Abstract
Background
Recently, many surgeons have chosen the quadriceps tendon (QT) as an autograft for anterior cruciate ligament (ACL) reconstruction. However, there have not been biomechanical studies that quantitatively evaluated knee function after reconstruction using a QT autograft.
Purpose
To measure the 6 degrees of freedom knee kinematics and in situ graft forces after reconstruction with a QT autograft compared with a quadrupled semitendinosus and gracilis (QSTG) tendon autograft.
Study Design
Controlled laboratory study.
Methods
Ten human cadaveric knees (age, 54–64 years) were tested in 3 conditions: (1) intact, (2) ACL deficient, and (3) after ACL reconstruction using a QT or QSTG autograft. With use of a robotic/universal force-moment sensor testing system, knee kinematics and in situ forces in the ACL and autografts were obtained at 5 knee flexion angles under externally applied loads: (1) 134-N anterior tibial load, (2) 134-N anterior tibial load with 200-N axial compression, and (3) 10-N·m valgus and 5-N·m internal tibial torque.
Results
Under the anterior tibial load, both autografts restored anterior tibial translation to within 2.5 mm of the intact knee and in situ forces to within 20 N of the intact ACL at 15°, 30°, and 60°. Adding compression did not change these findings. With the combined rotatory load, the anterior tibial translation and graft in situ forces were again not significantly different from the intact ACL. There were no significant differences between the grafts under any experimental condition.
Conclusion
Reconstruction of the ACL with a QT autograft restored knee function to similar levels as that reconstructed with a QSTG autograft under loads simulating clinical examinations.
Clinical Relevance
The positive biomechanical results of this cadaveric study lend support to the use of a QT autograft for ACL reconstruction, as it could restore knee function immediately after surgery under applied loads that mimic clinical examinations.
Keywords: ACL reconstruction, quadriceps tendon autograft, robotic/UFS testing system
The preferred autografts for anterior cruciate ligament (ACL) reconstruction are the bone–patellar tendon–bone (BPTB) and quadrupled semitendinosus and gracilis (QSTG) tendons. The BPTB autograft has been widely used since the 1960s24 partly because advocates preferred its high strength (ie, an ultimate load at failure for a graft width of 14 mm), measured to be twice that of the native ACL.39 Additionally, it has bone blocks on both ends that could facilitate good initial graft fixation for knee stability and osteointegration. However, there are a number of post-operative complications, including donor site morbidity, such as the risk of patellar tendon ruptures, loss of quadriceps function, and adhesions as well as knee extension deficits.27 A large number of patients also experienced anterior knee pain or kneeling pain,9,16,23 and many of them showed signs of osteoarthritis ≥8 years after surgery.1,44,51 In the last 3 decades, the QSTG autograft has gained popularity, as patients experienced less of the aforementioned postoperative issues.7,29 In addition, the ultimate load at failure of the QSTG graft matches well with that of a 10 mm–wide BPTB graft,52 while harvesting the QSTG graft is easier and requires a much smaller incision. Still, there are other shortcomings of the QSTG autograft that include increases in knee laxity,2,59 weakness in knee flexion,38 slower soft tissue–bone healing, bone tunnel enlargement,6,45 and fibrous tissue formation in the tunnel.
Recently, the quadriceps tendon (QT) has regained attention as an alternative autograft for ACL reconstruction, even though it was first advocated by Marshall et al35 in 1979 and Blauth3 in 1984. In those days, strength was the primary emphasis in orthopaedic sports medicine, and the ultimate load at failure for the QT graft was lower than that for a 14 mm–wide BPTB graft.39 In time, biomechanical studies have educated us that matching the stiffness of the graft is another and perhaps more important factor when evaluating the graft’s function.11,47 Furthermore, we have learned the importance of examining the structural properties of the entire graft-bone complex and its function.54 Meanwhile, laboratory studies have confirmed that a QT graft can be longer, wider, and thicker than the patellar tendon and that the QT-bone complex has tensile properties comparable with those of a PT-bone complex of the same width (mean stiffness: 474.9 ± 82.5 N/mm and 544.5 ± 113.3 N/mm, respectively; mean ultimate load: 2173 ± 618 N and 1953 ± 325 N, respectively).4,18,49
As such, Staubli and colleagues48,49 and Fulkerson et al8,13,32 have described their preferences for QT autografts. The fact that its use may require a smaller incision than the BPTB autograft during harvesting and that its bone block on one end would allow for bone-to-bone healing can be considered to be advantageous. Recent clinical studies showed that at 2- to 5.5-year follow-up, ACL reconstruction with a QT autograft has achieved good outcomes with fewer complications. Specifically, similar anterior knee stability, less donor site morbidity and anterior knee pain, and improved knee extension were found when compared with those with BPTB and QSTG autografts.5,15,17,21,25,28,30
Still, there is a lack of scientific data on knee function after ACL reconstruction using a QT autograft. This begs the research question of whether a QT autograft could restore knee mechanics at the time of surgery. We hypothesized that because of the good tensile properties of the QT-bone complex, it could successfully restore knee kinematics and graft function to levels comparable to those of a QSTG autograft. Thus, the objective of this study was to evaluate the multiple degrees of freedom (DOF) knee kinematics and in situ forces in the graft by a QT autograft after ACL reconstruction and compare with those using a QSTG autograft under identical external loading conditions that mimicked clinical examinations. We employed a robotic/universal force-moment sensor (UFS) testing system so that knee kinematics and forces in the grafts could be accurately measured after the same knee was reconstructed by a QT autograft and a QSTG autograft.42,55
MATERIALS AND METHODS
Specimen Preparation
Fresh-frozen human cadaveric knees were used in this study, with approval from the Committee for Oversight of Research and Clinical Training Involving Decedents. Each specimen was screened for a history of abnormal body mass index, lower limb trauma, and physical activity level. Additionally, upon dissection, any specimens with ligament damage, meniscal tears, or osteoarthritis were excluded. In the end, 10 knees (mean age, 57.4 ± 4.2 years; range, 54–64 years) were used in this study. The sample size was determined based on results of a power analysis performed using previous data to detect differences of 2 mm for anterior tibial translation and 20 N for in situ force measurements (power = .80; significance level = .05).55,57,60
Knee specimens were frozen at –20°C in airtight plastic bags until 24 hours before testing, when they were thawed at room temperature, and the grafts were harvested.36,56 The semitendinosus and gracilis tendons were harvested by an orthopaedic surgeon (N.S.) using a tendon stripper through a small incision at the anteromedial tibial surface. The QT autograft was harvested using a scalpel through a small incision at the anterior femoral site, removing a graft measuring approximately 10 mm wide and 70 to 80 mm long.4 Distally, a 6 mm– to 7 mm–thick and 20 mm–long bone block was removed from the patella using an oscillating saw. After harvest, the grafts were wrapped in double layers of saline-soaked gauze to limit dehydration.
