Abstract
Purpose
To report three cases of late recurrence of myopic foveoschisis (MF) after initial successful repair with pars plana vitrectomy (PPV) and membrane peeling to assess importance of internal limiting membrane peeling.
Methods
A retrospective, non-comparative case series was performed of patients who underwent a primary PPV by a single surgeon with successful resolution of MF, but eventually underwent repeat PPV for recurrent MF. Best corrected visual acuity, fundus photography and optical coherence tomography were obtained at each examination.
Results
3 eyes of 3 patients underwent PPV for recurrent MF. MF recurrence occurred 6, 3.5 and 12 years after the primary vitrectomy, respectively. Repeat vitrectomy with staining and additional peeling of the ILM resulted in good anatomical outcome and stabilization of visual acuity in all cases.
Conclusion
Late recurrence of MF after successful primary vitrectomy is described. Fibrocellular proliferation on residual cortical vitreous or incomplete internal limiting membrane peeling during the initial vitrectomy may underlie recurrence.
Keywords: maculopathy, myopia, retinal, schisis
Introduction
Myopic foveoschisis (MF), or myopic traction maculopathy, is a disorder of the vitreoretinal interface, characterized by retinoschisis of the posterior retina in highly myopic patients with posterior staphyloma.1-3 There are two subclasses described: a foveoschisis subclass in which the photoreceptor layer remains attached to retinal pigment epithelium (RPE) and the foveal detachment subclass in which the photoreceptor layer detaches from the RPE. MF occurs in 9-34 % of highly myopic eyes with posterior staphyloma.1-2, 4-5 The pathogenesis of MF remains unclear, however several etiologic factors have been suggested including tangential traction in the inner retina exerted by an epiretinal membrane (ERM) or residual vitreous cortex, rigidity of internal limiting membrane (ILM), thinning of the retina, stiffness of retinal vessels, and scleral curvature changes within the posterior staphyloma.4-10 OCT studies have depicted characteristic features of MF and the frequent association with other anomalies such as foveal detachment and macular holes (MH) with or without associated retinal detachment (RD).1, 6, 11-13 In general, 1/3 of the MF cases remain stable while 2/3 progress to MH with or without RD. Pars plana vitrectomy (PPV), a procedure that removes cortical vitreous from the posterior pole in combination with ILM peeling and gas tamponade, has become the standard of care for managing visually-significant MF with or without an associated MH.3,6,10,14-18 However, the long term outcomes of this procedure and the rate of MF recurrence are not well described in the literature. Herein, we present three cases of late recurrence of MF several years after a successful primary PPV.
Methods
A retrospective chart review of MF patients treated at Columbia University Medical Center was performed. We included MF patients who underwent a primary PPV with resolution of MF, but years later were noted with decline of visual acuity and MF recurrence requiring a second PPV. Surgical procedures were performed by a single surgeon (SC). At every clinic visit Snellen best corrected visual acuity (BCVA), slit lamp examination and funduscopic examination were recorded. Color fundus photographs and time domain optical coherence tomography (TD-OCT, Zeiss Stratus, for visits prior to 2007) or spectral domain OCT (SD-OCT, Zeiss Cirrus) imaging of the macula were obtained. Serial OCT scans were compared when available. The study had institutional review board approval by the Columbia University Institutional Review Board and complied with the Health Insurance Portability and Accountability Act of 1996.
Results
Three eyes of three patients who successfully underwent primary PPV for MF with late recurrence were identified. The primary PPV in all three cases consisted of a core vitrectomy, removal of cortical vitreous from the surface of posterior retina, fluid-air exchange (FAX) and gas tamponade. In two of the eyes, triamcinolone acetonide (TA) (Kenalog© 40 mg/ml, Bristol-Myers, New York, NY) was used to highlight the cortical vitreous layer, and partial ILM peeling was performed. The second PPV in all cases consisted of removal of any cortical vitreous tissue from the retinal surface assisted by TA, ILM peeling assisted by Brilliant Blue G, fluid-air exchange (FAX) and tamponade with 25% sulfur hexafluoride gas. Each case is detailed further below.
Case 1
A 53-year-old high myopic man (axial length 31.69 mm in the right eye (OD) and 31.59 mm in the left eye (OS)) was referred for OS recurrent MH associated to MF. He had history of a rhegmatogenous retinal detachment (RRD) repair with PPV OS, and two subsequent PPVs for a recurrent full thickness MH in the same eye. He also had uncomplicated cataract extraction in both eyes (OU). On initial examination, BCVA was 20/20 OD and 20/40 OS and anterior segment examination was unremarkable with posterior chamber intraocular lens (PCIOL) OU. OS dilated funduscopic examination (DFE) revealed myopic maculopathy with posterior staphyloma and a macular hole. OS OCT confirmed MF and MH.
