Abstract
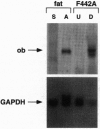
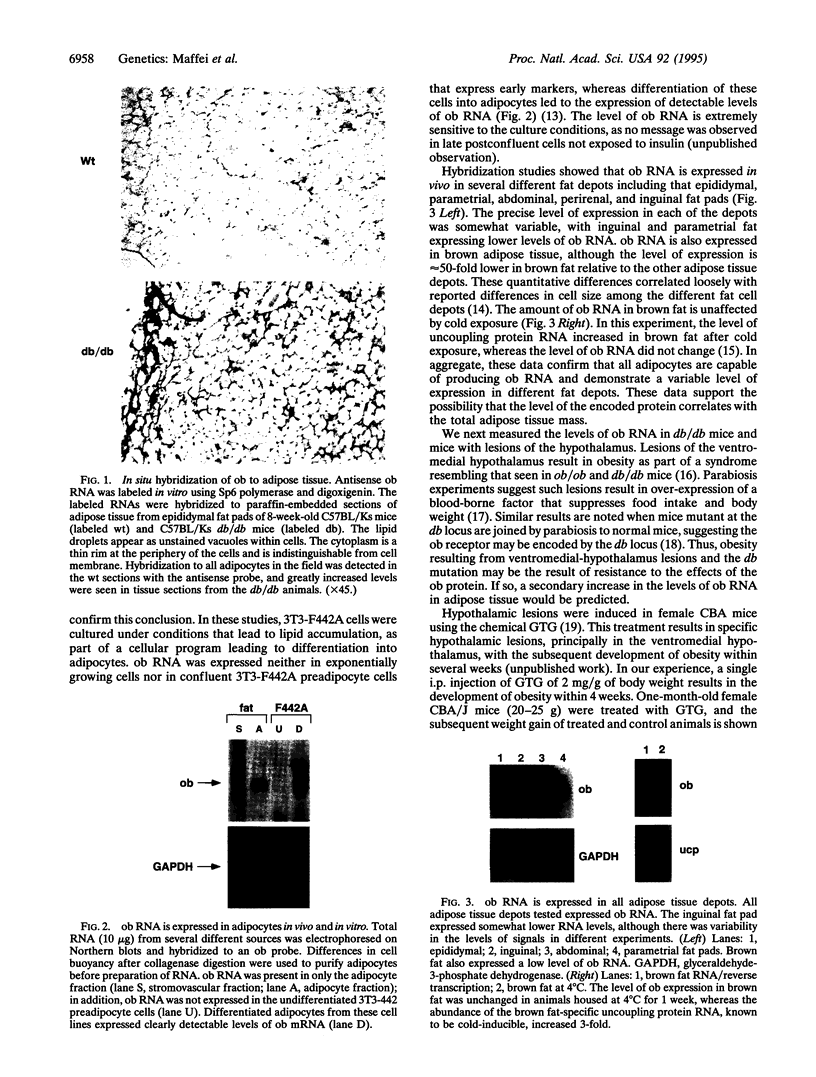
The gene product of the recently cloned mouse obese gene (ob) is important in regulating adipose tissue mass. ob RNA is expressed specifically by mouse adipocytes in vivo in each of several different fat cell depots, including brown fat. ob RNA is also expressed in cultured 3T3-442A preadipocyte cells that have been induced to differentiate. Mice with lesions of the hypothalamus, as well as mice mutant at the db locus, express a 20-fold higher level of ob RNA in adipose tissue. These data suggest that both the db gene and the hypothalamus are downstream of the ob gene in the pathway that regulates adipose tissue mass and are consistent with previous experiments suggesting that the db locus encodes the ob receptor. In db/db and lesioned mice, quantitative differences in expression level of ob RNA correlated with adipocyte lipid content. The molecules that regulate expression level of the ob gene in adipocytes probably are important in determining body weight, as are the molecules that mediate the effects of ob at its site of action.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell M., Meade C. J., Medawar P., Sowter C. Adipose tissue: contributions of nature and nurture to the obesity of an obese mutant mouse (ob/ob). Proc R Soc Lond B Biol Sci. 1977 Jan 14;195(1120):343–353. doi: 10.1098/rspb.1977.0014. [DOI] [PubMed] [Google Scholar]
- Ashwell M., Meade C. J. Obesity: do fat cells from genetically obese mice (C57BL/6J ob/ob) have an innate capacity for increased fat storage? Diabetologia. 1978 Dec;15(6):465–470. doi: 10.1007/BF02342871. [DOI] [PubMed] [Google Scholar]
- Bahary N., Leibel R. L., Joseph L., Friedman J. M. Molecular mapping of the mouse db mutation. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8642–8646. doi: 10.1073/pnas.87.21.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baura G. D., Foster D. M., Porte D., Jr, Kahn S. E., Bergman R. N., Cobelli C., Schwartz M. W. Saturable transport of insulin from plasma into the central nervous system of dogs in vivo. A mechanism for regulated insulin delivery to the brain. J Clin Invest. 1993 Oct;92(4):1824–1830. doi: 10.1172/JCI116773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray G. A., Campfield L. A. Metabolic factors in the control of energy stores. Metabolism. 1975 Jan;24(1):99–117. doi: 10.1016/0026-0495(75)90011-6. [DOI] [PubMed] [Google Scholar]
- Coleman D. L. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978 Mar;14(3):141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- Dani C., Bertrand B., Bardon S., Doglio A., Amri E., Grimaldi P. Regulation of gene expression by insulin in adipose cells: opposite effects on adipsin and glycerophosphate dehydrogenase genes. Mol Cell Endocrinol. 1989 May;63(1-2):199–208. doi: 10.1016/0303-7207(89)90096-8. [DOI] [PubMed] [Google Scholar]
