Abstract
Objective
To investigate the incidence and progression of age related m acular degeneration (AMD) and associated risk factors.
Design
Population-based prospective cohort study.
Participants
2868 participants from the Age Gene/Environment Susceptibility-Reykjavik Study with retinal data at baseline and five-year follow-up.
Methods
Digital macular photographs were graded for presence of AMD. Participants completed a questionnaire and extensive clinical battery. Biomarkers were assessed. Risk factors for AMD were analyzed using multivariate regression analysis with odds ratios (ORs) and 95% confidence intervals (CIs).
Main outcome measures
AMD, defined as early or late.
Results
Among 2196 participants free of AMD at baseline, 14.9% developed incident AMD. In multivariate models, incident AMD was significantly associated with age (OR per year 1.14 (95% CI 1.11, 1.17)), current smoking (OR 2.07 (1.38, 3.11)), former smoking (OR 1.36 (1.04, 1.79)), plasma high-density lipoprotein (HDL) cholesterol level (OR 1.62 per mmol/L (1.19, 2.22)), and body mass index (BMI) (OR 1.04 per kg/m2 (1.01, 1.07)). Among 563 participants with early AMD at baseline, 22.7% progressed to late AMD (11.0% pure geographic atrophy (GA) and 11.7% exudative AMD). In multivariate analyses, age was significantly associated with progression to GA (OR 1.14 (1.07, 1.21)) and exudative AMD (OR 1.08 (1.01, 1.14)). Adjusting for age, female sex was associated with exudative AMD (OR 2.10 (1.10, 3.98)) and plasma HDL cholesterol with GA (OR 2.03 per mmol/L (1.02, 4.05)).
Conclusion
By age 85 years, 57.4% of participants had signs of AMD. Age, smoking, plasma HDL cholesterol, BMI and female sex are associated with AMD. Elevated HDL cholesterol is associated with GA development.
Keywords: Age-related macular degeneration, risk, smoking, HDL cholesterol, gender, body mass index
Age-related macular degeneration (AMD) is a leading cause of blindness in the world1–5, accounting for up to 75% of incident non-preventable legal blindness6.Many population-based studies have reported on the prevalence of AMD in persons 40–85 years old at baseline7–9 and several have described the incidence of early AMD based on follow up visits 4–15 years later10–16. From these studies the most consistent risk factors for the development and progression of AMD have been age and cigarette smoking.
The predominantly Caucasian Icelandic population has among the highest life expectancies in Europe at 79.6 years for men, and 83.0 years for women17 making it ideal for selecting an old cohort in which to study the prevalence, incidence, and progression of AMD. The longitudinal Age, Gene/Environment Susceptibility-Reykjavik Study (AGES) with its array of biomarkers, clinical profiles, and genetic risk factors collected prospectively from participants who were ages 67 and older at the baseline study visit, builds on our previous report on the cross-sectional prevalence of AMD18 by describing the incidence and progression of AMD and their respective risk factors.
Methods
Study population
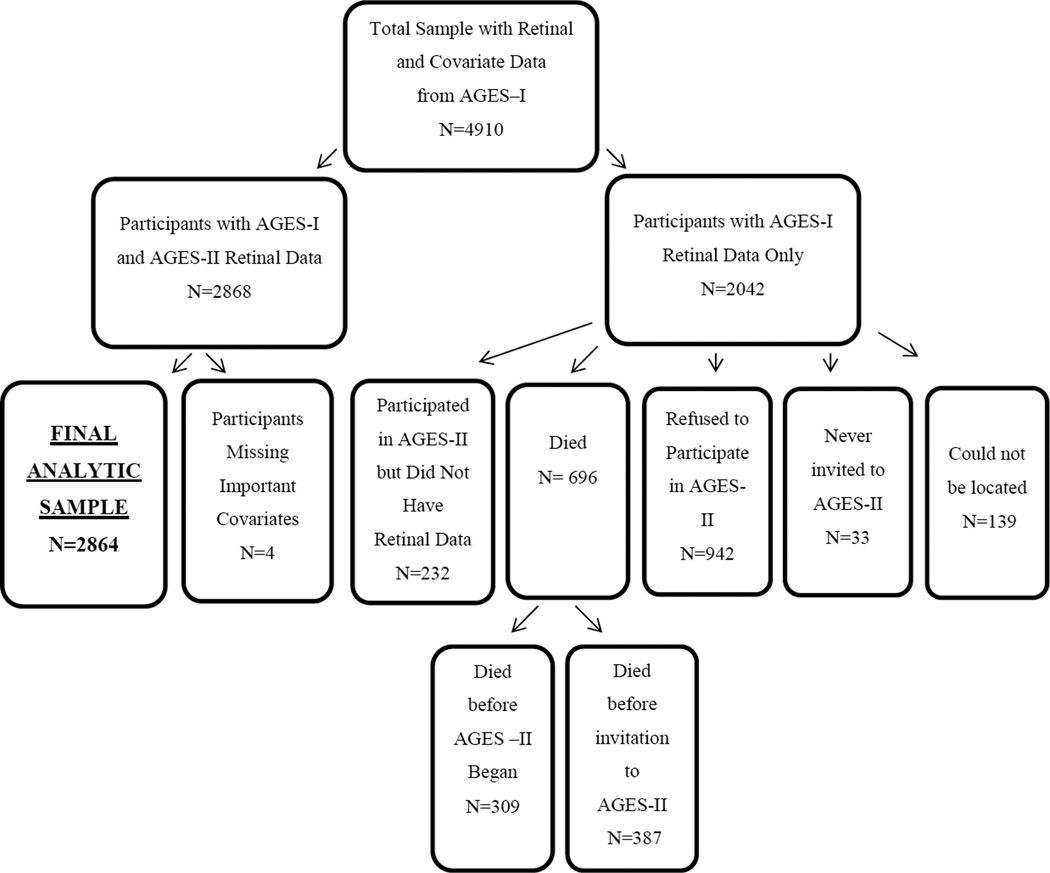
The Age, Gene/Environment Susceptibility-Reykjavik Study (AGES), as described in detail elsewhere, is a population-based study aimed to investigate genetic and environmental factors contributing to health, disability, and disease in older people born between 1907 and 193518,19. Between 2002 and 2006 at its baseline visit (AGES-I), 5764 participated in the AGES study, 5272 had readable AMD photographs of at least one eye18,20 and 4910 had data on AMD and covariates. Survivors were invited to participate in a 5-year follow up study visit (AGES-II) conducted between November 2007 and September 2011. The AGES-II visit protocol entailed a predetermined battery of tests held in two separate sessions on two different days. Retinal images were captured at the second session. Some of the individuals who agreed to participate in AGES II attended the first session and thereafter decided either not to return for the second session or agreed to participate only in select tests offered during the second session. Readable AMD photographs at both visits were available from 2868 people.