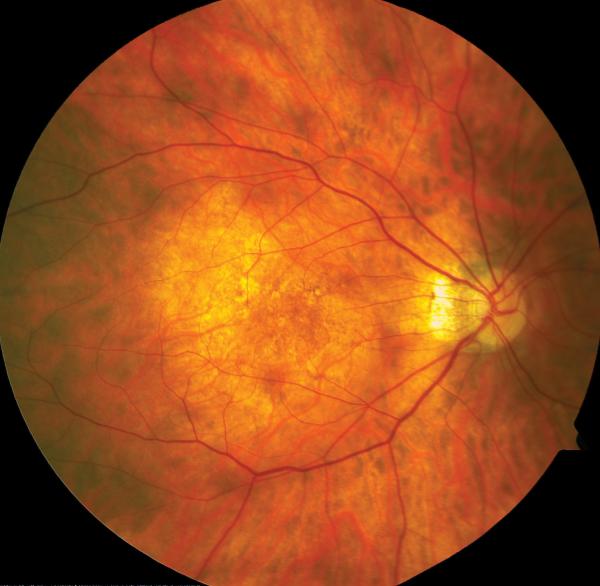
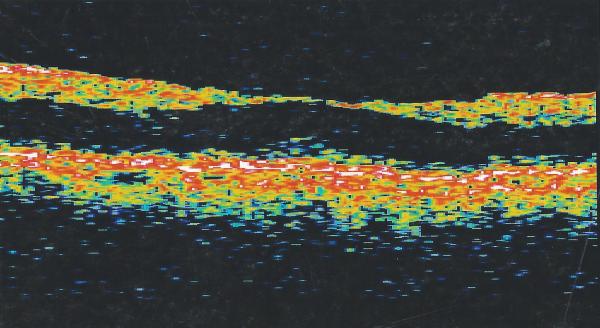
OS PPV, membrane peeling and gas tamponade was facilitated by TA to highlight cortical vitreous and ILM. This procedure resulted in a good anatomical outcome and BCVA OS was stable at 20/40 (Figs. 1A, 1B). However, 6 years later OS BCVA decreased to 20/60. Myopic traction maculopathy with posterior staphyloma and a lamellar hole was observed (Fig. 1C). SD-OCT revealed foveoschisis and a lamellar hole without signs of RD (Fig. 1D). After OS PPV with the peeling of residual cortical vitreous assisted by TA and additional peeling of ILM visualized after BBG staining, a complete resolution of MF (Fig. 1E) was obtained with OS BCVA improving to 20/40 four months postoperatively which has been maintained for 20 months of follow up (Fig. 1F).
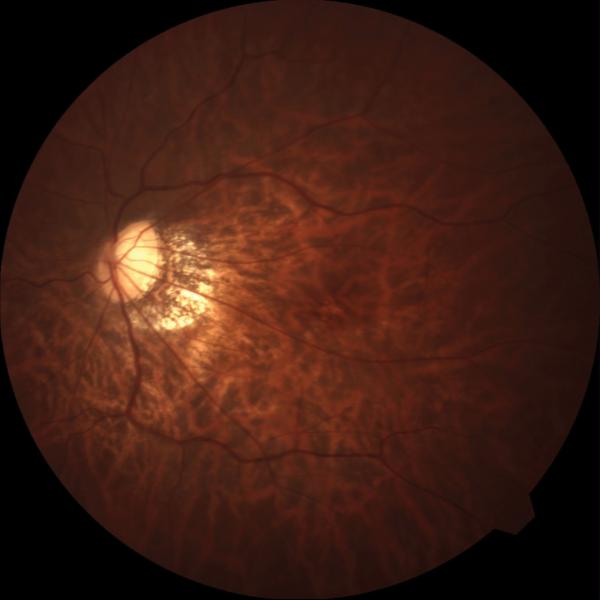
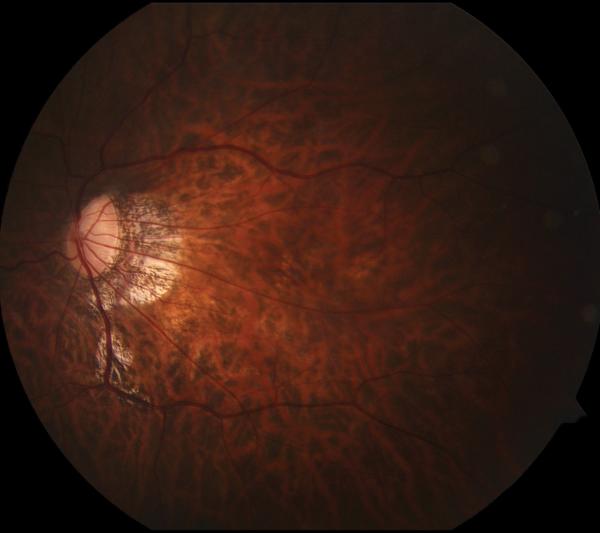
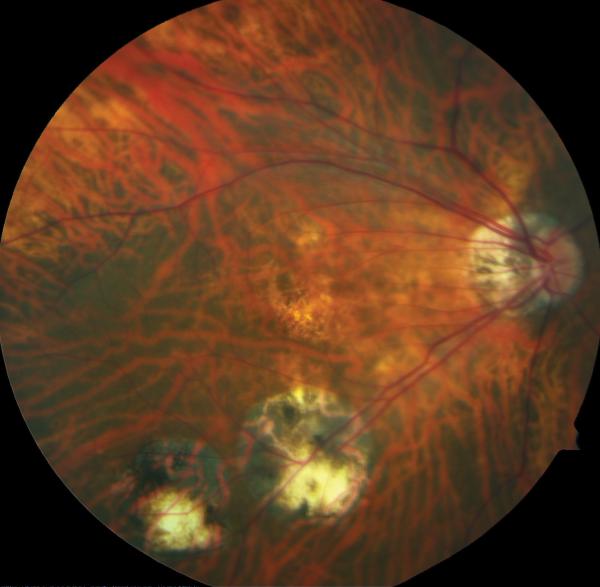