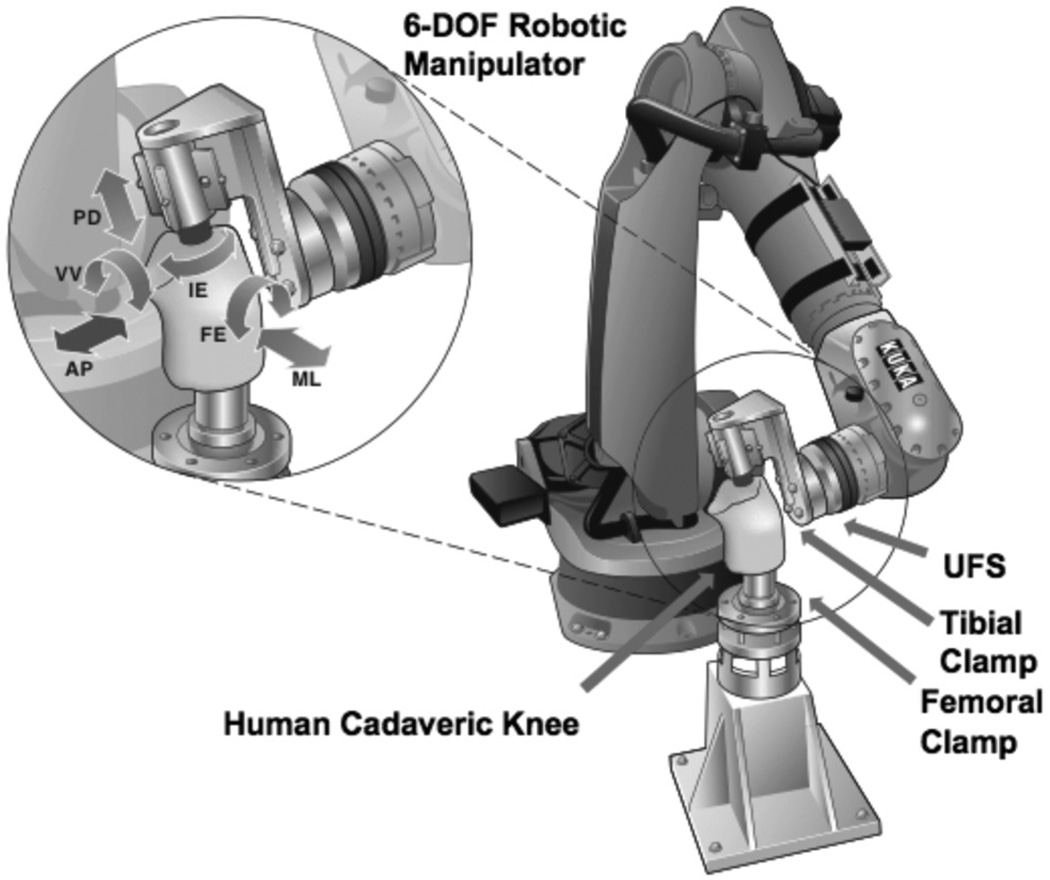
The specimens were then prepared for testing following an established protocol.26,58 Briefly, the femur and tibia were cut approximately 20 cm from the joint line, and soft tissues were removed approximately 10 cm away from the joint line, such that the femur and tibia could each be potted in a cylindrical epoxy mold (Fibre Glass, Evercoat, Cincinnati, Ohio, USA). The fibula was also cut 10 cm from the joint line and rigidly fixed to the tibia with a cortical screw to keep its anatomic position. The joints were attached to the robotic/UFS testing system by securing the potted bones within corresponding custommade aluminum clamps with transfixing bolts. The femoral side was rigidly mounted to the base of the robotic manipulator, while the tibial side was attached to the end effector of the robotic manipulator via the UFS system (Figure 1). The specimens were kept moist by regularly spraying with a 0.9% saline solution throughout the test.
Figure 1.
A schematic illustrating a human cadaveric knee joint being tested on the robotic/universal force-moment sensor testing system at preselected angles of knee flexion, providing 5 degrees of freedom joint motion (AP, anterior-posterior; FE, flexion-extension; IE, internal-external; ML, medial-lateral; PD, proximal-distal; VV, varus-valgus) at each prescribed angle of knee flexion.
Testing of Knee Kinematics and Graft Function
The robotic/UFS testing system was developed 2 decades ago to improve upon previous methodologies to study joint function.10,34,53 The UFS system (Model Theta; ATI Industrial Automation, Apex, North Carolina, USA) has a capacity of 1500 N of force and 240 N·m of torque and can measure 3 forces and 3 moments in a Cartesian coordinate system fixed with respect to the sensor with a repeatability of 0.5 N for force and 0.05 N·m for torque. The robotic manipulator (KUKA Model KR 210; KUKA Robotics Corp, Shelby Township, Michigan, USA) has a position and orientation repeatability of less than 0.1 mm and 0.1°. The high accuracy and repeatability, noncontact measurements, and full 6 DOF of joint motion are improvements over previously used systems, including the linkage system.20 In this study, the robotic/UFS testing system was operated in force, position, and hybrid control to obtain knee kinematics of the intact, ACL-deficient, and reconstructed knees as well as the in situ forces in the intact ACL and ACL reconstruction grafts from the same knee.33,43,55
The testing procedure and the data acquired are shown in Table 1. First, the path of passive flexion–extension of the intact knee was determined from full extension to 90° of flexion by moving the tibia in 1° increments while minimizing all external forces and moments. This path provided the starting position at each angle of flexion for the application of external tibial loads for the remainder of the test and served as the reference position by which to measure knee kinematics.10,26,34,57 In this study, 3 external loading conditions mimicking clinical examinations were applied to the knee joint:
a 134-N anterior tibial load with the knee at full extension and 15°, 30°, 60°, and 90° of knee flexion;
a 134-N anterior tibial load combined with 200-N axial compression with the knee at full extension and 15°, 30°, 60°, and 90° of flexion31,40; and
a combined 10-N·m valgus torque and 5-N·m internal tibial torque at 15° and 30° of knee flexion.34,58,60
TABLE 1.
