Abstract
Objective
To evaluate cost-effectiveness and cost utilities for treatment options for vitreomacular adhesions (VMA) and full thickness macular holes (MH).
Design
A Markov model of cost-effectiveness and utility.
Participants
There were no participants.
Methods
Outcomes of published clinical trials (index studies) of surgical treatment of VMA and MH, and a prospective, multicenter clinical trial of pharmaceutical vitreolysis with intravitreal ocriplasmin with saline control were used to generate a model for costs of treatment and visual benefits. All techniques were assumed to result in a 2.5 line visual benefit if anatomy was resolved. Markov analysis, with cost data from the Center of Medicare and Medicaid Services (CMS), was used to calculate imputed costs for each primary treatment modality in a facility setting with surgery performed in a hospital serving as the highest end of the range and non-facility setting with surgery performed in an ambulatory surgery center (ASC) serving as the lowest end of the range.
Main Outcome Measures
Imputed costs of therapy, cost per line saved, cost per line-year saved, cost per quality-adjusted life years (QALY).
Results
When PPV was selected as the primary procedure, the overall imputed cost ranged from $5,802-$7,931. The cost per line was $2,368-$3,237, the cost per line-year saved was $163-$233 and the cost per QALY was $5,444-$7,442. If intravitreal injection of ocriplasmin (IVO) was the primary procedure, the overall imputed cost was $8,767-$10,977. The cost per line ranged from $3,549-$4,456, the cost per line-year saved was $245-$307, and the cost per QALY was between $8,159-$10,244. If intravitreal saline injection (IVS) were used as a primary procedure, the overall imputed cost was $5,828-8,098. The cost per line was $2,374-3,299, the cost per line-year saved was $164-227 and the cost per QALY was $5,458-7,583.
Conclusions
PPV as a primary procedure was the most cost-effective therapy in this model. The other treatments had similar costs per QALY saved, and compare favorably to costs of therapy for other retinal diseases.
The role of persistent, progressive vitreomacular attachment (VMA) at the macula was most clearly defined clinically as a pathogenic step in macular hole (MH) formation.1-3 More subtle forms of VMA have been widely described and even categorized as its own entity distinct from MH as optical coherence tomography (OCT) has increased its detection.4,5
Pars plana vitrectomy (PPV) has been the gold standard of treatment for MH over the past twenty years.6,7 Treatment is highly effective with overall success rates reported in the range of 80-90% after a single surgery.8-14 The success rate in the earliest stage, smallest, most recent cases has been reported in excess of 90%.9-14 While some debates in the literature remain regarding the type of gas tamponade used,12-14 the necessity of peeling the internal limiting membrane (ILM),9-10,15-16 and the duration of positioning following surgery,11,17 there is widespread agreement that the procedure is effective.
Treatment of VMA without MH has presented more of a treatment quandary. VMA may progress to MH formation, it may resolve with spontaneous posterior vitreous detachment (PVD) and improved visual acuity, or it may remain dormant.18-20 There are no reliable predictors of its course, hence severity and progressive traction have factored most importantly into clinical decision making paradigms prompting intervention. Thus, eyes with moderately symptomatic VMA that fail to improve within a period of observation, or demonstrate progression of the traction effects, are commonly recommended for pars plana vitrectomy (PPV)-hitherto the sole therapeutic option.21-23
Data have recently been presented to suggest the benefit of an intravitreal injection of ocriplasmin (IVO) in patients with VMA, defined as vitreous adhesion to the macula within a 6-mm central retinal field surrounded by elevation of the posterior vitreous cortex on OCT, with or without MH less than 400 microns in diameter.24 The Microplasmin for Intravitreous Injection – Traction Release Without Surgical Treatment (MIVI-TRUST) study demonstrated that in these patients, adhesion was relieved at a rate of 26.5-40.6% thereby avoiding surgery in these patients.24 This treatment option, albeit carrying a lower success rate than vitrectomy, may provide an alternative for patients who have overriding travel needs that preclude a gas injection, difficulties with surgery and the post-operative management such as positioning, or in patients who would have a significant benefit from avoiding cataract surgery. Furthermore, its relatively lower invasiveness (compared to PPV) might prompt expanded treatment indications to patients with lesser degrees of symptoms or VMA.
Implicit in these considerations, of course, is that while an in-office injection might be very attractive compared to PPV, its lower success rate and relatively high cost per dose might diminish the overall cost-effectiveness.
The purpose of this report is to compare parameters of cost-effectiveness and cost-utility using a Markov decision-tree analysis for PPV, pharmacologic intervention with intravitreal ocriplasmin (IVO), and pharmacologic intervention with intravitreal saline (IVS) - the control group used in the MIVI-TRUST study.