Figure 1.
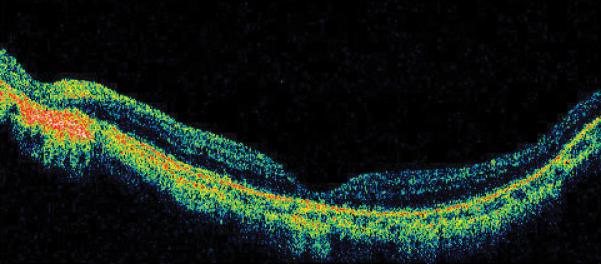
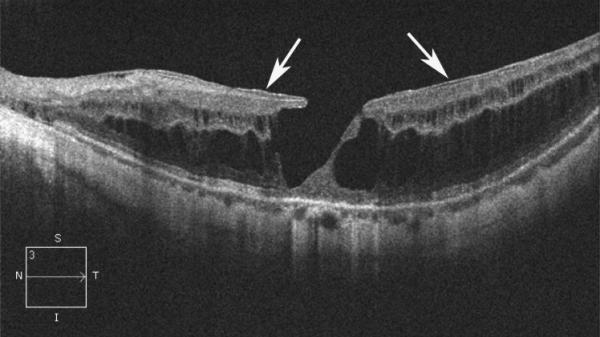
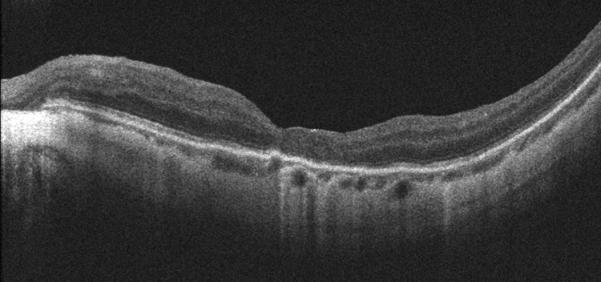
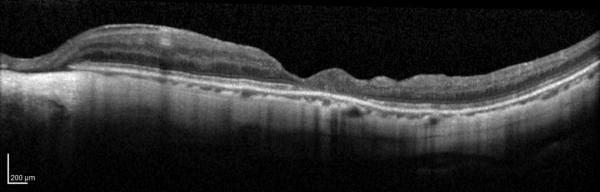
Case 1. Fundus photograph of the left eye (A) one month after pars plana vitrectomy (PPV) for myopic foveoschisis (MF) shows myopic maculopathy with posterior staphyloma, myopic conus and a tilted optic disc. Time-domain optical coherence tomograph (OCT, B) shows a flat macula with no evidence of MF, macular hole or residual traction. Fundus photograph of the left eye (C) 6 years after a primary PPV shows myopic maculopathy with posterior staphyloma, myopic conus and a tilted optic disc. Spectral-domain (SD) OCT (D) 6 years after a primary PPV shows recurrence of MF, a lamellar hole, and an epiretinal membrane (arrow). Four months after a second PPV there was a complete resolution of MF by SD-OCT (E), which remained stable at 20 months (F).