Robotic/UFS Testing Protocol and Data That Were Acquireda
| Data Acquired |
|||
|---|---|---|---|
| Knee State | Load/Kinematics Applied | Kinematics | In Situ Force |
| I. Intact | Path of passive flexion/extension | ||
Applied loads:
|
Intact knee | ||
| II. ACL deficient | Repeated intact knee kinematics | ACL | |
| Applied loads 1, 2, and 3 | ACL-deficient knee | ||
| III. QSTG reconstructedb | Applied loads 1, 2, and 3 | QSTG-reconstructed knee | |
| Released graft | Repeated QSTG-reconstructed knee kinematics | QSTG graft | |
| IV. QT reconstructedb | Applied loads 1, 2, and 3 | QT-reconstructed knee | |
| Released graft | Repeated QT-reconstructed knee kinematics | QT graft | |
ACL, anterior cruciate ligament; QSTG, quadrupled semitendinosus and gracilis; QT, quadriceps tendon; UFS, universal force-moment sensor.
Order of reconstructions was randomized.
The anterior tibial load mimicked the anterior drawer or Lachman test, which is commonly used to test ACL function. The simulated axial compression load added ground-reaction forces or active muscle contractions during daily activities.40 The combined rotatory load statically simulated the pivot-shift test, a diagnostic tool used to determine rotatory instability due to ACL deficiency.26
The 5 DOF kinematics (anterior-posterior, medial-lateral, and proximal-distal translations as well as internal-external and varus-valgus rotations) of the intact knee in response to the 3 externally applied loads were measured with the knee at full extension and 15°, 30°, 60°, and 90° of flexion (Figure 1). The in situ force in the ACL was determined by carefully transecting the ACL through its midsubstance using a medial mini-arthrotomy and then repeating the intact joint kinematics in position-control mode on the ACL-deficient knee. While the previous path and position were repeated, the UFS system measured a new set of forces and moments. Using the principle of superposition, the vector difference of the measured force between the intact and the ACL-deficient knee was the in situ force in the ACL.12,41 Next, the same external loading conditions (conditions 1–3 above) were applied to the ACL-deficient knee, and the resulting 5 DOF kinematics at each preselected angle of knee flexion were assessed to obtain the ACL-deficient kinematics. Kinematics were obtained only at full extension, 15°, and 30° with the addition of a compressive load because the ACL-deficient knee experienced a buckling effect at higher flexion angles that prevented data from being obtained.
Next, ACL reconstructions were performed using a medial parapatellar approach by 1 orthopaedic surgeon using QT and QSTG autografts in a randomized order. For the femoral tunnel, the position was aimed at the center of the insertion of the posterolateral bundle of the ACL using a guide wire.34,58 The tibial tunnel was created in the middle of the footprint of the insertion of the ACL using a Protac tibial guide set (Acufex; Smith & Nephew Inc, Andover, Massachusetts, USA).22 Both tunnels were drilled using a 10 mm–diameter cannulated drill over the guide wire. The graft was passed from the tibial to femoral bone tunnel (with the bone block inserted first in the QT autograft), with the femoral side fixed first using a titanium interference screw (8 × 25–mm RCI screw; Smith & Nephew Inc) while holding both ends of the graft and applying manual tension. The knee was then preconditioned by moving it through 5 cycles of the full range of knee flexion while applying a 44-N pretension on the graft. Finally, the tibial side was fixed with a double-spiked plate and fixation post and a staple (Mitek; Smith & Nephew Inc). In both reconstructions, the graft was fixed under the same initial loading condition (67 N of posterior tibial load and 44 N of initial graft tension at 30° of knee flexion)19.
Each reconstruction involved the use of the same single bone tunnel, fixation methods, and fixation loading conditions and took approximately 30 to 40 minutes to perform. After each reconstruction, the external loads were reapplied to the knee, and the resulting kinematics were recorded by the robotic/UFS testing system. The in situ forces in the grafts were obtained using the principle of superposition by releasing the graft and replaying the knee kinematics of the reconstructed knee to measure the vector changes in forces between the ACL-deficient state and the intact state.
Statistical Analysis
A 1-factor repeated-measures analysis of variance (ANOVA) was used for statistical analysis of knee kinematics and in situ forces at each flexion angle, with knee state (intact, ACL deficient, reconstructed) as the factor. Because all variables were measured on the same specimen, interspecimen variation was thus eliminated, which allowed for greater statistical power. A Bonferroni post hoc analysis was performed to make pairwise comparisons between specific knee states. The significance level was set at P < .05.
RESULTS
Anterior Tibial Load
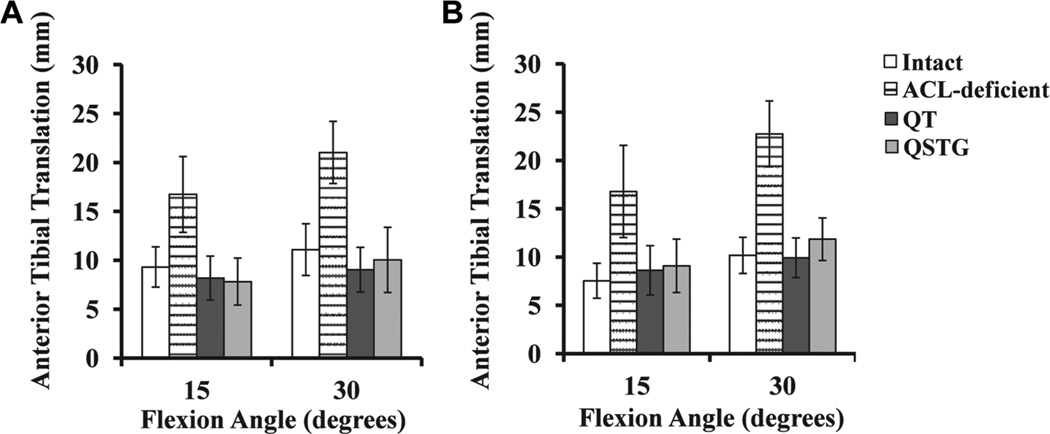
Under the 134-N anterior tibial load, anterior tibial translation increased as the joint was moved from full extension to 30° of knee flexion and then decreased at 60° and 90° (Figure 2A and Table 2). After transection of the ACL, anterior tibial translation increased significantly by 7.3 to 10.1 mm throughout the range of flexion angles tested over the intact joint (P < .05). After ACL reconstruction with either a QT or QSTG autograft, anterior tibial translation was reduced by 7.3 to 12.0 mm and was restored to within 2.1 mm of the intact joint. There were no significant differences between the 2 grafts at any of the flexion angles tested (P > .05), with a maximum difference between them being 1.1 mm at 60° of flexion. The corresponding axial tibial rotation was similar for all knee states and tested flexion angles and ranged from an average of 0.3° of external rotation to 2.5° of internal rotation.
Figure 2.
Mean anterior tibial translation of the intact, anterior cruciate ligament–deficient, and quadriceps tendon (QT)– or quadrupled semitendinosus and gracilis (QSTG)–reconstructed knee joint at 15° and 30° of knee flexion in response to (A) a 134-N anterior tibial load and (B) a 134-N anterior tibial load combined with 200-N axial compression.