Methods
Success rates for the treatment of VMA and MH were derived from index studies that evaluated pharmacologic24 and surgical treatment.6-17 For anatomic success, previous reports suggest that the closure rates for small MH are at least 90% with single surgery.8-14 Outcomes for IVO were derived from the MIVI-TRUST study - VMA was relieved with a success rate of 26.5% and the MH closure rate was 40.6%.24 An assumption of 2.5 lines saved (i.e. regained and prevented loss) was made for both PPV and IVO successes. These estimates might be lower than actual considering that many treated patients would likely have lost additional VA if left untreated, but a principle of the methodology of the current study model was to err on the side of underestimating utility. The MIVI-TRUST study did not directly report VA improvement as an outcome measure.24 The post hoc subgroup analysis showed that patients with VA < 20/50 gained 3 lines of vision and those with better vision gained fewer lines and so the 2.5 lines estimate is consistent with these data.24 Studies of MH repair typically include larger holes but do not describe VA outcomes for subgroups with smaller MHs, but those patients would probably have lost additional VA if not treated (hence the input of “lines saved”).
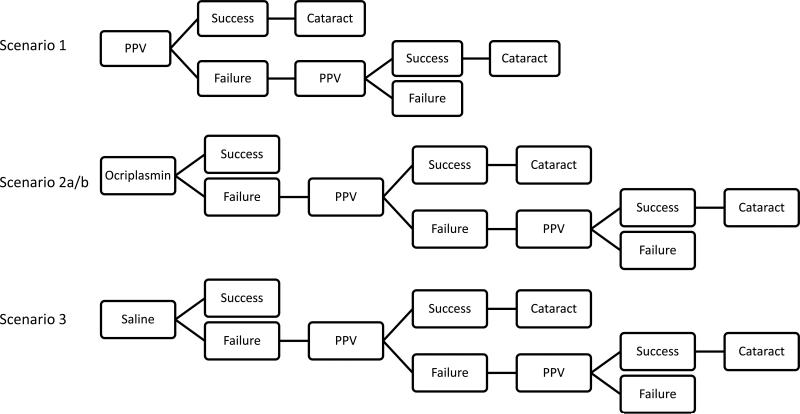
Medicare fee data were acquired from the Centers of Medicare and Medicaid Services (CMS) for the number of relative value units (RVU) and cost in United States (US) dollars associated with each surgical procedure, injection, imaging study or office visit.25-29 Calculations were made for a facility practice in which surgery was done in a hospital operating room and a non-facility practice in which surgery was done in an ambulatory surgical center (ASC) in the geographic area of Miami, Florida. These two practice care settings constituted a high and low end estimate of costs. Professional fees and facility fees, where applicable, were included in the calculations. The current rate of $34.023 US dollars per RVU was applied to calculate CMS reimbursements. Four different clinical scenarios were then reviewed using a Markov decision analysis30 based on these index studies (Figure 1). The Markov-style analysis was selected for the ability to represent transitions between different states.30
Figure 1.
Decision Model Used for Markov Analysis
In scenario 1, the initial treatment was PPV (90% anatomic success rate), with failures treated by an additional PPV. Scenario 2 modeled treatment with IVO, with failures treated with PPV, and subsequent failures treated with second PPV. Scenarios 2a and 2b were calculated using two different initial success rates - one to evaluate the overall study group (26.5% success rate) and the second for small (<400 microns, as considered in the MIVI-TRUST studies) MHs (40.5% success rate). A third group, scenario 3, was a model based on initial treatment with IVS (10.9% success rate), the control group for the MIVI-TRUST trial, with failures treated with PPV. The subsequent failures as in scenarios 2 and 3, were treated with PPV, and repeat PPV for persistent failures. An assumed baseline of 62.9% phakic patients, the MIVI-TRUST study population,24 was used in order to model costs for treating induced cataract formation after 100% of all eyes undergoing PPV. Another assumption was that 2% of patients undergoing PPV would develop a retinal detachment (RD) that would be treated with PPV. Patients who developed RRD were assumed to have no lines saved, and this is reflected in each of the scenarios.
Current procedural terminology (CPT) codes were used to calculate professional fee inputs (including the use of the appropriate modifiers to 70% of the allowable fee for a repeat PPV) (Tables 1 and 2). Professional anesthesia fees were calculated based on a sum of base and time units, multiplied by the conversion factor $25.52. In the case of CPT code 00145, 6 base units and 4 time units (one hour) was used to estimate the professional fee of $255. For CPT code 00142, 4 base units and 2 time units (30 minutes) were used to estimate a total of $153 in anesthesia professional fees.
Table 5.