Case 2
A 56-year-old highly myopic woman (axial length 30.1 mm OD and 30.62 mm OS) presented with 3 months of decreased visual acuity OD with metamorphopsia. On initial examination, BCVA was 20/40 OD and 20/25-2 OS, and near visual acuity was Jaeger 5 in each eye. The anterior segment examination was unremarkable. OD DFE revealed a posterior staphyloma (Fig. 2A). SD-OCT showed foveoschisis without MH or RD (Fig. 2B). OD PPV was performed consisting of a core vitrectomy, removal of cortical vitreous from the posterior pole surface assisted by TA, ILM peeling assisted by indocyanine green (IC-Green©, Akorn Pharmaceuticals, Lake Forest, IL) and FAX. Two months postoperatively, residual foveoschisis was noted (Fig. 2C). 13 months after primary PPV, BCVA improved to 20/20 with full recovery of macular anatomy (Figs. 2D, 2E). However, 42 months postoperatively, OD BCVA decreased to 20/60 with metamorphopsia. SD-OCT evidenced MF recurrence (Figs. 2F, 2G). After a second PPV, which consisted of peeling of minimal cortical vitreous remnants assisted by TA and complete ILM peeling (after BBG staining) within the area of the posterior staphyloma, total flattening of the macula was obtained. Four months postoperatively, OD BCVA improved to 20/30 (Figs. 2H, 2I), which was maintained at 1 year postoperatively (Fig. 2J).
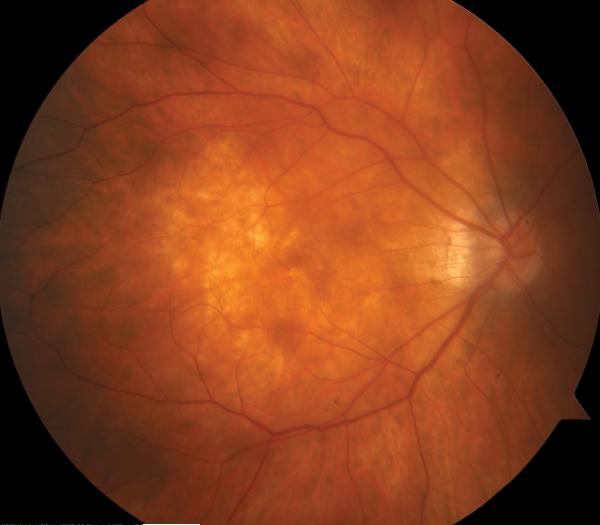
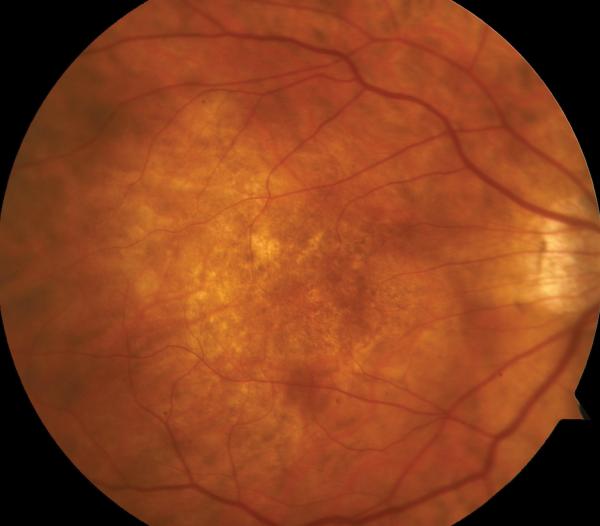
Figure 2.