Interviews and examinations
The AGES Study methods, examination protocols and characteristics of the cohort have been described in detail elsewhere19,20. In brief: during baseline and follow-up assessment at the Icelandic Heart Association (IHA) Research Center, participants completed a standardized protocol including a detailed interview and an extensive battery of clinical tests and imaging studies19. Blood specimens were drawn and a biomarker profile was assessed18,20,21. The study offered to participants the option of providing free transport to the clinic. The AGES Study was approved by the Icelandic National Bioethics Committee (VSN: 00-063), which acts as the institutional review board for the IHA, and by the Institutional Review Board for the U.S. National Institute of Aging, National Institutes of Health.
Assessment of AMD
The same standardized study protocol was followed at baseline and at follow up. Fundus photography, after pharmacologic dilation of the pupils, was performed as described in detail previously18. In brief, two photographic fields were taken of each eye, the first centered on the optic disc and the second centered on the fovea using a 45-degree, 6.3-megapixel digital nonmydriatic camera (Canon, Lake Success, NY).
Using a modification of the Wisconsin Age-Related Maculopathy Grading scheme, retinal images were evaluated twice by trained graders at the University of Wisconsin Ocular Epidemiology Reading Center, who were masked to the health status of the participant. Images were graded using EyeQ Lite software (an image-processing database for storage, retrieval, and manipulation of digital images)8,22. As published previously, early AMD was defined by the presence of any soft drusen and pigmentary abnormalities (increased or decreased retinal pigment) or the presence of large soft drusen ≥125µm in diameter with a large drusen area >500µm in diameter or large ≥125µm indistinct soft drusen in the absence of signs of late AMD. Late AMD was defined by the presence of any of the following: geographic atrophy (GA) or exudative AMD including subretinal hemorrhage, subretinal fibrous scar, retinal pigment epithelial detachment, or serous detachment of the sensory retina or signs of treatment for neovascular AMD18. Persons suspected of having had intravitreal treatment for AMD as determined from retinal image grading or indicating it in the questionnaire, had their treatment subsequently confirmed or rejected by cross-validating with the database maintained by the only center administering such treatment in Iceland.
Characterization of Possible Risk Factors
All factors reported in the literature as possible mediators of AMD risk for which AGES collected data were considered as covariates. Body mass index (BMI) was calculated as measured weight (kg) divided by height (meters) squared. Smoking status was categorized as never smoker, former smoker or current smoker. Cod liver oil use was based on self-report. Hypertension was defined as self-reported history of hypertension, use of anti-hypertension drugs, or blood pressure ≥140/90mm Hg. Pulse pressure was measured using arterial tonometry, expressed in units of mm Hg, and later categorized by quartiles. Diabetes mellitus was defined as self-reported history of diabetes, use of glucose-lowering medications or fasting blood glucose of ≥7.0mmol/L. Blood samples were drawn after overnight fasting, and total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, and high-sensitivity C-reactive protein (hsCRP) were measured in the IHA laboratory using standard methods. Unless otherwise noted, all variables investigated as risk factors were assessed at the baseline visit.
