Abstract
Systemic and gastrointestinal infection can be established in infant mice after intragastric challenge with Candida albicans. Differences in virulence of the six strains tested were noted. As early as 3 h after infection, some but not all livers, spleens, and kidneys contained C. albicans, but the peak number of colony-forming units in these organs was seen at 6 h. The early colonization of the organs could not be attributed to aspiration of the inoculum since about 90% of lungs and livers tested yielded no colony-forming units at 10 to 15 min postinfection. In animals with systemic infections, lungs, livers, kidneys, and spleens showed similar numbers of colony-forming units within the organs during the first 6 h postinfection- and then the number declined progressively up to 72 h. The gastrointestinal tract was colonized throughout a 20-day period of study. Counts made at intervals beyond day 1 yielded between 10(5) and 10(6) colony-forming units in the stomach, ileum, and cecum. Preparatory techniques for scanning electron microscopy preserved the yeast, intestinal mucus layer, and epithelial surface and made it possible to visualize the association between the pathogen and host tissues within the digestive tract.
Full text
PDF


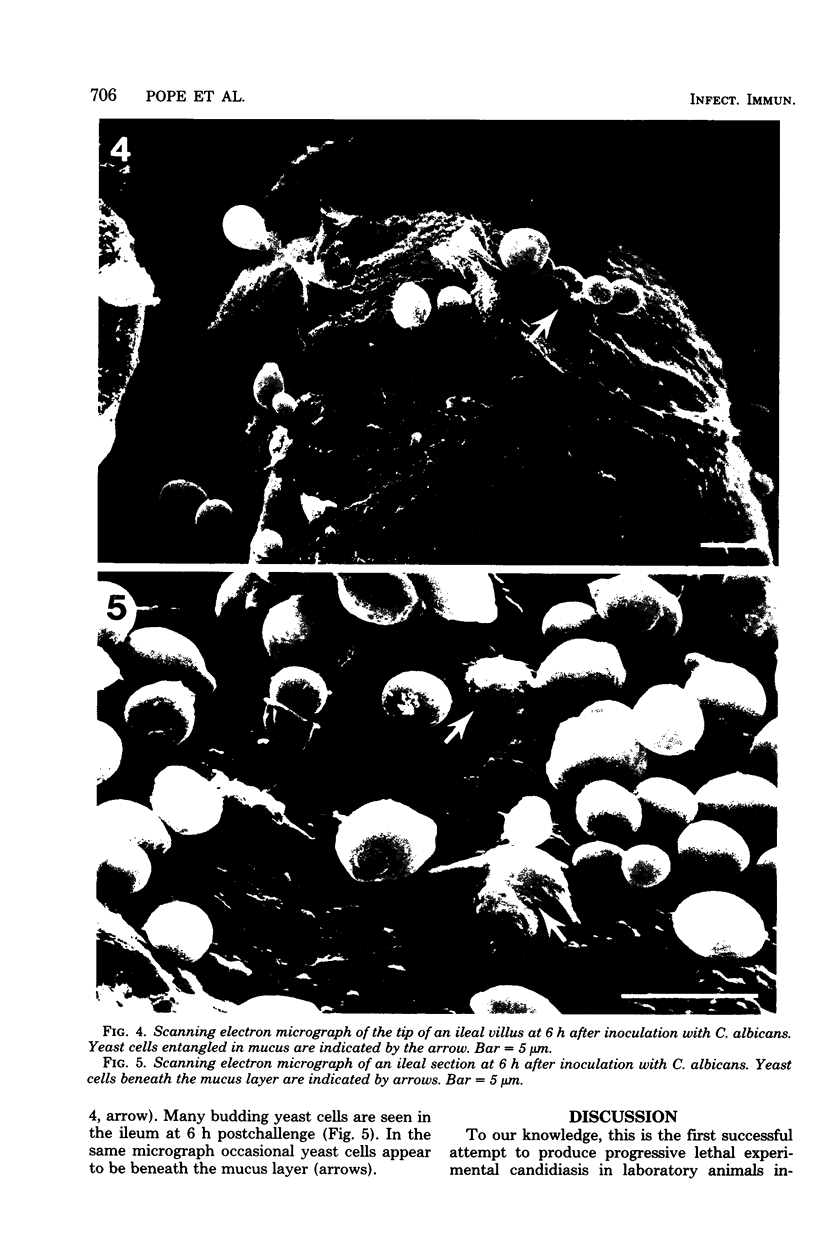

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baselski V. S., Parker C. D. Intestinal distribution of Vibrio cholerae in orally infected infant mice: kinetics of recovery of radiolabel and viable cells. Infect Immun. 1978 Aug;21(2):518–525. doi: 10.1128/iai.21.2.518-525.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselski V., Briggs R., Parker C. Intestinal fluid accumulation induced by oral challenge with Vibrio cholerae or cholera toxin in infant mice. Infect Immun. 1977 Mar;15(3):704–712. doi: 10.1128/iai.15.3.704-712.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON J. M. The fate of bacteria in the small intestine. J Pathol Bacteriol. 1960 Jan;79:131–140. doi: 10.1002/path.1700790116. [DOI] [PubMed] [Google Scholar]
- Davis C. P. Preservation of gastrointestinal bacteria and their microenvironmental associations in rats by freezing. Appl Environ Microbiol. 1976 Feb;31(2):304–312. doi: 10.1128/aem.31.2.304-312.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaria A., Buckley H., von Lichtenberg F. Gastrointestinal candidiasis in rats treated with antibiotics, cortisone, and azathioprine. Infect Immun. 1976 Jun;13(6):1761–1770. doi: 10.1128/iai.13.6.1761-1770.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eras P., Goldstein M. J., Sherlock P. Candida infection of the gastrointestinal tract. Medicine (Baltimore) 1972 Sep;51(5):367–379. doi: 10.1097/00005792-197209000-00002. [DOI] [PubMed] [Google Scholar]