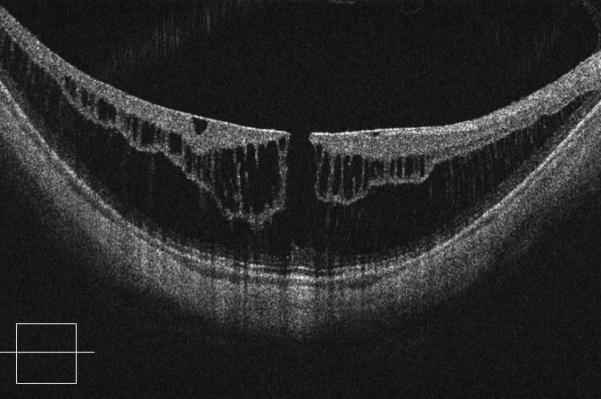
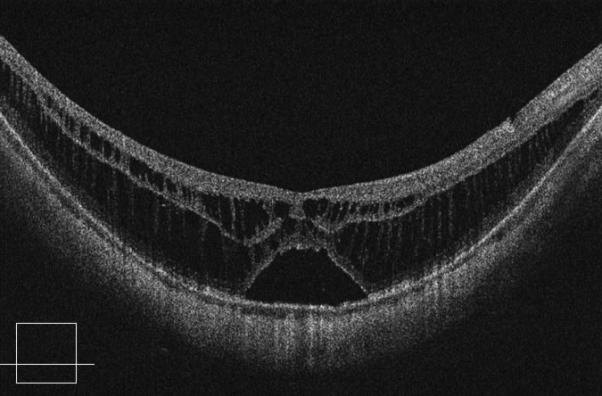
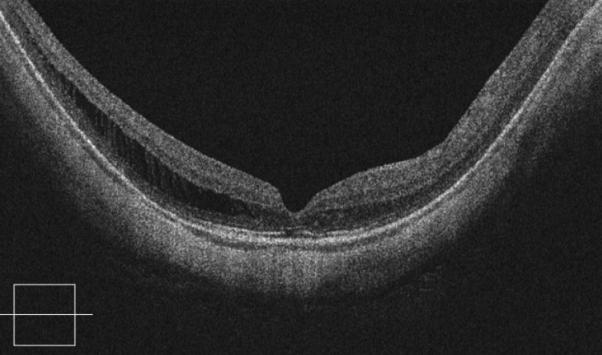
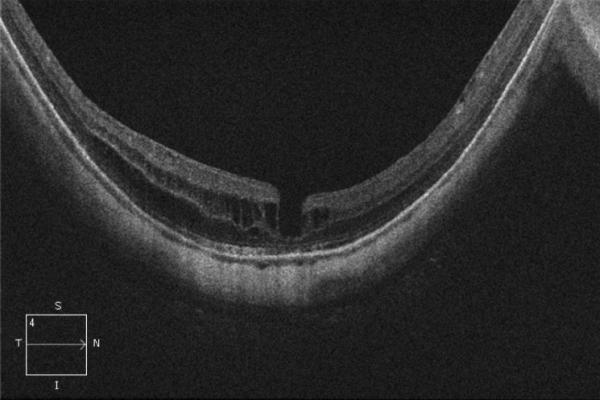
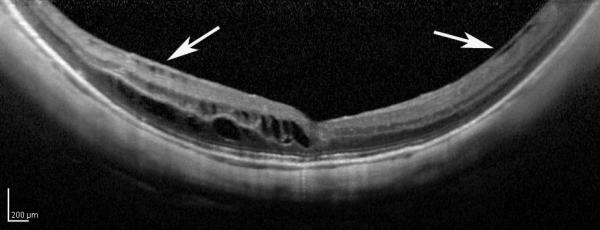
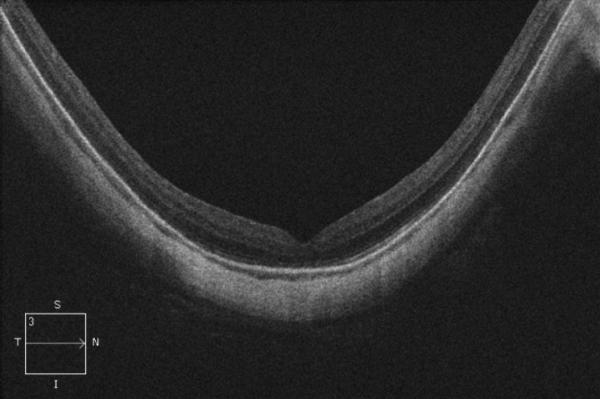
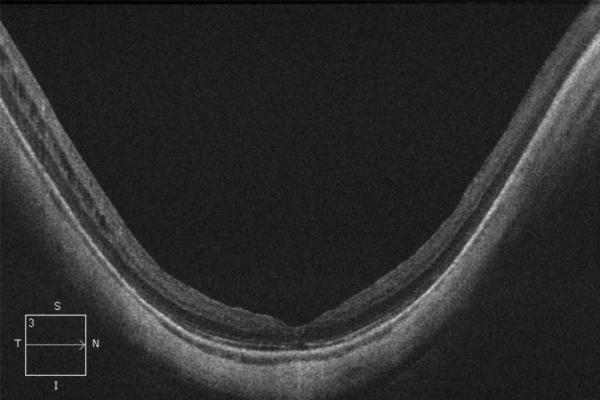
Case 2. Fundus photograph of the right eye (OD) at presentation (A) shows myopic maculopathy with a posterior staphyloma, myopic conus and a tilted optic disc. Spectral domain optical coherence tomograph (SD-OCT, B) shows myopic foveoschisis (MF) with an epiretinal membrane, but without macular hole (MH) or associated retinal detachment. SD-OCT two months after pars plana vitrectomy (PPV, C) shows residual MF. OD fundus photograph (D) and SD-OCT (E) 13 months after primary PPV shows almost complete resolution of MF with persistence of MF only in the temporal aspect and minimal ellipsoidal layer defect subfoveally. SD-OCT (Cirrus) 42 months after primary PPV (F) shows MF recurrence. SD-OCT (Spectralis) 42 months after primary PPV (G) shows MF recurrence with residual epiretinal tissue (arrows). Fundus photograph four months after the second PPV (H) was similar to the clinical appearance 13 months after the primary PPV (D). SD-OCT (Cirrus) four months after secondary PPV (I) shows resolution of foveoschisis, which was maintained at 1 year (J).