Cost per Line-Years Saved and Cost per QALY (US $)
| Facility Billing | Non-Facility Billing | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial Procedure | Second Procedure | Lines saved | Mean age | Years remaining | Cost per line | Cost per line-year saved | Cost per QALY | Cost per line | Cost per line-year saved | Cost per QALY |
| PPV | PPV | 2.45 | 71 | 14.5 | 3,237 | 223 | 7,442 | 2,368 | 163 | 5,444 |
| Ocriplasmin (27%) | PPV | 2.46 | 71 | 14.5 | 4,456 | 307 | 10,244 | 3,666 | 253 | 8,429 |
| Ocriplasmin (41%) | PPV | 2.47 | 71 | 14.5 | 4,301 | 297 | 9,887 | 3,549 | 245 | 8,159 |
| Saline | PPV | 2.46 | 71 | 14.5 | 3,299 | 227 | 7,583 | 2,374 | 164 | 5,458 |
QALY = quality adjusted life years, US = United States, PPV = pars plana vitrectomy, Ocriplasmin (27%) = 27% initial success rate with ocriplasmin, Ocriplasmin (41%) = 41% initial success rate with ocriplasmin
The number of clinic visits, procedures and imaging studies used to generate an imputed cost in each given scenario for one year of treatment follow-up care are listed in Table 3. Patients were assumed to receive one level 4 new patient exam and two level 3 follow-up visits. They were also assumed to receive at least two OCT imaging tests. Patients with pharmacologic intervention were assumed to have two additional clinic visits and one more OCT session than those who strictly had surgery, since there was not a global period attached to their follow-up care.
The cost of ocriplasmin is currently listed as $3,950. Saline is not currently marketed for intravitreal injection, but this estimated cost was $100, based on the costs of similarly compounded products, such as intravitreal bevacizumab.31 A drug maintenance cost of 6% of the medication cost was applied to the injection groups. In the case of hospital, facility billing, this cost was considered part of the hospital fee. In the case of non-facility, ASC billing, this cost was considered part of the professional fee because there are no facility charges for injections. All injections were assumed to be done in office, so no ASC or hospital operating room or anesthesia charges were applied in the model.
Microsoft Excel (Microsoft Corporation, Seattle, WA) software was used to perform calculations and analysis. The average age used was the same as that in the MIVI-TRUST trial, 71 years.24 The actuarial tables of the Social Security Administration were used to average the male and female life expectancies to a total of 14.5 years.32 A previously published formula to calculate quality-adjusted life year (QALY) data was used and the conversion of 0.03 QALYs per line-year of vision saved was applied.33
Results
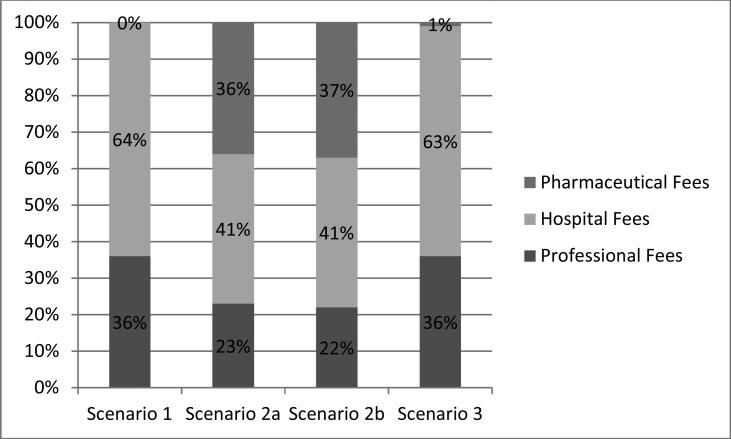
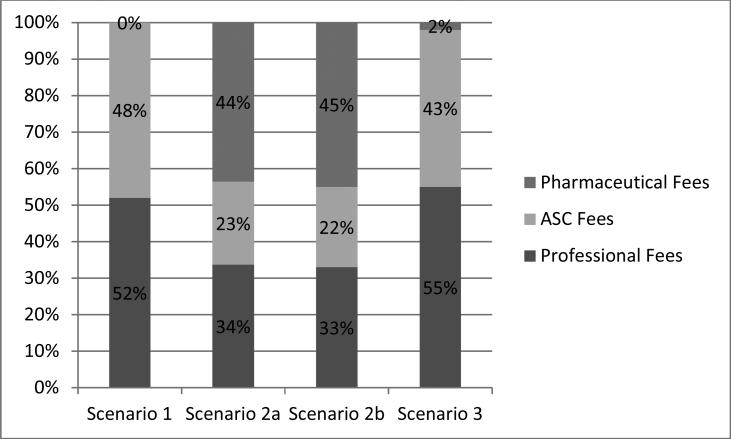
Imputed costs were calculated for each procedure with categorization of fees (Table 4, Figures 2-3) and cost-utility measures (Table 5).
Figure 2.
Distribution of fees based facility billing
Figure 3.
Distribution of fees based on non-facility/ASC billing
Scenario 1: Pars plana vitrectomy
In this scenario, as described in methods, PPV was used as the initial procedure for treatment. For facility billing with hospital surgery, the total imputed cost was $7,931, composed of $2,859 in professional fees (36%) and $5,073 in hospital fees (64%). There were no pharmaceutical fees as no intravitreal injections would be given. The cost per line of vision saved was $3,237. The cost per line-year saved was $223 and the cost per QALY was $7,442. In a non-facility scenario with ASC surgery, the total imputed cost was $5,802, with $3,027 in professional fees (52%) and $2,775 in ASC fees (48%). The cost per line was $2,368, cost per line-year saved was $163 and cost per QALY was $5,444.