TABLE 2.
Anterior Tibial Translation of the Intact, ACL-Deficient, and QT- and QSTG-Reconstructed Knee Jointa
| Flexion Angle |
|||||
|---|---|---|---|---|---|
| Translation, mm | Full Extension | 15° | 30° | 60° | 90° |
| 134-N anterior tibial load | |||||
| Intact | 6.8 ± 1.2 | 9.3 ± 2.0 | 11.1 ± 2.6 | 9.8 ± 2.7 | 8.0 ± 2.8 |
| ACL deficient | 14.1 ± 3.3b | 16.7 ± 3.9b | 21.0 ± 3.2b | 19.9 ± 5.5b | 17.0 ± 6.7b |
| QT reconstructed | 7.2 ± 1.9 | 8.2 ± 2.4 | 9.0 ± 2.3 | 10.1 ± 2.8 | 9.3 ± 2.8 |
| QSTG reconstructed | 6.8 ± 1.7 | 7.8 ± 2.4 | 10.0 ± 3.3 | 11.2 ± 2.4 | 10.1 ± 3.0 |
| 134-N anterior tibial load + 200-N axial compression | |||||
| Intact | 5.7 ± 1.1 | 7.5 ± 1.8 | 10.2 ± 1.9 | 10.8 ± 1.7 | 9.1 ± 2.2 |
| ACL deficient | 13.5 ± 3.1b | 16.8 ± 4.8b | 22.8 ± 3.4b | — | |
| QT reconstructed | 7.4 ± 1.6 | 8.6 ± 2.6 | 9.9 ± 2.1 | 11.3 ± 2.9 | 10.2 ± 2.6 |
| QSTG reconstructed | 7.2 ± 1.8 | 9.1 ± 2.8 | 11.9 ± 2.2 | 12.1 ± 2.6 | 11.6 ± 2.1 |
Values are expressed as mean ± standard deviation. In response to a 134-N anterior tibial load and a 134-N anterior tibial load combined with 200-N axial compression. ACL, anterior cruciate ligament; QSTG, quadrupled semitendinosus and gracilis; QT, quadriceps tendon.
Significant difference from all other conditions (P < .05).
The magnitude of the in situ force in the ACL remained relatively constant from full extension to 30° of knee flexion (Table 3) and then decreased at 60° and 90°. This trend was also observed in both the QT and QSTG grafts. At full extension, 15°, 30°, 60°, and 90°, there were no significant differences between the in situ forces in the 2 grafts. The same was true for each graft compared with the intact ACL from full extension to 30° (P > .05). However, at 60° and 90° of knee flexion, there were significant differences (20–22 N and 26–29 N, respectively) compared with the intact ACL (P < .05).
TABLE 3.
In Situ Force of the Intact ACL, QT Autograft, and QSTG Autografta
| Flexion Angle |
|||||
|---|---|---|---|---|---|
| In Situ Force, N | Full Extension | 15° | 30° | 60° | 90° |
| 134-N anterior tibial load | |||||
| Intact ACL | 116 ± 26 | 109 ± 22 | 115 ± 24 | 88 ± 26 | 72 ± 23 |
| QT graft | 108 ± 23 | 108 ± 21 | 101 ± 32 | 68 ± 22b | 47 ± 17b |
| QSTG graft | 106 ± 23 | 99 ± 15 | 90 ± 35 | 67 ± 24b | 44 ± 20b |
| 134-N anterior tibial load 1 200-N axial compression | |||||
| Intact ACL | 122 ± 49 | 119 ± 34 | 120 ± 27 | 102 ± 25 | 80 ± 32 |
| QT graft | 94 ± 25 | 110 ± 30 | 132 ± 42 | 87 ± 33 | 54 ± 33 |
| QSTG graft | 96 ± 28b | 107 ± 14 | 136 ± 58 | 88 ± 41 | 46 ± 31b |
Values are expressed as mean ± standard deviation. In response to a 134-N anterior tibial load and a 134-N anterior tibial load combined with 200-N axial compression. ACL, anterior cruciate ligament; QSTG, quadrupled semitendinosus and gracilis; QT, quadriceps tendon.
Significant difference from intact ACL (P < .05).
Combined Anterior Tibial Load and Axial Compression
With the addition of 200-N axial compression to the 134-N anterior tibial load, the anterior tibial translation of the intact knee increased from full extension to 30° of knee flexion and then remained relatively constant between 60° and 90° of flexion (Figure 2B and Table 2). For the ACL-deficient joint, these values increased by 7.8 to 12.6 mm compared with the intact knee, and the increases followed a similar pattern as those under the anterior tibial load (P < .05). After ACL reconstruction with a QT or QSTG graft, the anterior tibial translation was reduced by 6.3 to 12.9 mm and was actually restored to within 2.5 mm of the intact knee. Furthermore, there were no significant differences between the 2 grafts at any tested angle (P > .05). The axial tibial rotation remained similar for all knee states, although there was an increasing trend in the average internal tibial rotation from full extension (range, −1.2° to 2.7°) to 90° of knee flexion (range, 6.4°–8.6°).
The in situ forces in the ACL remained constant from full extension to 30° of knee flexion and then decreased steadily at 60° and 90° (Table 3). For both of the ACL replacement grafts, the in situ forces in the QT and QSTG grafts were slightly lower at full extension and increased at 15° and 30°. Then, the values decreased steadily at 60° and 90°. At full extension, 15°, 30°, 60°, and 90°, there were no significant differences between the in situ forces in the 2 grafts (P > .05). The same was true for each graft compared with the intact ACL from 15° to 60° (P > .05). At full extension and 90°, there were significant differences (26–28 N and 26–34 N, respectively) between the QSTG graft and the intact ACL (P < .05).
Combined Rotatory Load
Under the combined rotatory load, anterior tibial translation increased from 15° to 30° of knee flexion with all knee conditions (Table 4). After transection of the ACL, anterior tibial translation increased by 2.8 to 3.7 mm compared with the intact knee (P < .05 at 30°). After ACL reconstruction, anterior tibial translation with either the QT or QSTG graft was within 0.3 mm of the intact joint at both 15° and 30° of knee flexion (P > .05). Further, no significant differences could be detected between the 2 grafts or between either graft and the intact knee (P > .05).
TABLE 4.