Case 3
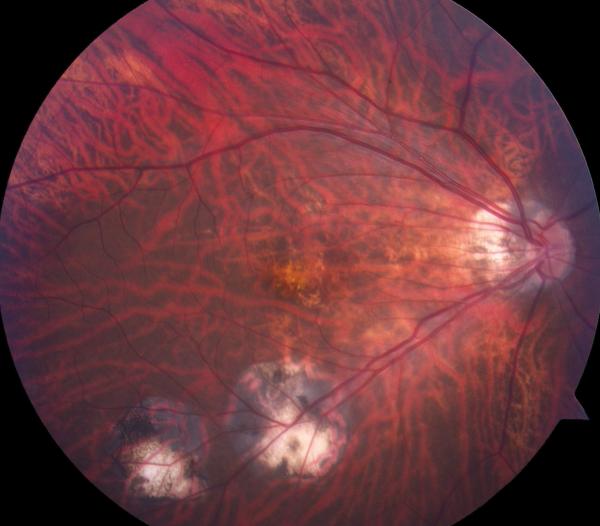
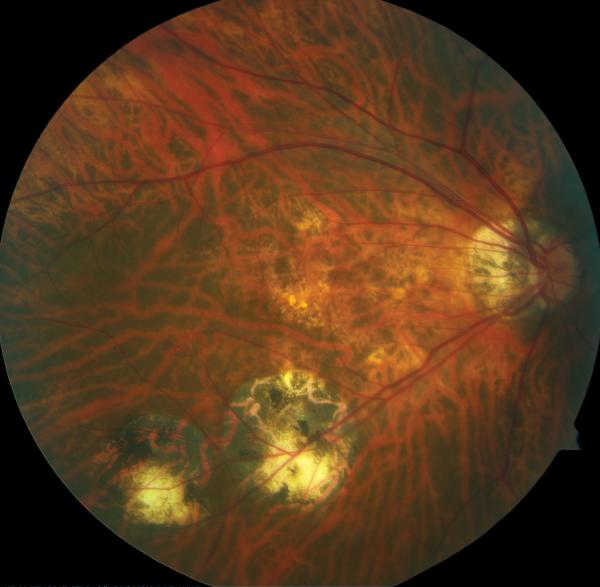
In 1999, a 56-year-old highly myopic woman (axial length 29.92 mm OD and 29.93 mm OS) presented with decreased OD vision. She had history of OS recurrent RRD secondary to MH status post four PPVs that was reattached with silicone oil tamponade. The silicone oil was later removed successfully. On initial examination, BCVA was 20/40 OD and 20/400 OS and anterior segment examination was unremarkable. OD DFE revealed myopic traction maculopathy within a posterior staphyloma. TD-OCT showed the splitting of the retinal layers (Fig. 3A). OD primary PPV was performed for MF. No adjuvant was used at that time to enhance cortical vitreous or ILM removal. Only a thin epiretinal membrane was found and removed. Ten months later, BCVA was 20/25 and TD-OCT showed a flat macula (Fig. 3B) with stability at seven years (Fig. 3C, 3D). During that period of time patient underwent uncomplicated cataract extraction and developed glaucoma OU. Twelve years after primary PPV, BCVA OD decreased to 20/70. On exam, OD posterior staphyloma and laser photocoagulation scars surrounding old retinal breaks were noted (Fig. 3E). SD-OCT showed foveoschisis without MH or RD (Fig. 3F). She underwent a second PPV OD, including removal of cortical vitreous remnants from the posterior pole surface around the staphyloma assisted by TA, ILM peeling assisted by BBG 0.25%, FAX and gas tamponade with SF6 20%. Five months postoperatively, OD BCVA improved to 20/50 with flattening of the macula (Figs. 3G, 3H) and has maintained OD BCVA at the 20/40 level for 20 months postoperatively (Fig. 3I).
Figure 3.