Scenario 2: Intravitreal injection of ocriplasmin
Two scenarios were examined based on the overall group and the MH subset of the MIVI-Trust study. In the overall IVO group (scenario 2a) the total imputed cost of facility setting with hospital surgery was $10,977; professional fees were $2,505 (23%), hospital fees were $4,522 (41%) and pharmaceutical fees were $3,950 (36%). The cost per line saved was $4,456, the cost per line-year saved was $307 and the cost per QALY was $10,244.
In the non-facility setting with ASC surgery, the imputed cost was $9,031; professional fees were $3,042 (34%), ASC fees were $2,039 (23%) and pharmaceutical fees were $3,950 (44%). The cost per line, cost per line-year saved and cost per QALY were $3,666, $253 and $8,429, respectively.
The MH subgroup (scenario 2b) first had IVO treatment, followed by PPV if indicated, resulting in an imputed cost of $10,624 for facility setting with hospital surgery. The professional fees were $2,342 (22%), the hospital fees were $4,332 (41%) and the pharmaceutical fee was $3,950 (37%) The cost per line saved was calculated to be $4,301, the cost per line-year saved was $297 and the cost per QALY was $9,887.
For non-facility with ASC surgery, the imputed cost was $8,767 with 33% to professional fees ($2,879), 22% to ASC fees ($1,938) and 45% ($3,950) for pharmaceutical fees. The cost per line was $3,549, cost per line-year was $245 and cost per QALY was $8,159.
Scenario 3: Intravitreal injection of saline
The intravitreal saline control group of the MIVI-TRUST trial, had a 10.9% success rate; failures received PPV, and this model considered a further PPV if the first PPV failed. The imputed cost for this scenario in a facility setting with hospital surgery was $8,098. This included 36% in professional fees ($2,925), 63% in hospital fees ($5,073), and 1% in pharmaceutical fees ($100). The cost per line saved was $3,299, the cost per line-year was $227 and the cost per QALY saved was $7,583.
In the non-facility setting with ASC surgery, the imputed cost of IVS was $5,828, with 55% in professional fees ($3,231), 43% in ASC fees ($2,497) and 2% in pharmaceutical fees ($100). The cost per line, cost per line-year saved and cost per QALY were $2,374, $164 and $5,458, respectively.
Discussion
This study uses Markov modeling of the costs of several scenarios of treatment of VMA or MH. The lowest cost with highest utility was the scenario in which PPV was the initial modality of treatment in both facility and non-facility. This suggests that surgery is still the most cost-effective therapy for patients with VMA regardless of practice or surgical setting.
If the efficacy of IVO was modeled to increase to 71%, then the cost per QALY would be equivalent to that of PPV in the facility setting with hospital surgery. In a non-facility setting with ASC surgery, the efficacy of IVO would have to reach 87% to be equivalent to that of PPV in the same setting. Considered another way, if the initial efficacy rate of IVO was 40.6%, as reported for macular hole closure, then the cost of ocriplasmin would have to be decreased to $1,500 under facility setting with hospital surgery, and $1,300 with non-facility setting with ASC surgery in order for the imputed cost of initial IVO to equal the cost and cost utility of PPV. If the initial efficacy rate of IVO of 26.5% is used, in order to reach the same cost per QALY as PPV, the cost of ocriplasmin would have to be less than $350.
Almost by definition, the various scenarios have very different proportional mixes of where the treatment costs are apportioned. In the non-facility settings, the professional fees and pharmaceutical fees made up a larger percentage of the total costs because facility charges are not associated with injections and clinical visits in this setting. The scenarios with IVO involve a sizeable percentage of overall costs going to the pharmaceutical fees (36% to 45%), which is not present in the PPV scenario. In contrast, the percentage of costs as professional fees (36%-52%) in the primary PPV scenario was greater than that of the primary IVO scenario (22-34%). On the other hand, if one could assume a large pool of patients to treat, a physician could conceivably perform a greater number of injections in the same amount of time as opposed to PPV, so perhaps opportunity cost considerations might make this a more economically attractive option from the physician's perspective.
Cost and utility analyses have been performed on other retina therapies.34-39 The cost per QALY saved of IVO in this model ranged from $8,159 to $10,244. In comparison, the cost per QALY for the treatment of diabetic macular edema (DME) with anti-vascular endothelial growth factor (VEGF) agents was $4,160-$23,119 depending on the agent or protocol used in one study,36 and another Markov analysis of treatment for DME showed a cost per QALY ranging from $11,138-89,903.38 Evaluations of intravitreal injections for retinal vein occlusion (RVO) or age-related macular degeneration (AMD) show similar data.37 In contrast, the cost per QALY of retinal detachment repairs is $554-$2,243.39 From this analysis, IVO is on the lower end of the range of therapy as compared to pharmacologic-based treatment of these other chronic retinal diseases, with the added benefit of being a lifelong durable effect with a one-time therapy. Still, treatment of these conditions have in common the greater share of costs going to pharmaceutical manufacturers and a lower share going to professional and facility fees.