Statistical Methods
Baseline characteristics of participants who completed both retinal examinations and participants who did not return for the follow up visit were compared using analysis of covariance and logistic regression with adjustment for age and sex.
Incident AMD and progression to late AMD were calculated for each potential risk factor. Multivariate logistic regression was then used to examine the association between the characteristics and incident AMD and progression to late AMD. Covariates in regression models included age (in years), female sex (yes/no), current smoker (yes/no), former smoker (yes/no), use of cod liver oil (yes/no), hypertension (yes/no), diabetes mellitus (yes/no), body mass index (kg/m2), total cholesterol (mmol/L), HDL cholesterol (mmol/L) and hsCRP (mg/L). All potential risk factors were included as covariates in the multivariate analyses and retained regardless of their significance in order to facilitate comparisons with results from other studies. Odds ratios (OR) and 95% confidence intervals (CI) were calculated for each variable and P values < 0.05 were considered significant. Statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, North Carolina).
Results
At baseline 4910 AGES participants had gradable AMD photographs and information on covariates. Of these, 2868 (58%) completed a follow-up visit on average, 5 years, after the baseline examination and constitute the sample for this analysis. Their mean age at baseline was 74.7 years and 42.4% were male. Figure 1 provides a flow chart depicting reasons for non-participation in the follow-up visit.
Figure 1.
Flowchart depicting sample selection, including reasons for non-participation in the Age, Gene/Environment Susceptibility-Reykjavik Study (AGES)-II examination.
Characteristics at baseline for participants in AGES-II and those who did not return beyond AGES-I are presented in Table 1 along with p-values depicting relevant differences. Those who did not return for follow up were significantly more likely to be older, and, adjusting for age, more likely to be smokers, have diabetes, have elevated levels of hsCRP, and less likely to be regular users of cod-liver oil. People with AMD signs at baseline were slightly less likely to participate in the follow up visit, reflecting perhaps their older mean age at baseline.
Table 1.
Baseline Characteristics for Participants who Completed the AGES-I Examination (N=4910), Stratified by Inclusion or Exclusion from the AGES-II Follow-Up Examination
| Baseline Characteristics | Participants from AGES-I and Participating in AGES-II Examination (N=2868) |
Participants from AGES-I and Not Participating in AGES-II Examination (N=2042) |
P Value* |
|---|---|---|---|
| Mean Age (SD) | 74.7 (4.8) | 78.7 (5.6) | < 0.01 |
| Sex % Males (N) | 42.4% (1217) | 44.0% (899) | 0.53 |
| Smoking Status | |||
| Former Smoker % (N) | 45.6% (1306) | 44.1% (898) | 0.48 |
| Current Smoker % (N) | 11.0% (315) | 13.7% (278) | < 0.01 |
| Cod Liver Oil Use % (N) | 58.9% (1688) | 56.2% (1147) | 0.01 |
| Diabetes % (N) | 9.9% (284) | 14.1% (288) | < 0.01 |
| Hypertension % (N) | 78.6% (2253) | 84.1% (1717) | 0.08 |
| Mean DBP, mmHg (SD) | 74.3 (9.4) | 73.5 (10.1) | 0.28 |
| Mean SBP, mmHg (SD) | 141.2 (19.5) | 144.1 (21.2) | 0.04 |
| Mean Pulse Pressure, mmHg (SD) | 66.9 (17.3) | 70.6 (19.1) | 0.07 |
| Mean BMI, kg/m2 (SD) | 27.3 (4.2) | 26.7 (4.7) | 0.32 |
| Mean Total Cholesterol, mmol/L (SD) | 5.6 (1.1) | 5.6 (1.2) | 0.80 |
| Mean HDL Cholesterol, mmol/L (SD) | 1.6 (0.4) | 1.6 (0.5) | 0.25 |
| Mean C-Reactive Protein, mg/L (SD) | 3.3 (5.6) | 4.1 (7.0) | < 0.01 |
| AMD Sign in Worse Eye at Baseline % (N) | |||
| Large Drusen (≥ 125µm) | 27.2% (780) | 36.1% (738) | 0.83 |
| Soft Distinct | 34.5% (988) | 45.0% (919) | 0.84 |
| Soft Indistinct | 16.2% (464) | 23.1% (470) | 0.87 |
| Soft Drusen Area ≥ 500µm | 14.2% (407) | 22.1% (451) | 0.48 |
| Any Soft Drusen | 37.1% (1064) | 47.6% (972) | 0.93 |
| Increased Retinal Pigment | 17.8% (510) | 25.5% (521) | 0.86 |
| RPE Depigmentation | 9.2% (265) | 15.0% (306) | 0.66 |
| Retinal and Pigmentary Abnormalities | 17.9% (514) | 25.6% (523) | 0.79 |
| Early AMD | 19.6% (563) | 24.5% (501) | 0.46 |
| Subretinal New Vessels | 0.4% (11) | 0.8% (17) | 0.44 |
| Subretinal Hemorrhage | 0.9% (26) | 2.1% (42) | 0.65 |
| Pure Geographic Atrophy | 1.5% (42) | 3.8% (73) | 0.26 |
| Any Geographic Atrophy | 2.6% (74) | 5.6% (114) | 0.82 |
| Exudative AMD | 2.3% (67) | 4.7% (95) | 0.76 |
| Late AMD | 3.8% (109) | 8.2% (168) | 0.32 |
AGES=Age, Gene/Environment Susceptibility-Reykjavik Study; SD=standard deviation; DBP=diastolic blood pressure; SBP=systolic blood pressure; BMI=body mass index; HDL=high-density lipoprotein; AMD=age-related macular degeneration
Comparison of participants observed to have completed the AGES-II follow-up examination and participants who did not complete the follow-up examination, adjusted for age and sex.