- Guentzel M. N., Berry L. J. Motility as a virulence factor for Vibrio cholerae. Infect Immun. 1975 May;11(5):890–897. doi: 10.1128/iai.11.5.890-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentzel M. N., Field L. H., Eubanks E. R., Berry L. J. Use of fluorescent antibody in studies of immunity to cholera in infant mice. Infect Immun. 1977 Feb;15(2):539–548. doi: 10.1128/iai.15.2.539-548.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges D. J. Enteric pathogen--normal flora interactions. Am J Clin Nutr. 1970 Nov;23(11):1451–1456. doi: 10.1093/ajcn/23.11.1451. [DOI] [PubMed] [Google Scholar]
- Koupal L. R., Deibel R. H. Assay, characterization, and localization of an enterotoxin produced by Salmonella. Infect Immun. 1975 Jan;11(1):14–22. doi: 10.1128/iai.11.1.14-22.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause W., Matheis H., Wulf K. Fungaemia and funguria after oral administration of Candida albicans. Lancet. 1969 Mar 22;1(7595):598–599. doi: 10.1016/s0140-6736(69)91534-7. [DOI] [PubMed] [Google Scholar]
- Nishikawa T., Hatano H., Ohnishi N., Sasaki S., Nomura T. Establishment of Candida albicans in the alimentary tract of the germ-free mice and antagonism with Escherichia coli after oral inoculation. Jpn J Microbiol. 1969 Sep;13(3):263–276. doi: 10.1111/j.1348-0421.1969.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Nolting S., Fegeler K., Koralewski F., Ludwig G. Einfluss von Antibiotika und Cytostatika auf die experimentelle Candidose der Maus. Mykosen. 1975 Jul;18(7):309–313. [PubMed] [Google Scholar]
- Phillips A. W., Balish E. Growth and invasiveness of Candida albicans in the germ-free and conventional mouse after oral challenge. Appl Microbiol. 1966 Sep;14(5):737–741. doi: 10.1128/am.14.5.737-741.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington J. S., Anderson S. E., Jr Pneumocystis and fungal infection in patients with malignancies. Int J Radiat Oncol Biol Phys. 1976 Jan-Feb;1(3-4):313–315. doi: 10.1016/0360-3016(76)90059-6. [DOI] [PubMed] [Google Scholar]
- Turner J. R., Butler T. F., Johnson M. E., Gordee R. S. Colonization of the intestinal tract of conventional mice with Candida albicans and treatment with antifungal agents. Antimicrob Agents Chemother. 1976 May;9(5):787–792. doi: 10.1128/aac.9.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umenai T. Systemic candidiasis produced by oral Candida administration in mice. Tohoku J Exp Med. 1978 Oct;126(2):173–175. doi: 10.1620/tjem.126.173. [DOI] [PubMed] [Google Scholar]
- Walsh J. H., Yalow R., Berson S. A. Detection of Australia antigen and antibody by means of radioimmunoassay techniques. J Infect Dis. 1970 May;121(5):550–554. doi: 10.1093/infdis/121.5.550. [DOI] [PubMed] [Google Scholar]