In the broader medical perspective, the cost per QALY considered to be acceptable has been widely published as $50,000-$100,000.33 Both PPV and IVO are well below this acceptable threshold. For example, PPV with membrane peeling for macular pucker was estimated to have a cost per QALY of $5,454, and this cost did not include clinic visits or weighting for additional procedures such as cataract surgery.34 The cost per QALY of VMA treatments falls between laser treatment of retinopathy of prematurity ($1,053/QALY) and pegaptanib therapy in neovascular AMD ($76,217/QALY).34 As compared with other medical conditions, the cost per QALY saved of VMA treatment is in a similar range to that of use of beta blockers for systemic hypertension ($7,389/QALY) and drug maintenance of recurrent depression ($8,449/QALY).34
This model is based on a number of assumptions, one of which is the number of lines of vision saved from therapy. The number of lines saved is on the conservative side, and if a larger number of lines saved were used, such as 5 lines, then all the cost utility values would be decreased (improved) by one-half and all therapies would be well within the $50,000-100,000 range of acceptable cost utility.33
A previous study evaluated the cost-utility of membrane peeling in PPV for MH in Great Britain.40 The authors did not perform a Markov analysis or include clinic visits or cataract formation into the cost per patient. They found that on average, the cost of MH repair was between £2,550-2,974 ($4,105-4,788) when converted for 2011 exchange rates. This cost does not differ greatly from our unadjusted CMS reimbursement of PPV and anesthesia for MH alone, which was $4,957, suggesting there some degree of robustness to our model's assumptions and methodologies.
This model of costs does have some limitations. As in any model, there were assumptions made, including average age, life expectancy, success of treatment options, and lens status. Most of these assumptions were designed to err on the side of being favorable towards the intravitreal injection of ocriplasmin. The dataset is based on a facility practice in Miami, Florida, a relatively high cost sector. Despite this, the relative relationships of the costs do not vary by region and the actual costs do not vary by greater than 10%.39 If anything, the pharmaceutical cost is stable in all regions and it is the CMS reimbursement portions that would be lower in other regions. An observation arm was also not included in this evaluation, and reports suggest that a sizeable, but unpredictable, proportion of a select group of patients with VMA may resolve with observation alone.18 Additional costs associated with surgery as opposed to intravitreal injection were not assigned values, such reduced productivity from missed work, or morbidity from face-down positioning because these are difficult to quantify. The benefits of avoiding an operating room procedure for some patients may make up any difference in cost. Finally, IVO is a relatively new therapy and long-term effects may not be well understood in the way that they are for PPV, which may also affect the cost model presented.
Intravitreal ocriplasmin is a promising medication that has efficacy in some cases – a higher proportion of patients may benefit in selected cases. As a primary treatment for VMA, its lower success rate and relatively high cost confer less cost-effectiveness and less cost-utility than PPV. This type of cost modeling may be useful for evaluation of other ophthalmic and medical conditions.
Table 1.
Medicare-Allowable Charges Per Procedure for Hospital-Based Billing
| Medicare Allowables (US $) | |||||
|---|---|---|---|---|---|
| Procedure | Code | Professional Fees | Hospital Fees | Pharmaceutical Fees | Total |
| New patient visit (Level 4) | 99204 | 145 | 128 | -- | 273 |
| Follow up visit (Level 3) | 99213 | 55 | 74 | -- | 128 |
| PPV for MH | 67042 | 1,788 | 2,914 | -- | 4,702 |
| Intravitreal injection | 67028 | 118 | 232 | -- | 350 |
| OCT macula | 92134 | 50 | 48 | -- | 99 |
| Ocriplasmin | -- | -- | -- | 3,950 | 3,950 |
| Saline | -- | -- | -- | 100 | 100 |
| Maintenance fee ocriplasmin (6% of drug cost) | -- | -- | 237 | -- | 237 |
| Maintenance fee saline (6% of drug cost) | -- | -- | 6 | -- | 6 |
| Phacoemulsification of cataract | 66984 | 769 | 1,730 | -- | 2499 |
| IOL Biometry | 92136 | 98 | 74 | -- | 173 |
| Repair of retinal detachment | 67108 | 1,892 | 2,914 | -- | 4,806 |
| Anesthesia fees for VR surgery | 00145 | -- | 255 | -- | 255 |
| Anesthesia fees for cataract surgery | 00142 | -- | 153 | -- | 153 |
US = United States, PPV = pars plana vitrectomy, MH = macular hole, OCT = optical coherence tomography, IOL = intraocular lens, VR = vitreoretinal
Table 2.