- Dani C., Doglio A., Amri E. Z., Bardon S., Fort P., Bertrand B., Grimaldi P., Ailhaud G. Cloning and regulation of a mRNA specifically expressed in the preadipose state. J Biol Chem. 1989 Jun 15;264(17):10119–10125. [PubMed] [Google Scholar]
- Debons A. F., Krimsky I., Maayan M. L., Fani K., Jemenez F. A. Gold thioglucose obesity syndrome. Fed Proc. 1977 Feb;36(2):143–147. [PubMed] [Google Scholar]
- Faust I. M., Johnson P. R., Hirsch J. Surgical removal of adipose tissue alters feeding behavior and the development of obesity in rats. Science. 1977 Jul 22;197(4301):393–396. doi: 10.1126/science.877564. [DOI] [PubMed] [Google Scholar]
- Faust I. M., Johnson P. R., Stern J. S., Hirsch J. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am J Physiol. 1978 Sep;235(3):E279–E286. doi: 10.1152/ajpendo.1978.235.3.E279. [DOI] [PubMed] [Google Scholar]
- HERVEY G. R. The effects of lesions in the hypothalamus in parabiotic rats. J Physiol. 1959 Mar 3;145(2):336–352. doi: 10.1113/jphysiol.1959.sp006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson A., Stadler U., Glotzer M. A., Kozak L. P. Mitochondrial uncoupling protein from mouse brown fat. Molecular cloning, genetic mapping, and mRNA expression. J Biol Chem. 1985 Dec 25;260(30):16250–16254. [PubMed] [Google Scholar]
- Johnson A. K., Gross P. M. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993 May;7(8):678–686. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- Johnson P. R., Hirsch J. Cellularity of adipose depots in six strains of genetically obese mice. J Lipid Res. 1972 Jan;13(1):2–11. [PubMed] [Google Scholar]
- Liebelt R. A., Nicholson N., Ichinoe S. Regulatory influences of adipose tissue on food intake and body weight. Ann N Y Acad Sci. 1965 Oct 8;131(1):559–582. doi: 10.1111/j.1749-6632.1965.tb34820.x. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. Receptor-mediated peptide transport through the blood-brain barrier. Endocr Rev. 1986 Aug;7(3):314–330. doi: 10.1210/edrv-7-3-314. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Richardson R. L., Wright J. T., Kim J. W., Hausman G. J. Expression of transforming growth factor-beta (TGF-beta 1) and insulin-like growth factor II (IGF-II) messenger RNA in the developing subcutaneous tissue (SQ) of the fetal pig. Growth Dev Aging. 1992 Fall;56(3):149–157. [PubMed] [Google Scholar]
- Schaeren-Wiemers N., Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry. 1993 Dec;100(6):431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994 Dec 1;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]