Knee Kinematics of the Intact, ACL-Deficient, and QT- and QSTG-Reconstructed Knee Jointa
| ACL-Reconstructed Knee |
||||
|---|---|---|---|---|
| ACL-Intact Knee | ACL-Deficient Knee | QT | QSTG | |
| Anterior tibial translation, mm | ||||
| 15° of flexion | 1.3 ± 2.7 | 4.1 ± 5.3 | 1.0 ± 2.1 | 1.0 ± 1.8 |
| 30° of flexion | 1.6 ± 4.8b | 5.3 ± 5.3 | 1.3 ± 2.4 | 1.5 ± 2.6 |
| Internal tibial rotation, deg | ||||
| 15° of flexion | 15.4 ± 8.7 | 17.5 ± 13.0 | 14.6 ± 10.7 | 14.9 ± 10.3 |
| 30° of flexion | 21.5 ± 9.7 | 22.6 ± 13.3 | 19.6 ± 12.1 | 15.7 ± 10.6 |
Values are expressed as mean ± standard deviation. In response to a combined 10-N·m valgus tibial torque and 5-N·m internal tibial torque. ACL, anterior cruciate ligament; QSTG, quadrupled semitendinosus and gracilis; QT, quadriceps tendon.
Significant difference compared with the ACL-deficient knee (P < .05).
The tibial rotation also increased from 15° to 30° of knee flexion under all knee conditions (Table 4). After reconstruction, the values were within 0.9° to 1.9° of the intact joint with the QT graft and 0.5° to 5.8° with the QSTG graft. However, these differences were not statistically significant (P > .05).
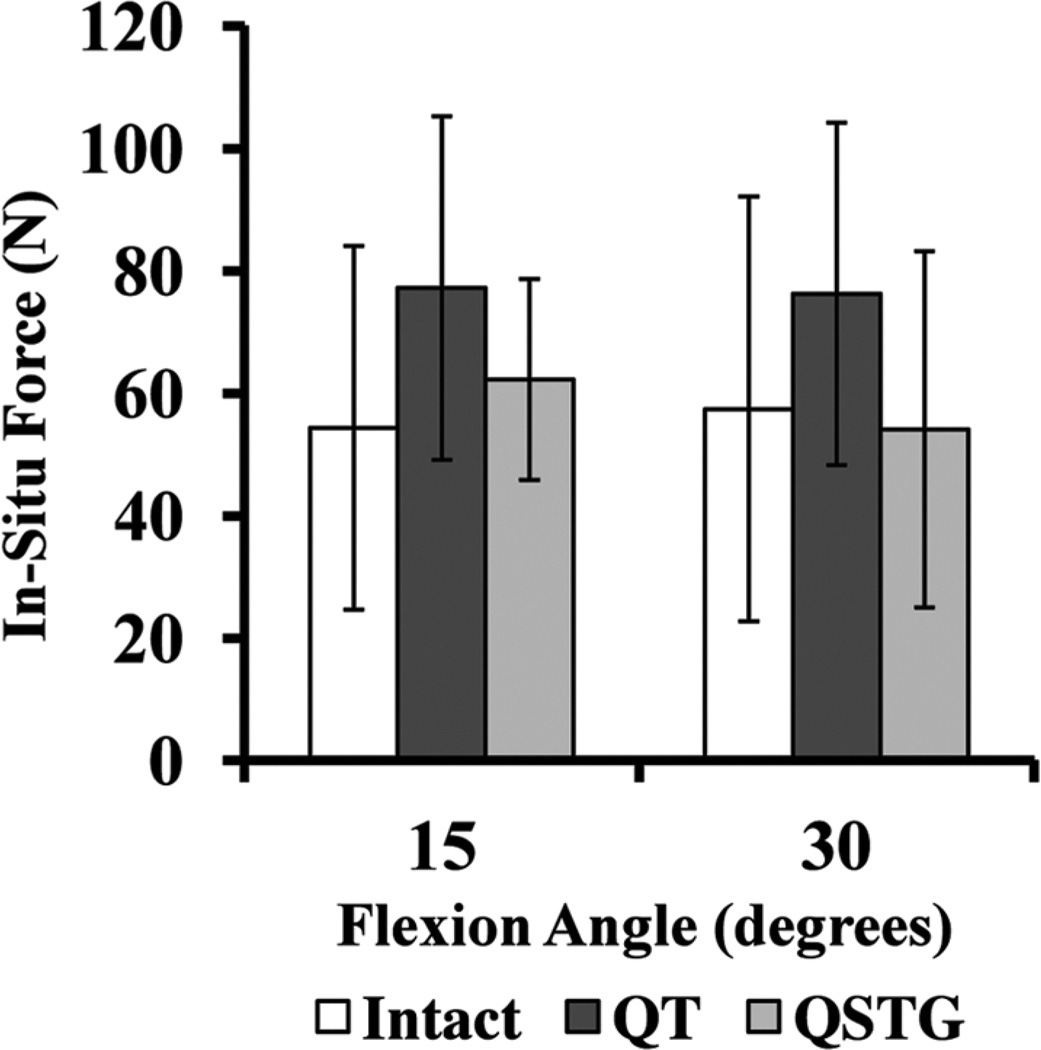
The magnitude of the in situ force in the ACL under the combined rotatory load was similar at 15° and 30° of knee flexion (54 ± 30 N and 57 ± 35 N, respectively) (Figure 3). This trend was also observed in the QT graft (77 ± 28 N and 76 ± 28 N at 15° and 30°, respectively) and QSTG graft (62 ± 16 N and 54 ± 29 N, respectively). There were no significant differences between the 2 grafts at either flexion angle (P > .05). The same was true for each graft compared with the ACL (P > .05).
Figure 3.
Mean in situ force of the intact anterior cruciate ligament, quadriceps tendon (QT) autograft, and quadrupled semitendinosus and gracilis (QSTG) autograft at 15° and 30° of knee flexion in response to a combined 10-N-m valgus and 5-N·m internal tibial torque.
DISCUSSION
In this study, quantitative biomechanical data from human cadaveric knees on knee kinematics and in situ forces in the intact ACL, a QT autograft, and a QSTG autograft were obtained using external loading conditions mimicking those used in clinical examinations. Data on the kinematics of the intact knee (ie, anterior tibial translation and internal tibial rotation) as well as the in situ forces in the intact ACL are consistent with published data from our research center and others using similar testing conditions.14,26,46,55,57,60
The robotic/UFS testing system employed in this study is advantageous, as it allows 6 DOF kinematics to be recorded without artificially constraining the knee and enables noncontact measurement of the in situ forces in the ACL and ACL replacement grafts. This system is also an improvement over traditional methods such as the linkage system20 because the starting position and path of motion can be repeated. Using this system under 3 external loading conditions (anterior tibial load, combined axial compressive load, and combined rotatory load), data from each testing condition could be accurately obtained from the same knee. Most importantly, the methods employed allowed us to obtain data for all knee states (ie, intact joint, ACL-deficient joint, and reconstructed joint) from each specimen, which eliminated interspecimen variation and allowed for much greater statistical power.