Among 2196 individuals without signs of AMD at baseline, 14.9% (328 persons) developed incident AMD by AGES-II, of whom 14.2% (312) developed early AMD and 0.7% (16) late AMD (Table 2, available at www.aaojournal.org). Table 2 shows the distribution of baseline values for possible risk characteristics among incident cases. Among persons aged 85 years and older, 38.6% developed incident AMD over 5 years follow up period. An increased incidence of AMD was observed in smokers and persons with higher BMI, HDL cholesterol, and CRP compared to persons with lower levels. However, after adjusting for age and sex in a multivariate model, only increasing age, smoking, greater BMI and higher plasma HDL cholesterol levels were significant independent risk factors associated with the development of incident AMD (Table 3). Females were somewhat but not significantly more likely than males to develop incident AMD (OR=1.31; 95% CI: 0.98, 1.74, p=0.07, Table 3).
Table 3.
Association between Incident Age-Related Macular Degeneration and Risk Factors in Multivariate Logistic Regression Analyses among AGES Participants who Completed both Retinal Examinations (N=2868)
| Incident AMD (N=328) | ||
|---|---|---|
| Baseline Characteristic | OR (95% CI) | P value |
| Age, per year | 1.14 (1.11, 1.17) | < 0.01 |
| Sex, female vs. male | 1.31 (0.98, 1.74) | 0.07 |
| Current smoker, yes vs. no | 2.07 (1.38, 3.11) | < 0.01 |
| Former smoker, yes vs. no | 1.36 (1.04, 1.79) | 0.03 |
| Cod liver oil use, yes vs. no | 1.00 (0.78, 1.28) | 0.98 |
| Hypertension, yes vs. no | 0.88 (0.63, 1.23) | 0.46 |
| Pulse Pressure, per mm Hg | 1.00 (0.99, 1.01) | 0.92 |
| Diabetes, yes vs. no | 1.05 (0.69, 1.59) | 0.84 |
| BMI, per kg/m2 | 1.04 (1.01, 1.07) | 0.01 |
| Total cholesterol, per mmol/L | 0.95 (0.85, 1.07) | 0.37 |
| HDL cholesterol, per mmol/L | 1.62 (1.19, 2.22) | < 0.01 |
| C-reactive protein | ||
| Moderate (1–3 mg/L) vs. Low (< 1 mg/L) | 1.05 (0.77, 1.43) | 0.77 |
| High (> 3mg/L) vs. Low (< 1 mg/L) | 1.11 (0.79, 1.57) | 0.55 |
OR=odds ratio; CI=confidence interval
AGES=Age, Gene/Enviroment Susceptibility-Reykjavik Study; AMD=age-related macular degeneration; BMI=body mass index; HDL=high-density lipoprotein
Incidence defined as exhibiting no AMD at baseline examination but presenting with early or late AMD at the follow-up examination.
Among the 563 AGES-I participants with early AMD, 128 (22.7%) progressed to late AMD at AGES-II (Table 4, available at www.aaojournal.org). Progression of AMD occurred more often in females compared to males and among persons with higher levels of HDL cholesterol or CRP compared with persons with lower levels and among persons who did not use cod liver oil (Table 4, available at www.aaojournal.org). We compared mean differences in plasma HDL cholesterol measurements taken at AGES-I and II visits to discern if we could identify any significant difference in levels over time for incident cases and, separately, for people whose AMD progressed. No differences in mean plasma HDL cholesterol level over time were found for either group (data not shown). We examined t he possible interaction for HDL cholesterol and C-reactive protein in separate logistic reaction models for prevalence, incidence and progression of AMD and, after adjustment for covariates, found no statistically significant association (p=0.68, p=0.41 and p=0.77, respectively). We employed the same analysis to test for possible interaction between use of cod liver oil use and statins and again found no significant interaction (p=0.71, p=0.32 and p=0.79, respectively).
After multivariate logistic regression, only per year increase in age (OR=1.12; 95% CI: 1.06, 1.17) and female sex (OR=1.70; 95% CI: 1.05, 2.75) were significant predictors of progression from early to late AMD (Table 5). When considering specific late AMD lesions separately, higher levels of HDL cholesterol were significantly associated with GA (OR=2.03; 95% CI: 1.02, 4.05) and female sex was associated with exudative disease (OR=1.70; 95% CI: 1.10, 3.98).