Medicare-Allowable Charges Per Procedure for Non-Facility/ASC Billing
| Medicare Allowables (US $) | |||||
|---|---|---|---|---|---|
| Procedure | Code | Professional Fees | ASC Fees | Pharmaceutical Fees | Total |
| New patient visit (Level 4) | 99204 | 183 | -- | -- | 183 |
| Follow up visit (Level 3) | 99213 | 120 | -- | -- | 120 |
| PPV for MH | 67042 | 1,788 | 1,635 | -- | 3,423 |
| Intravitreal injection | 67028 | 120 | -- | -- | 120 |
| OCT macula | 92134 | 50 | -- | -- | 50 |
| Ocriplasmin | -- | -- | -- | 3,950 | 3,950 |
| Saline | -- | -- | -- | 100 | 100 |
| Maintenance fee ocriplasmin (6% drug cost) | -- | 237 | -- | -- | 237 |
| Maintenance fee saline (6% drug cost) | -- | 6 | -- | -- | 6 |
| Phacoemulsification of cataract | 66984 | 769 | 971 | -- | 1,740 |
| IOL Biometry | 92136 | 98 | -- | -- | 98 |
| Repair of retinal detachment | 67108 | 1,892 | 1,635 | -- | 3,527 |
| Anesthesia fees for VR surgery | 00145 | -- | 255 | -- | 255 |
| Anesthesia fees for cataract surgery | 00142 | -- | 153 | -- | 153 |
US = United States, ASC = ambulatory surgery center, PPV = pars plana vitrectomy, MH = macular hole, OCT = optical coherence tomography, IOL = intraocular lens, VR = vitreoretinal
Table 3.
Estimated Use of Resources Based on Initial Procedure
| Procedure | CPT Code | Scenario 1, PPV | Scenario 2a, ocriplasmin 26.5% | Scenario 2b, ocriplasmin 40.5% | Scenario 3, Saline |
|---|---|---|---|---|---|
| New patient visit, level 4 | 99204 | 1 | 1 | 1 | 1 |
| Follow up visit, level 3 | 99213 | 2 | 4 | 4 | 4 |
| Intravitreal injection | 67028 | 0 | 1 | 1 | 1 |
| PPV | 67042 | 1.1 | 0.81 | 0.65 | 0.99 |
| OCT imaging | 92134 | 2.15 | 3.74 | 3.06 | 3.9 |
| Ocriplasmin drug | 0 | 1 | 1 | 0 | |
| Saline drug | 0 | 0 | 0 | 1 | |
| Drug maintenance fee | 0 | 1 | 1 | 1 | |
| Anesthesia for VR surgery | 00145 | 1.12 | 0.82 | 0.67 | 1.01 |
| Cataract surgery | 66984 | 0.63 | 0.46 | 0.63 | 0.57 |
| IOL Biometry | 92136 | 0.63 | 0.46 | 0.63 | 0.57 |
| Anesthesia for cataract surgery | 00142 | 0.63 | 0.46 | 0.63 | 0.57 |
| RD repair | 67108 | 0.02 | 0.01 | 0.01 | 0.02 |
CPT = current procedural terminology , PPV = pars plana vitrectomy, MH = macular hole, OCT = optical coherence tomography, VR = vitreoretinal, IOL = intraocular lens, RD = retinal detachment
Table 4.
Weighted Costs by Initial Procedure in Treatment of VMA (US $) for Facility and Non-Facility Billing
| Facility Billing | Non-Facility Billing | |||||||
|---|---|---|---|---|---|---|---|---|
| Initial Procedure | Total Professional Fees | Total Hospital Fees | Total Pharmaceutical Fees | Total fees | Total Professional Fees | Total ASC Fees | Total Pharmaceutical Fees | Total fees |
| Scenario 1, PPV | 2,859 | 5,073 | 0 | 7,931 | 3,027 | 2,775 | 0 | 5,802 |
| Scenario 2a (ocriplasmin 26.5%) | 2,505 | 4,522 | 3,950 | 10,977 | 3,042 | 2,039 | 3,950 | 9,031 |
| Scenario 2b (ocriplasmin 41%) | 2,342 | 4,332 | 3,950 | 10,624 | 2,879 | 1,938 | 3,950 | 8,767 |
| Scenario 3, saline | 2,925 | 5,073 | 100 | 8,098 | 3,231 | 2,497 | 100 | 5,828 |
VMA = vitreomacular adhesion, US = United States, ASC = ambulatory surgery center, PPV = pars plana vitrectomy, Ocriplasmin (27%) = 27% initial success rate with ocriplasmin, Ocriplasmin (41%) = 41% initial success rate with ocriplasmin
Acknowledgments
Financial Support:
Supported by NIH Center Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant, Department of Defense (DOD- Grant#W81XWH-09-1-0675).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
JSC: none
WES: Consultant: Alimera
Reference
- 1.Gass JD. Idiopathic senile macular hole. Its early stages and pathogenesis. Arch Ophthalmol. 1988;106:629–39. doi: 10.1001/archopht.1988.01060130683026. [DOI] [PubMed] [Google Scholar]
- 2.Uchino E, Uemura A, Ohba N. Initial stages of posterior vitreous detachment in healthy eyes of older persons evaluated by optical coherence tomography. Arch Ophthalmol. 2001;119:1475–9. doi: 10.1001/archopht.119.10.1475. [DOI] [PubMed] [Google Scholar]
- 3.Chauhan DS, Antcliff RJ, Rai PA, et al. Papillofoveal traction in macular hole formation: the role of optical coherence tomography. Arch Ophthalmol. 2000;118:32–8. doi: 10.1001/archopht.118.1.32. [DOI] [PubMed] [Google Scholar]
- 4.Gallemore RP, Jumper JM, McCuen BW II, et al. Diagnosis of vitreoretinal adhesion in macular disease with optical coherence tomography. Retina. 2000;20:115–20. [PubMed] [Google Scholar]
- 5.Witkin AJ, Ko TH, Fujimoto JG, et al. Vitreofoveal attachment causing metamorphopsia: an ultrahigh-resolution optical coherence tomography finding. Retina. 2006;26:1085–7. doi: 10.1097/01.iae.0000254885.17777.0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991;109:654–9. doi: 10.1001/archopht.1991.01080050068031. [DOI] [PubMed] [Google Scholar]