It was found that both the QT and QSTG autografts could restore knee kinematics to within 2.5 mm of those of the intact knee, and the in situ forces in both of the grafts were mostly within 10 N of the intact ACL at the time of surgery. Notably, no statistically significant differences were found in the knee kinematics or in situ forces in the grafts under all of these experimental conditions. Thus, these data support our hypothesis that the QT autograft could restore knee function to the same level as a QSTG graft. The differences in the in situ forces in the grafts and the intact ACL at 60° and 90° are also consistent with those published in the literature58. It has been shown that posterolateral graft placement could not completely restore the intact ACL’s function at higher flexion angles because the anteromedial bundle of the ACL was not restored.
In a previous study using the porcine knee as a model, the effect of a combined anterior tibial load and axial compression on knee kinematics and in situ forces in the ACL using the robotic/UFS testing system was examined. It was shown that such combined loading led to significant increases in anterior tibial translation and in situ forces in the ACL compared with anterior tibial loading alone.31 The present study provides new information on knee function under this loading condition in humans, showing that the anterior tibial translation and in situ forces in the ACL or ACL replacement grafts were similar under the anterior tibial load with compression compared with the anterior tibial load alone.
In the present study, no statistically significant difference was found between the intact, ACL-deficient, or reconstructed joint under the combined rotatory load, except anterior tibial translation between the intact and ACL-deficient joint at 30°. These findings are consistent with those of Seon et al,46 who found differences of less than 3.5° of internal tibial rotation among all tested knee states under the same anterior tibial load used in the current study. The values obtained are also similar to those found in other published studies.14,60
The limitations of the present study include the use of cadaveric joints for in vitro testing. As such, the data obtained could only represent the condition immediately after surgery. Further, the average age of the donors in this study was higher than that of the patients who require ACL reconstruction. The loading conditions used were also representations of those used in clinical examinations to test for ACL and ACL graft function and do not represent the complex in vivo loading conditions. To solve this problem, we suggest additional studies that use biplanar fluoroscopy to record accurate in vivo knee kinematics37,50 after ACL reconstruction with either graft and compare in a controlled clinical trial.
To conclude, this study yielded new, quantitative scientific data on knee function when using the QT as an autograft for ACL reconstruction. Our positive findings support the clinical results reported for patients who had undergone ACL reconstruction using a QT autograft by showing that the QT autograft could restore knee function as well as a QSTG graft under externally applied loads simulating clinical tests for ACL function at time zero. Together, these in vitro biomechanical data and in vivo clinical results suggest that the QT could be a good alternative graft choice for patients undergoing ACL reconstruction. Further, the QT autograft could be used in revision ACL surgery in which an earlier autograft has already been harvested and used.
ACKNOWLEDGMENT
One or more of the authors has declared the following potential conflict of interest or source of funding: Financial support from the McGowan Institute for Regenerative Medicine, Commonwealth of Pennsylvania, National Institute of Health (T32 EB000392), and Asian♦American Institute for Research and Education is acknowledged.
REFERENCES
- 1.Ahn JH, Kim JG, Wang JH, et al. Long-term results of anterior cruciate ligament reconstruction using bone-patellar tendon-bone: an analysis of the factors affecting the development of osteoarthritis. Arthroscopy. 2012;28(8):1114–1123. doi: 10.1016/j.arthro.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 2.Barrett GR, Noojin FK, Hartzog CW, et al. Reconstruction of the anterior cruciate ligament in females: a comparison of hamstring versus patellar tendon autograft. Arthroscopy. 2002;18(1):46–54. doi: 10.1053/jars.2002.25974. [DOI] [PubMed] [Google Scholar]
- 3.Blauth W. [A new drill template for the operative treatment of injuries of the anterior cruciate ligament] Unfallheilkunde. 1984;87(11):463–466. [PubMed] [Google Scholar]
- 4.Chen CH, Chen WJ, Shih CH. Arthroscopic anterior cruciate ligament reconstruction with quadriceps tendon-patellar bone autograft. J Trauma. 1999;46(4):678–682. doi: 10.1097/00005373-199904000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Chen CH, Chuang TY, Wang KC, et al. Arthroscopic anterior cruciate ligament reconstruction with quadriceps tendon autograft: clinical outcome in 4–7 years. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1077–1085. doi: 10.1007/s00167-006-0111-0. [DOI] [PubMed] [Google Scholar]
- 6.Clatworthy MG, Annear P, Bulow JU, et al. Tunnel widening in anterior cruciate ligament reconstruction: a prospective evaluation of hamstring and patella tendon grafts. Knee Surg Sports Traumatol Arthrosc. 1999;7(3):138–145. doi: 10.1007/s001670050138. [DOI] [PubMed] [Google Scholar]
- 7.Cooley VJ, Deffner KT, Rosenberg TD. Quadrupled semitendinosus anterior cruciate ligament reconstruction: 5-year results in patients without meniscus loss. Arthroscopy. 2001;17(8):795–800. doi: 10.1016/s0749-8063(01)90001-5. [DOI] [PubMed] [Google Scholar]
- 8.DeAngelis JP, Fulkerson JP. Quadriceps tendon: a reliable alternative for reconstruction of the anterior cruciate ligament. Clin Sports Med. 2007;26(4):587–596. doi: 10.1016/j.csm.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Feller JA, Webster KE, Gavin B. Early post-operative morbidity following anterior cruciate ligament reconstruction: patellar tendon versus hamstring graft. Knee Surg Sports Traumatol Arthrosc. 2001;9(5):260–266. doi: 10.1007/s001670100216. [DOI] [PubMed] [Google Scholar]