Table 5.
Association between Progression of Age-Related Macular Degeneration and Risk Factors in Multivariate Logistic Regression Analyses among AGES Participants who Completed both Retinal Examinations (N=2868)
| Pure Geographic Atrophy (N=62) |
Exudative AMD (N=66) |
Late AMD (N=128) |
||||
|---|---|---|---|---|---|---|
| Baseline Characteristic | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value |
| Age, per year | 1.14 (1.07, 1.21) | < 0.01 | 1.08 (1.01, 1.14) | < 0.01 | 1.12 (1.06, 1.17) | < 0.01 |
| Sex, female vs. male | 1.42 (0.75, 2.67) | 0.28 | 2.10 (1.10, 3.98) | 0.02 | 1.70 (1.05, 2.75) | 0.03 |
| Current smoker, yes vs. no | 1.56 (0.62, 3.90) | 0.35 | 0.97 (0.39, 2.42) | 0.95 | 1.27 (0.63, 2.54) | 0.50 |
| Former smoker, yes vs. no | 1.34 (0.73, 2.49) | 0.35 | 0.93 (0.52, 1.65) | 0.80 | 1.13 (0.72, 1.78) | 0.59 |
| Cod liver oil use, yes vs. no | 0.64 (0.37, 1.13) | 0.12 | 0.88 (0.51, 1.50) | 0.63 | 0.72 (0.47, 1.09) | 0.12 |
| Hypertension, yes vs. no | 0.57 (0.27, 1.20) | 0.14 | 1.03 (0.48, 2.21) | 0.94 | 0.73 (0.41, 1.28) | 0.27 |
| Pulse Pressure, per mmHg | 0.99 (0.98, 1.01) | 0.50 | 1.00 (0.99, 1.02) | 0.81 | 1.00 (0.99, 1.01) | 0.83 |
| Diabetes, yes vs. no | 1.30 (0.50, 3.39) | 0.59 | 1.29 (0.56, 2.98) | 0.55 | 1.26 (0.64, 2.48) | 0.50 |
| BMI, per kg/m2 | 1.03 (0.96, 1.11) | 0.40 | 0.96 (0.89, 1.04) | 0.30 | 1.00 (0.94, 1.06) | 0.93 |
| Total cholesterol, per mmol/L | 0.80 (0.60, 1.07) | 0.13 | 1.03 (0.80, 1.32) | 0.82 | 0.91 (0.74, 1.11) | 0.35 |
| HDL cholesterol, per mmol/L | 2.03 (1.02, 4.05) | 0.04 | 0.67 (0.32, 1.40) | 0.28 | 1.25 (0.73, 2.14) | 0.43 |
| C-reactive protein | ||||||
| Moderate (1–3 mg/L) vs. Low (< 1 mg/L) | 1.29 (0.61, 2.72) | 0.51 | 1.06 (0.52, 2.18) | 0.87 | 1.19 (0.69, 2.06) | 0.54 |
| High (> 3mg/L) vs. Low (< 1 mg/L) | 1.25 (0.54, 2.89) | 0.60 | 1.31 (0.60, 2.86) | 0.50 | 1.32 (0.72, 2.43) | 0.37 |
OR=odds ratio; CI=confidence interval
AGES=Age, Gene/Enviroment Susceptibility-Reykjavik Study; AMD=age-related macular degeneration; BMI=body mass index; HDL=high-density lipoprotein
Progression defined as exhibiting early AMD at baseline examination but exhibiting late AMD at the follow-up examination.
By age 85 years, 275 of the 479 AGES-II participants (57.4%) had signs of AMD (44.5% had early AMD signs and 16% had signs of late AMD).
Discussion
In the aged AGES cohort we estimated the incidence of AMD to be approximately 3% per year. Over 95% of the incident cases were classified as early lesions. The rate of progression of AMD from early to late was approximately 4.5% per year distributed in roughly equal proportions as GA and exudative disease. More than 55% of adults aged 85 years and older exhibit signs of AMD in at least one eye. Risk factors for incident AMD included age, BMI, smoking, and high levels of HDL cholesterol whereas risk factors for AMD progression were mainly age, with females more likely than males to progress to exudative disease and those with higher HDL cholesterol levels more likely than those with l ower levels to progress to GA.
The results of our study are generally comparable to incidence and progression rates reported for other populations of European ancestry, particularly the Beaver Dam Eye Study, the Rotterdam Eye Study and the Blue Mountain Eye Study11–13. The Beaver Dam Eye Study reported, among persons ages 75 years and older, an incidence of 2% per year and of progression at 4% per year23. Increasing age at baseline was the most significant predictor of AMD status and after adjusting for age, AMD incidence did not differ by sex in the Beaver Dam cohort24. AGES found women to have a somewhat higher risk of incident AMD compared to men as well as a higher risk of AMD progression to exudative AMD, however, we cannot rule out the possibility that selective survival may explain this finding. Similar to previous studies,26–28 we find smoking is a risk factor for incident AMD whereby former smokers seem to be at lower risk than current smokers. Notably, smoking was not associated with AMD progression in the AGES sample. Men, particularly those who smoked at baseline and had other adverse health conditions, may have died, consistent with the suggestion of selective survival and this may explain, in part, the inconsistent findings on sex differences reported in the literature25.