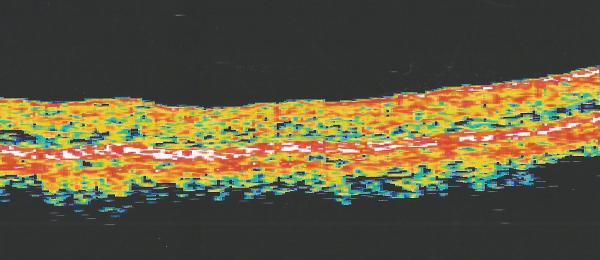
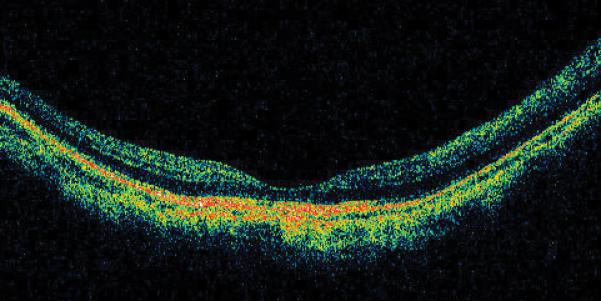
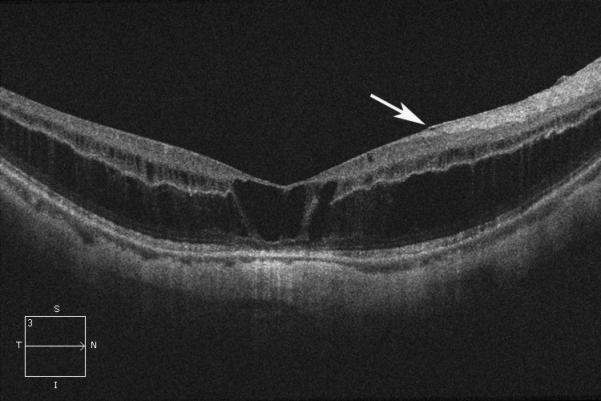
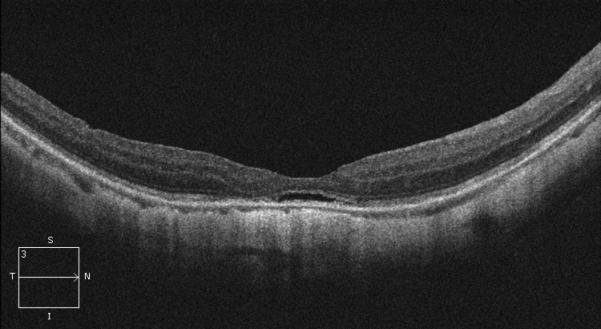
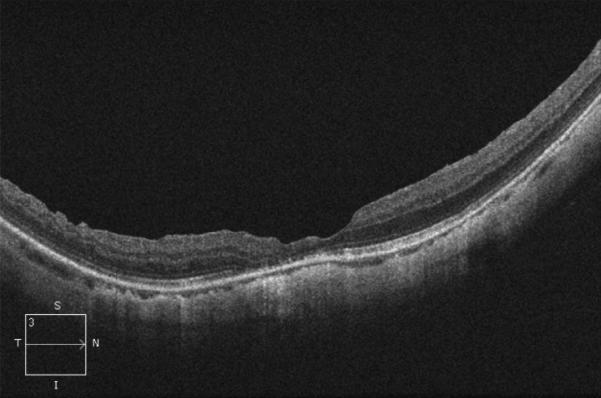
Case 3. Right eye (OD) macular time domain optical coherence tomography (OCT) shows foveoschisis with no signs of macular hole or retinal detachment (A). OCT 10 months after primary PPV shows a flat macula (B). OD fundus photograph 7 years after primary PPV (C) shows myopic maculopathy with posterior staphyloma, myopic conus, tilted optic disc and two posterior scars along the inferotemporal arcade. OD OCT 7 years postoperative (D) shows a flat macula with no evidence of myopic foveoschisis (MF) or residual traction. OD fundus photograph 12 years postoperative (E) was unchanged in comparison to 7 years (C), however, SD-OCT 12 years postoperative (F) revealed MF, with evident epiretinal tissue in the nasal aspect of the scan (arrow), but without signs of macular hole or associated retinal detachment. Fundus photograph five months after secondary PPV (G) was similar to the clinical appearance 7 years after the primary PPV (C). SD-OCT (H) at five months after secondary PPV shows resolution of MF, which was stable at 20 months postoperative (I).
Discussion
We describe three patients who underwent successful primary vitrectomy but experienced late recurrence of foveoschisis. After a second vitrectomy with more complete removal of epiretinal proliferation and ILM peeling, resolution of the MF and improvement of visual acuity was achieved and maintained for four months of follow-up.