- 10.Fox RJ, Harner CD, Sakane M, et al. Determination of the in situ forces in the human posterior cruciate ligament using robotic technology: a cadaveric study. Am J Sports Med. 1998;26(3):395–401. doi: 10.1177/03635465980260030901. [DOI] [PubMed] [Google Scholar]
- 11.Frank C, Amiel D, Woo SLY, et al. Normal ligament properties and ligament healing. Clin Orthop Relat Res. 1985;196:15–25. [PubMed] [Google Scholar]
- 12.Fujie H, Livesay GA, Woo SLY, et al. The use of a universal force-moment sensor to determine in-situ forces in ligaments: a new methodology. J Biomech Eng. 1995;117(1):1–7. doi: 10.1115/1.2792266. [DOI] [PubMed] [Google Scholar]
- 13.Fulkerson JP, Langeland R. An alternative cruciate reconstruction graft: the central quadriceps tendon. Arthroscopy. 1995;11(2):252–254. doi: 10.1016/0749-8063(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 14.Gadikota HR, Seon JK, Kozanek M, et al. Biomechanical comparison of single-tunnel-double-bundle and single-bundle anterior cruciate ligament reconstructions. Am J Sports Med. 2009;37(5):962–969. doi: 10.1177/0363546508330145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geib TM, Shelton WR, Phelps RA, et al. Anterior cruciate ligament reconstruction using quadriceps tendon autograft: intermediate-term outcome. Arthroscopy. 2009;25(12):1408–1414. doi: 10.1016/j.arthro.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Gerhard P, Bolt R, Duck K, et al. Long-term results of arthroscopically assisted anatomical single-bundle anterior cruciate ligament reconstruction using patellar tendon autograft: are there any predictors for the development of osteoarthritis? Knee Surg Sports Traumatol Arthrosc. 2013;21:957–964. doi: 10.1007/s00167-012-2001-y. [DOI] [PubMed] [Google Scholar]
- 17.Gorschewsky O, Klakow A, Putz A, et al. Clinical comparison of the autologous quadriceps tendon (BQT) and the autologous patella tendon (BPTB) for the reconstruction of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2007;15(11):1284–1292. doi: 10.1007/s00167-007-0371-3. [DOI] [PubMed] [Google Scholar]
- 18.Harris NL, Smith DA, Lamoreaux L, et al. Central quadriceps tendon for anterior cruciate ligament reconstruction, part I: morphometric and biomechanical evaluation. Am J Sports Med. 1997;25(1):23–28. doi: 10.1177/036354659702500105. [DOI] [PubMed] [Google Scholar]
- 19.Hoher J, Kanamori A, Zeminski J, et al. The position of the tibia during graft fixation affects knee kinematics and graft forces for anterior cruciate ligament reconstruction. Am J Sports Med. 2001;29(6):771–776. doi: 10.1177/03635465010290061601. [DOI] [PubMed] [Google Scholar]
- 20.Hollis JM, Takai S, Adams DJ, et al. The effects of knee motion and external loading on the length of the anterior cruciate ligament (ACL): a kinematic study. J Biomech Eng. 1991;113(2):208–214. doi: 10.1115/1.2891236. [DOI] [PubMed] [Google Scholar]
- 21.Howe JG, Johnson RJ, Kaplan MJ, et al. Anterior cruciate ligament reconstruction using quadriceps patellar tendon graft, part I: long-term followup. Am J Sports Med. 1991;19(5):447–457. doi: 10.1177/036354659101900505. [DOI] [PubMed] [Google Scholar]
- 22.Jackson DW, Gasser SI. Tibial tunnel placement in ACL reconstruction. Arthroscopy. 1994;10(2):124–131. doi: 10.1016/s0749-8063(05)80079-9. [DOI] [PubMed] [Google Scholar]
- 23.Jarvela T, Kannus P, Jarvinen M. Anterior knee pain 7 years after an anterior cruciate ligament reconstruction with a bone-patellar tendon-bone autograft. Scand J Med Sci Sports. 2000;10(4):221–227. doi: 10.1034/j.1600-0838.2000.010004221.x. [DOI] [PubMed] [Google Scholar]
- 24.Jones KG. Reconstruction of the anterior cruciate ligament: a technique using the central 1/3 of the patellar ligament. J Bone Joint Surg Am. 1963;45(5):925–932. [PubMed] [Google Scholar]
- 25.Joseph M, Fulkerson J, Nissen C, et al. Short-term recovery after anterior cruciate ligament reconstruction: a prospective comparison of three autografts. Orthopedics. 2006;29(3):243–248. doi: 10.3928/01477447-20060301-14. [DOI] [PubMed] [Google Scholar]
- 26.Kanamori A, Zeminski J, Rudy TW, et al. The effect of axial tibial torque on the function of the anterior cruciate ligament: a biomechanical study of a simulated pivot shift test. Arthroscopy. 2002;18(4):394–398. doi: 10.1053/jars.2002.30638. [DOI] [PubMed] [Google Scholar]
- 27.Kartus J, Magnusson L, Stener S, et al. Complications following arthroscopic anterior cruciate ligament reconstruction: a 2–5-year follow-up of 604 patients with special emphasis on anterior knee pain. Knee Surg Sports Traumatol Arthrosc. 1999;7(1):2–8. doi: 10.1007/s001670050112. [DOI] [PubMed] [Google Scholar]
- 28.Kim SJ, Kumar P, Oh KS. Anterior cruciate ligament reconstruction: autogenous quadriceps tendon-bone compared with bone-patellar tendon-bone grafts at 2-year follow-up. Arthroscopy. 2009;25(2):137–144. doi: 10.1016/j.arthro.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Laxdal G, Kartus J, Hansson L, et al. A prospective randomized comparison of bone-patellar tendon-bone and hamstring grafts for anterior cruciate ligament reconstruction. Arthroscopy. 2005;21(1):34–42. doi: 10.1016/j.arthro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Lee S, Seong SC, Jo H, et al. Outcome of anterior cruciate ligament reconstruction using quadriceps tendon autograft. Arthroscopy. 2004;20(8):795–802. doi: 10.1016/j.arthro.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Li GA, Rudy TW, Allen C, et al. Effect of combined axial compressive and anterior tibial loads on in situ forces in the anterior cruciate ligament: a porcine study. J Orthop Res. 1998;16(1):122–127. doi: 10.1002/jor.1100160121. [DOI] [PubMed] [Google Scholar]
- 32.Lippe J, Armstrong A, Fulkerson JP. Anatomic guidelines for harvesting a quadriceps free tendon autograft for anterior cruciate ligament reconstruction. Arthroscopy. 2012;28(7):980–984. doi: 10.1016/j.arthro.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Livesay GA, Fujie H, Kashiwaguchi S, et al. Determination of the insitu forces and force distribution within the human anterior cruciate ligament. Ann Biomed Eng. 1995;23(4):467–474. doi: 10.1007/BF02584446. [DOI] [PubMed] [Google Scholar]
- 34.Loh JC, Fukuda Y, Tsuda E, et al. Knee stability and graft function following anterior cruciate ligament reconstruction: comparison between 11 o’clock and 10 o’clock femoral tunnel placement. 2002 Richard O’Connor Award Paper. Arthroscopy. 2003;19(3):297–304. doi: 10.1053/jars.2003.50084. [DOI] [PubMed] [Google Scholar]