There is increasing evidence that HDL cholesterol is involved in inflammation and is associated with AMD29–31. Higher levels of plasma HDL cholesterol, but not total cholesterol, LDL cholesterol or triglycerides were significantly associated with the incidence of AMD in AGES, as well as in the Rotterdam study32. In Beaver Dam, the association with HDL cholesterol was most striking in persons with pure GA23 similar to the present study. The age and sex-adjusted relative risk of HDL cholesterol was positively associated with the incidence of early AMD in the Blue Mountain Eye Study but was not statistically significant, possibly due to reduced power. There was also no significant association of HDL cholesterol with incident late AMD, although the relative risk of increasing HDL cholesterol appeared to be marginally protective33. In recent years, it has become evident that HDL cholesterol concentration as such does not necessarily reflect the protective action of HDL cholesterol. Measurement of circulating HDL particle load may be more important divided into cholesterol rich HDL particles and particles containing small amount34. Furthermore, certain polymorphisms in the hepatic and endothelial lipase genes resulting in low or high HDL cholesterol may not correspond to expected differences in risk30,35.
The impact of immune dysfunction and complement dysregulation on AMD pathogenesis is well established, whereby common and rare variants in multiple members of a pro-inflammatory alternative pathway of the innate immunity are associated with AMD through overactivation36. Genetic variants generally confer similar risk for both late AMD forms37. We have reported AMD genetic profile for this cohort elsewhere, with similar results as in other white populations38. A recent article from Iceland reported a rare variant in the C3 to be associated with high risk of AMD. This variant, associated with inflammation, was found to be significantly more common in GA than exudative AMD patients39. The association of HDL cholesterol and GA may thus be associated with the anti-inflammatory properties of HDL cholesterol29.
BMI was significantly associated with incident AMD; however, it was not associated with progression to late AMD in our multivariate models. Obesity may be a marker for reduced physical activity and increase in inflammation both associated with AMD in the literature26,40. In AGES, high hsCRP was associated with AMD incidence and progression in bivariate analysis; however, after covariate adjustment, hsCRP was no longer associated with either incidence or progression of AMD. This is not consistent with results from a recent pooled meta-analysis of data from several studies, and a direct role for CRP in AMD causation remains a topic of research41.
Several factors reported by some other studies as risk indicators including hypertension, diabetes, total cholesterol, triglycerides and Omega-3fatty acids/cod-liver oil were investigated in our analysis and were found to be unrelated to AMD status after considering age, sex, and smoking. Icelanders commonly consume cod-liver oil on a daily basis, in addition to having a high intake of fish and fish products42. Studies from populations where consumption of fish and fish products is much less common have reported omega-3 fatty acids to be protective against the development of early AMD although findings from a recent clinical trial did not confirm this supposition43,44. It is possible that below a threshold, Omega-3 fatty acids may be beneficial with respect to AMD, but in our cohort where the intake of Omega-3 fatty acids is high, there was no association with AMD incidence or progression.
Our study offers several advantages in that it contains a sizeable number of old individuals, the participants were drawn from a population sample without regard for AMD status, and the cohort is well studied for a variety of health conditions common in old age. Fundus photographs were taken by radiographers trained to take retinal images according to a standardized protocol and were graded by an independent reading center using well-established methods. Image quality was good18. It is also worth noting some limitations. AGES was designed to study diseases common in old age, and therefore we do not have data in mid-life with which to report incidence for younger ages or identify risk factors which may prevent the development or progression of AMD later in life. In addition, although free transport to the clinic was offered to all participants, frailty, morbidity and mortality did impact on continued participation beyond the baseline AGES exam in this aged cohort. Therein, our estimates of incidence and progression are likely to be underestimates and our assessment of risk factors (e.g. smoking, hypertension) may be imprecise.
In conclusion, AMD is very common in people aged 85 years or older. In addition to age, we confirm relationships of smoking, increased BMI, and higher HDL cholesterol levels with increased AMD risk. Smoking has been related to numerous adverse health conditions and since former smokers appear to have lower risk, smoking cessation even in late life is a reasonable recommendation to reduce risk of AMD. The relationship of higher plasma HDL cholesterol with risk of early AMD and with progression to GA needs further study. Determining whether different processes or biomarkers, tracked over time, influence the development or progression of the two advanced forms of AMD may be important for prognosis, prevention and future treatment.
Supplementary Material
Acknowledgments
Financial Support
This work was supported by the National Institute of Health (Intramural Research Program of the National Institute of Aging and the National Eye Institute, ZIAEY00401), National Institute of Health contract number N01-AG-1-2100, the Icelandic Heart Association, the Icelandic Parliament, the University of Iceland Research Fund and the Helga Jonsdottir and Sigurlidi Kristjansson Research Fund. The funders had no role in data collection, management, analysis and interpretation of the data, preparation, writing and approval of the manuscript, and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no proprietary or commercial interest in any materials discussed in this article.
