K. In vivo evaluation of biodegradable magnesium alloy bone implant in the first 6 months implantation. J. Biomed. Mater. Res. A. 2009;90A:882–893. doi: 10.1002/jbm.a.32132. [DOI] [PubMed] [Google Scholar]
- Zierold AA. Reaction of bone to various metals. Arch. Surg. 1924;9:365–412. [Google Scholar]