- 7.Freeman WR, Azen SP, Kim JW, et al. Vitrectomy for Treatment of Macular Hole Study Group. Vitrectomy for the treatment of full-thickness stage 3 or 4 macular holes. Results of a multicentered randomized clinical trial. Arch Ophthalmol. 1997;115:11–21. doi: 10.1001/archopht.1997.01100150013002. [DOI] [PubMed] [Google Scholar]
- 8.Wendel RT, Patel AC, Kelly NE, et al. Vitreous surgery for macular holes. Ophthalmology. 1993;100:1671–6. doi: 10.1016/s0161-6420(93)31419-3. [DOI] [PubMed] [Google Scholar]
- 9.Brooks HL., Jr Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology. 2000;107:1939–48. doi: 10.1016/s0161-6420(00)00331-6. discussion 1948-9. [DOI] [PubMed] [Google Scholar]
- 10.Tadayoni R, Gaudric A, Haouchine B, Massin P. Relationship between macular hole size and the potential benefit of internal limiting membrane peeling. Br J Ophthalmol. 2006;90:1239–41. doi: 10.1136/bjo.2006.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tadayoni R, Vicaut E, Devin F, et al. A randomized controlled trial of alleviated positioning after small macular hole surgery. Ophthalmology. 2011;118:150–5. doi: 10.1016/j.ophtha.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 12.Kim SS, Smiddy WE, Feuer WJ, Shi W. Outcomes of sulfur hexafluoride (SF6) versus perfluoropropane (C3F8) gas tamponade for macular hole surgery. Retina. 2008;28:1408–15. doi: 10.1097/IAE.0b013e3181885009. [DOI] [PubMed] [Google Scholar]
- 13.Thompson JT, Smiddy WE, Glaser BM, et al. Intraocular tamponade duration and success of macular hole surgery. Retina. 1996;16:373–82. doi: 10.1097/00006982-199616050-00002. [DOI] [PubMed] [Google Scholar]
- 14.Park DW, Sipperley JO, Sneed SR, et al. Macular hole surgery with internal-limiting membrane peeling and intravitreous air. Ophthalmology. 1999;106:1392–7. doi: 10.1016/S0161-6420(99)00730-7. discussion 1397-8. [DOI] [PubMed] [Google Scholar]
- 15.Spiteri Cornish K, Lois N, Scott N, et al. Vitrectomy with internal limiting membrane (ILM) peeling versus vitrectomy with no peeling for idiopathic full-thickness macular hole (FTMH). Cochrane Database Syst Rev. 2013;6:CD009306. doi: 10.1002/14651858.CD009306.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lois N, Burr J, Norrie J, et al. Full-thickness Macular Hole and Internal Limiting Membrane Peeling Study (FILMS) Group. Internal limiting membrane peeling versus no peeling for idiopathic full-thickness macular hole: a pragmatic randomized controlled trial. Invest Ophthalmol Vis Sci. 2011;52:1586–92. doi: 10.1167/iovs.10-6287. [DOI] [PubMed] [Google Scholar]
- 17.Tornambe PE, Poliner LS, Grote K. Macular hole surgery without face-down positioning. A pilot study. Retina. 1997;17:179–85. doi: 10.1097/00006982-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 18.John VJ, Flynn HW, Jr, Smiddy WE, et al. Clinical course of vitreomacular adhesion managed by initial observation. Retina. 2014;34:442–6. doi: 10.1097/IAE.0b013e3182a15f8b. [DOI] [PubMed] [Google Scholar]
- 19.Stalmans P, Duker JS, Kaiser PK, et al. OCT-based interpretation of the vitreomacular interface and indications for pharmacologic vitreolysis. Retina. 2013;33:2003–11. doi: 10.1097/IAE.0b013e3182993ef8. [DOI] [PubMed] [Google Scholar]
- 20.Duker JS, Kaiser PK, Binder S, et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction and macular hole. Ophthalmology. 2013;120:2611–9. doi: 10.1016/j.ophtha.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 21.Smiddy WE, Michels RG, Glaser BM, deBustros S. Vitrectomy for macular traction caused by incomplete vitreous separation. Arch Ophthalmol. 1988;106:624–8. doi: 10.1001/archopht.1988.01060130678025. [DOI] [PubMed] [Google Scholar]
- 22.Davis RP, Smiddy WE, Flynn HW, Puliafito CA. Surgical management of vitreofoveal traction syndrome: optical coherence tomographic evaluation and outcomes. Ophthalmic Surg Lasers Imaging. 2010;41:150–6. doi: 10.3928/15428877-20100303-01. [DOI] [PubMed] [Google Scholar]
- 23.Jackson TL, Nicod E, Angelis A, et al. Pars plana vitrectomy for vitreomacular traction syndrome: a systematic review and metaanalysis of safety and efficacy. Retina. 2013;33:2012–7. doi: 10.1097/IAE.0b013e3182a6b3e2. [DOI] [PubMed] [Google Scholar]
- 24.Stalmans P, Benz MS, Gandorfer A, et al. MIVI-TRUST Study Group. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med. 2012;367:606–15. doi: 10.1056/NEJMoa1110823. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Medicare and Medicaid Services Physician Fee Schedule. 2013 revised release. ANES2013.xls, GPCI2013.xls, PPRVU13_V1226_UP0.xlsx [within RVU13AR.zip]. Available at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Relative-Value-Files-Items/RVU13AR.html. Accessed March 17, 2014.