References
- 1.Klein R, Lee KE, Gangnon RE, Klein BE. Incidence of visual impairment over a 20-year period: the Beaver Dam Eye Study. Ophthalmology. 2013;120:1210–1219. doi: 10.1016/j.ophtha.2012.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klaver CW, Wolfs RW, Vingerling JR, et al. Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Arch Ophthalmol. 1998;116:653–658. doi: 10.1001/archopht.116.5.653. [DOI] [PubMed] [Google Scholar]
- 3.Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 4.Gunnlaugsdottir E, Arnarsson A, Jonasson F. Prevalence and causes of visual impairment and blindness in Icelanders aged 50 years and older: the Reykjavik Eye Study. Acta Ophthalmol. 2008;86:778–785. doi: 10.1111/j.1755-3768.2008.01191.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang JJ, Foran S, Mitchell P. Age-specific prevalence and causes of bilateral and unilateral visual impairment in older Australians: the Blue Mountains Eye Study. Clin Experiment Ophthalmol. 2000;28:268–273. doi: 10.1046/j.1442-9071.2000.00315.x. [DOI] [PubMed] [Google Scholar]
- 6.Gunnlaugsdottir E, Arnarsson A, Jonasson F. Five-year incidence of visual impairment and blindness in older Icelanders: the Reykjavik Eye Study. Acta Ophthalmol. 2010;88:358–366. doi: 10.1111/j.1755-3768.2008.01445.x. [DOI] [PubMed] [Google Scholar]
- 7.Jonasson F, Arnarsson A, Sasaki H, et al. The prevalence of age-related maculopathy in Iceland: Reykjavik Eye Study. Arch Ophthalmol. 2003;121:379–385. doi: 10.1001/archopht.121.3.379. [DOI] [PubMed] [Google Scholar]
- 8.Klein R, Klein BE, Knudtson MD, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the Multi-ethnic Study of Atherosclerosis. Ophthalmology. 2006;113:373–380. doi: 10.1016/j.ophtha.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99:933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 10.Jonasson F, Arnarsson A, Peto T, et al. 5-year incidence of age-related maculopathy in the Reykjavik Eye Study. Ophthalmology. 2005;112:132–138. doi: 10.1016/j.ophtha.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:7–21. doi: 10.1016/s0161-6420(97)30368-6. [DOI] [PubMed] [Google Scholar]
- 12.Klaver CC, Assink JJ, van Leeuwen R, et al. Incidence and progression rates of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci. 2001;42:2237–2241. [PubMed] [Google Scholar]
- 13.Mitchell P, Wang JJ, Foran S, Smith W. Five-year incidence of age-related maculopathy lesions: the Blue Mountains Eye Study. Ophthalmology. 2002;109:1092–1097. doi: 10.1016/s0161-6420(02)01055-2. [DOI] [PubMed] [Google Scholar]
- 14.Varma R, Foong AW, Lai MY, et al. Los Angeles Latino Eye Study Group. Four-year incidence and progression of age-related macular degeneration: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2010;149:741–751. doi: 10.1016/j.ajo.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang JJ, Rochtchina E, Lee AJ, et al. Ten-year incidence and progression of age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology. 2007;114:92–98. doi: 10.1016/j.ophtha.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Klein R, Klein BE, Knudtson MD, et al. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114:253–262. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 17.Aspelund T, Gudnason V, Magnusdottir BT, et al. Analysing the large decline in coronary heart disease mortality in the Icelandic population aged 25–74 between the years 1981 and 2006. [Accessed March 3, 2014];PLoS ONE. 2010 5:e13957. doi: 10.1371/journal.pone.0013957. [serial online] Available at: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0013957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonasson F, Arnarsson A, Eiríksdottir G, et al. Prevalence of age-related macular degeneration in old persons: Age, Gene/environment Susceptibility Reykjavik Study. Ophthalmology. 2011;118:825–830. doi: 10.1016/j.ophtha.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu C, Cotch MF, Sigurdsson S, et al. Retinal and cerebral microvascular signs and diabetes: the Age, Gene/Environment Susceptibility-Reykjavik Study. Diabetes. 2008;57:1645–1650. doi: 10.2337/db07-1455. [DOI] [PubMed] [Google Scholar]
- 21.Qiu C, Cotch MF, Sigurdsson S, et al. Cerebral microbleeds and age-related macular degeneration: the AGES-Reykjavik Study. Neurobiol Aging. 2012;33:2935–2937. doi: 10.1016/j.neurobiolaging.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein R, Meuer SM, Moss SE, et al. Detection of age-related macular degeneration using a nonmydriatic digital camera and a standard film fundus camera. Arch Ophthalmol. 2004;122:1642–1646. doi: 10.1001/archopht.122.11.1642. [DOI] [PubMed] [Google Scholar]
- 23.Klein R, Klein BE, Tomany SC, Cruickschanks KJ. The association of cardiovascular disease with the long-term incidence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 2003;110:1273–1280. doi: 10.1016/S0161-6420(03)00599-2. [DOI] [PubMed] [Google Scholar]