- 35.Marshall JL, Warren RF, Wickiewicz TL, et al. Anterior cruciate ligament: technique of repair and reconstruction. Clin Orthop Relat Res. 1979;143:97–106. [PubMed] [Google Scholar]
- 36.Moon DK, Woo SLY, Takakura Y, et al. The effects of refreezing on the viscoelastic and tensile properties of ligaments. J Biomech. 2006;39(6):1153–1157. doi: 10.1016/j.jbiomech.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 37.Myers CA, Torry MR, Shelburne KB, et al. In vivo tibiofemoral kinematics during 4 functional tasks of increasing demand using biplane fluoroscopy. Am J Sports Med. 2012;40(1):170–178. doi: 10.1177/0363546511423746. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura N, Horibe S, Sasaki S, et al. Evaluation of active knee flexion and hamstring strength after anterior cruciate ligament reconstruction using hamstring tendons. Arthroscopy. 2002;18(6):598–602. doi: 10.1053/jars.2002.32868. [DOI] [PubMed] [Google Scholar]
- 39.Noyes FR, Butler DL, Grood ES, et al. Biomechanical analysis of human ligament grafts used in knee-ligament repairs and reconstructions. J Bone Joint Surg Am. 1984;66(3):344–352. [PubMed] [Google Scholar]
- 40.Papageorgiou CD, Ma CB, Abramowitch SD, et al. A multidisciplinary study of the healing of an intraarticular anterior cruciate ligament graft in a goat model. Am J Sports Med. 2001;29(5):620–626. doi: 10.1177/03635465010290051501. [DOI] [PubMed] [Google Scholar]
- 41.Rudy TW, Livesay GA, Woo SLY, et al. A combined robotic/universal force sensor approach to determine in situ forces of knee ligaments. J Biomech. 1996;29(10):1357–1360. doi: 10.1016/0021-9290(96)00056-5. [DOI] [PubMed] [Google Scholar]
- 42.Sakane M, Fox RJ, Woo SLY, et al. In situ forces in the anterior cruciate ligament and its bundles in response to anterior tibial loads. J Orthop Res. 1997;15(2):285–293. doi: 10.1002/jor.1100150219. [DOI] [PubMed] [Google Scholar]
- 43.Sakane M, Livesay GA, Fox RJ, et al. Relative contribution of the ACL, MCL, and bony contact to the anterior stability of the knee. Knee Surg Sports Traumatol Arthrosc. 1999;7(2):93–97. doi: 10.1007/s001670050128. [DOI] [PubMed] [Google Scholar]
- 44.Salmon LJ, Russell VJ, Refshauge K, et al. Long-term outcome of endoscopic anterior cruciate ligament reconstruction with patellar tendon autograft: minimum 13-year review. Am J Sports Med. 2006;34(5):721–732. doi: 10.1177/0363546505282626. [DOI] [PubMed] [Google Scholar]
- 45.Segawa H, Omori G, Tomita S, et al. Bone tunnel enlargement after anterior cruciate ligament reconstruction using hamstring tendons. Knee Surg Sports Traumatol Arthrosc. 2001;9(4):206–210. doi: 10.1007/s001670100201. [DOI] [PubMed] [Google Scholar]
- 46.Seon JK, Gadikota HR, Wu JL, et al. Comparison of single- and double-bundle anterior cruciate ligament reconstructions in restoration of knee kinematics and anterior cruciate ligament forces. Am J Sports Med. 2010;38(7):1359–1367. doi: 10.1177/0363546510361494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith BA, Livesay GA, Woo SLY. Biology and biomechanics of the anterior cruciate ligament. Clin Sports Med. 1993;12(4):637–670. [PubMed] [Google Scholar]
- 48.Staubli HU, Jakob RP. Central quadriceps tendon for anterior cruciate ligament reconstruction, part I: morphometric and biochemical evaluation. Am J Sports Med. 1997;25(5):725–727. [PubMed] [Google Scholar]
- 49.Staubli HU, Schatzmann L, Brunner P, et al. Quadriceps tendon and patellar ligament: cryosectional anatomy and structural properties in young adults. Knee Surg Sports Traumatol Arthrosc. 1996;4(2):100–110. doi: 10.1007/BF01477262. [DOI] [PubMed] [Google Scholar]
- 50.Torry MR, Shelburne KB, Peterson DS, et al. Knee kinematic profiles during drop landings: a biplane fluoroscopy study. Med Sci Sports Exerc. 2011;43(3):533–541. doi: 10.1249/MSS.0b013e3181f1e491. [DOI] [PubMed] [Google Scholar]
- 51.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63(3):269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson TW, Zafuta MP, Zobitz M. A biomechanical analysis of matched bone-patellar tendon-bone and double-looped semitendinosus and gracilis tendon grafts. Am J Sports Med. 1999;27(2):202–207. doi: 10.1177/03635465990270021501. [DOI] [PubMed] [Google Scholar]
- 53.Woo SLY, Abramowitch SD, Kilger R, et al. Biomechanics of knee ligaments: injury, healing, and repair. J Biomech. 2006;39(1):1–20. doi: 10.1016/j.jbiomech.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 54.Woo SLY, Hollis JM, Adams DJ, et al. Tensile properties of the human femur-anterior cruciate ligament-tibia complex: the effects of specimen age and orientation. Am J Sports Med. 1991;19(3):217–225. doi: 10.1177/036354659101900303. [DOI] [PubMed] [Google Scholar]
- 55.Woo SLY, Kanamori A, Zeminski J, et al. The effectiveness of reconstruction of the anterior cruciate ligament with hamstrings and patellar tendon: a cadaveric study comparing anterior tibial and rotational loads. J Bone Joint Surg Am. 2002;84(6):907–914. doi: 10.2106/00004623-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 56.Woo SLY, Orlando CA, Camp JF, et al. Effects of postmortem storage by freezing on ligament tensile behavior. J Biomech. 1986;19(5):399–404. doi: 10.1016/0021-9290(86)90016-3. [DOI] [PubMed] [Google Scholar]
- 57.Yagi M, Wong EK, Kanamori A, et al. Biomechanical analysis of an anatomic anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30(5):660–666. doi: 10.1177/03635465020300050501. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto Y, Hsu WH, Woo SLY, et al. Knee stability and graft function after anterior cruciate ligament reconstruction: a comparison of a lateral and an anatomical femoral tunnel placement. Am J Sports Med. 2004;32(8):1825–1832. doi: 10.1177/0363546504263947. [DOI] [PubMed] [Google Scholar]
- 59.Yunes M, Richmond JC, Engels EA, et al. Patellar versus hamstring tendons in anterior cruciate ligament reconstruction: a meta-analysis. Arthroscopy. 2001;17(3):248–257. doi: 10.1053/jars.2001.21242. [DOI] [PubMed] [Google Scholar]
- 60.Zamarra G, Fisher MB, Woo SLY, et al. Biomechanical evaluation of using one hamstrings tendon for ACL reconstruction: a human cadaveric study. Knee Surg Sports Traumatol Arthrosc. 2010;18(1):11–19. doi: 10.1007/s00167-009-0911-0. [DOI] [PubMed] [Google Scholar]