- 26.Centers for Medicare and Medicaid Services Addendum B-Final OPPS Payment by HCPCS Code for CY 2013. [January 2013 Web Addendum B.01.01.13.xlsx]. Available at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Addendum-A-and-Addendum-B-Updates-Items/January-2013-addendum-B.html?DLPage=1&DLSort=2&DLSortDir=descending. Accessed March 17, 2014.
- 27.Centers for Medicare and Medicaid Services CY <year?> Physician Fee Schedule. Available at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/index.html. Accessed October 28, 2013. AQ: are you citing CY 2014 (page link opens) or CY 2013? If CY 2013, use this link: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1590-FC.html, and cite specific download zip file and give file name within zip file.
- 28.Centers for Medicare and Medicaid Services Ambulatory Surgical Center (ASC) Payment. Addenda Updates. July 2013 ASC Approved HCPCS Code and Payment Rates. July_2013_ASC_web_addenda_06.17.2013.xls [within: 2013-July-ASC-Addenda.zip]. Available at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ASCPayment/11_Addenda_Updates.html. Accessed March 17, 2014.
- 29.Centers for Medicare and Medicaid Services Medicare Claims Processing Manual. Physicians/Nonphysician Practitioners. 2013:121–9. Rev 2714. [Google Scholar]
- 30.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–38. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 31.Patel JJ, Mendes MA, Bounthavong M, et al. Cost-utility analysis of bevacizumab versus ranibizumab in neovascular age-related macular degeneration using a Markov model. J Eval Clin Pract. 2012;18:247–55. doi: 10.1111/j.1365-2753.2010.01546.x. [DOI] [PubMed] [Google Scholar]
- 32.United States Social Security Administration Actuarial Life Table. Period Life Table, 2009. Available at: http://ssa.gov/OACT/STATS/table4c6.html. Accessed March 17, 2014.
- 33.Brown MM, Brown GC, Sharma S, Landy J. Health care economic analyses and value-based medicine. Surv Ophthalmol. 2003;48:204–23. doi: 10.1016/s0039-6257(02)00457-5. [DOI] [PubMed] [Google Scholar]
- 34.Brown MM, Brown GC, Lieske HB, Lieske PA. Preference-based comparative effectiveness and cost-effectiveness: a review and relevance of value-based medicine for vitreoretinal interventions. Curr Opin Ophthalmol. 2012;23:163–74. doi: 10.1097/ICU.0b013e3283523fc1. [DOI] [PubMed] [Google Scholar]
- 35.Smiddy WE. Clinical applications of cost analysis of diabetic macular edema treatments. Ophthalmology. 2012;119:2558–62. doi: 10.1016/j.ophtha.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Smiddy WE. Relative cost of a line of vision in age-related macular degeneration. Ophthalmology. 2007;114:847–54. doi: 10.1016/j.ophtha.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 37.Smiddy WE. Economic considerations of macular edema therapies. Ophthalmology. 2011;118:1827–33. doi: 10.1016/j.ophtha.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stein JD, Newman-Casey PA, Kim DD, et al. Cost-effectiveness of various interventions for newly diagnosed diabetic macular edema. Ophthalmology. 2013;120:1835–42. doi: 10.1016/j.ophtha.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang JS, Smiddy WE. Cost-effectiveness of retinal detachment repair. Ophthalmology. doi: 10.1016/j.ophtha.2013.11.003. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ternent L, Vale L, Boachie C, et al. Full-Thickness Macular Hole and Internal Limiting Membrane Peeling Study (FILMS) Group. Cost-effectiveness of internal limiting membrane peeling versus no peeling for patients with an idiopathic full-thickness macular hole: results from a randomised controlled trial. Br J Ophthalmol. 2012;96:438–43. doi: 10.1136/bjophthalmol-2011-300402. [DOI] [PubMed] [Google Scholar]