- 24.Klein R, Myers CE, Meuer SM, et al. Risk alleles in CFH and ARMS2 and the long-term natural history of age-related macular degeneration: the Beaver Dam Eye Study. JAMA Ophthalmol. 2013;131:383–392. doi: 10.1001/jamaophthalmol.2013.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choudhury F, Varma R, McKean-Cowdin R, et al. Los Angeles Latino Eye Study Group. Risk factors for four-year incidence and progression of age-related macular degeneration: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2011;152:385–395. doi: 10.1016/j.ajo.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Age-Related Eye Disease Study Research Group. Risk factors for the incidence of advanced age-related macular degeneration in the Age-Related Eye Disease Study (AREDS): AREDS report no. 19. Ophthalmology. 2005;112:533–539. doi: 10.1016/j.ophtha.2004.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein R, Knudtson MD, Cruickshanks KJ, Klein BE. Further observations on the association between smoking and the long-term incidence and progression of age-related macular degeneration: the Beaver Dam Eye Study. Arch Ophthalmol. 2008;126:115–121. doi: 10.1001/archopht.126.1.115. [DOI] [PubMed] [Google Scholar]
- 28.Chakravarthy U, Augood C, Bentham GC, et al. Cigarette smoking and age-related macular degeneration in the EUREYE Study. Ophthalmology. 2007;114:1157–1163. doi: 10.1016/j.ophtha.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 29.Barter PJ, Nicholls S, Rye KA, et al. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 30.Yu Y, Reynolds R, Fagerness J, et al. Association of variants in the LIPC and ABCA1 genes with intermediate and large drusen and advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:4663–4670. doi: 10.1167/iovs.10-7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fauser S, Smailhodzic D, Caramoy A, et al. Evaluation of serum lipid concentrations and genetic variants at high-density lipoprotein metabolism loci and TIMP3 in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:5525–5528. doi: 10.1167/iovs.10-6827. [DOI] [PubMed] [Google Scholar]
- 32.van Leeuwen R, Klaver CC, Vingerling JR, et al. Cholesterol and age-related macular degeneration: is there a link? Am J Ophthalmol. 2004;137:750–752. doi: 10.1016/j.ajo.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Wang JJ, Rochtchina E, Smith W, et al. Combined effects of complement factor H genotypes, fish consumption, and inflammatory markers on long-term risk for age-related macular degeneration in a cohort. Am J Epidemiol. 2009;169:633–641. doi: 10.1093/aje/kwn358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mora S, Glynn RJ, Ridker PM. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 2013;128:1189–1197. doi: 10.1161/CIRCULATIONAHA.113.002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nature Rev Immunol. 2013;13:438–450. doi: 10.1038/nri3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cameron JD, Yang Z, Gibbs D, et al. HTRA1 variant confers similar risks to geographic atrophy and neovascular age-related macular degeneration. Cell Cycle. 2007;6:1122–1125. doi: 10.4161/cc.6.9.4157. [DOI] [PubMed] [Google Scholar]
- 38.Holliday EG, Smith AV, Cornes BK, et al. Wellcome Trust Case Control Consortium. Insights into the genetic architecture of early stage age-related macular degeneration: a genome-wide association study meta-analysis. [Accessed March 3, 2014];PLoS ONE. 2013 8:e53830. doi: 10.1371/journal.pone.0053830. [serial online] Available at: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0053830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Helgason H, Sulem P, Duvvari MR, et al. A rare nonsynonymous sequence variant in C3 is associated with high risk of age related macular degeneration. Nat Genet. 2013;45:1371–1374. doi: 10.1038/ng.2740. [DOI] [PubMed] [Google Scholar]
- 40.Seddon JM, Cote J, Davis N, Rosner B. Progression of age-related macular degeneration: association with body mass index, waist circumference, and waist-hip ratio. Arch Ophthalmol. 2003;121:785–792. doi: 10.1001/archopht.121.6.785. [DOI] [PubMed] [Google Scholar]
- 41.Mitta VP, Christen WG, Glynn RJ, et al. C-reactive protein and the incidence of macular degeneration: pooled analysis of 5 cohorts. JAMA Ophthalmol. 2013;131:507–513. doi: 10.1001/jamaophthalmol.2013.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnarsson A, Sverrisson T, Stefánsson E, et al. Risk factors for five-year incident age-related macular degeneration: the Reykjavik Eye Study. Am J Ophthalmol. 2006;142:419–428. doi: 10.1016/j.ajo.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 43.SanGiovanni JP, Chew EY, Agrón E, et al. Age-Related Eye Disease Study Research Group. The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23. Arch Ophthalmol. 2008;126:1274–1279. doi: 10.1001/archopht.126.9.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.