Myopic foveoschisis, or myopic traction maculopathy, has been postulated to precede the formation of a macular hole with or without an associated retinal detachment resulting in marked decrease in visual acuity in a subgroup of highly myopic patients with posterior staphyloma.19-21 Complete posterior vitreous detachment, confirmed by biomicroscopy or ultrasound, does not prevent the cortical vitreous remnants from adhering to the retina. The exertion of tangential tensile forces on a thin retina, perhaps due to the persistence of pre-retinal vitreous remnants or ILM, might explain the recurrence of MF. This may be difficult to visualize clinically, and on examination, the retina appears to have a cystic appearance. Patients may subjectively notice decreased vision, most often when reading. SD-OCT demonstrates the splitting of the retinal layers.
Vitrectomy is a well-established surgical approach in treating MF.3,6,10,14-18 However, there are several surgical challenges in highly myopic eyes. Because these eyes are often more than 30 mm in axial length, standard vitreous instruments may not readily reach the posterior pole and manipulation of these instruments may be challenging. Additionally, chorioretinal atrophy may be present at the posterior pole in highly myopic patients may lead to low contrast and therefore difficulty identifying and visualizing a thin retina. Reported complications described in these eyes include macular hole formation and extrafoveal retinal holes.6,15,22 The vitreous structure in highly myopic eyes are also unusual. Vitreoschisis may be present, which appears as a layer of cortical vitreous, adherent to the surface of the retina. This layer of vitreous is not always visualized on OCT, and best seen at the time of vitrectomy using TA to highlight the cortical vitreous remnants and epiretinal membranes overlying the retinal surface.23 Adjuvants such as BBG, a selective ILM-staining dye, allows the identification, delimitation, and enhances the peeling of ILM in a more complete and safe fashion.24
In our cases showing late recurrence of MF, we have observed fibrocellular proliferation on the scaffold of cortical vitreous remnants and residual ILM during the subsequent PPV. These layers were not always visualized on SD-OCT imaging prior to the reoperation and were only clearly visible intraoperatively using triamcinolone and BBG staining. This suggests these patients require careful primary surgery with removal of the ILM within the limits of the posterior staphyloma. If residual layers of cortical vitreous remain after primary surgery, they may become compressed against the retinal surface and later result in recurrent tangential traction and recurrence of MF, or failure of MH closure. For example, in case 2, ICG was used to enhance the visualization of the ILM during the primary PPV. However, in the subsequent PPV, it was evident that remnants of ILM remained. ICG staining of ILM during the primary PPV was light and BBG stained the ILM more distinctly during the second PPV. Reproliferation of epiretinal membranes on residual cortical vitreous or incomplete ILM peeling during primary PPV could underlie the recurrence in our patients. For that reason, it is our assertion that the key factor to the success of this surgical procedure is the thorough identification of persistent cortical vitreous remnants and the complete peeling of ILM, allowing the complete removal of any possible tractional components and myofibroblasts on the ILM.25,26 Sayanagi et al. also reported that 2 eyes with persistent MF after primary vitrectomy without ILM peeling were successfully treated by reoperation including complete vitreous cortex removal and ILM peeling.27 These surgical goals are more readily attained employing the co-adjuvants TA and BBG. The cases presented here contradict prior reports that myopic foveoschisis can be treated effectively without internal limiting membrane peel.17-18 Additionally, a recent report suggests that foveal-sparing ILM peeling surgery may decrease the occurrence of postoperative MH in cases of MF.28
In summary, we describe a series of highly myopic patients with late recurrence of MF after successful primary vitrectomy. Fibrocellular proliferation on the scaffold of residual cortical vitreous or incompletely peeled ILM during the initial vitrectomy may be the cause. To reduce the chance of recurrence, complete release of vitreoretinal traction including ILM peeling is essential. In our experience, this surgical approach has yielded the best long-term visual outcomes in myopic foveoschisis. Given the long duration at which recurrence occurred in these cases (up to 12 years in Case 3), long-term follow up and studies are needed.
Summary Statement.
Pars plana vitrectomy with membrane peeling is an established surgical therapy developed to manage myopic foveoschisis. We present three cases with late recurrence of myopic foveoschisis after initial successful primary vitrectomy procedures. Fibrocellular proliferation on the scaffold of residual cortical vitreous and internal limiting membrane may underlie recurrence.
Acknowledgments
This publication was supported in part by an unrestricted award to the Harkness Eye Institute, Department of Ophthalmology, Columbia University College of Physicians and Surgeons from Research to Prevent Blindness and by a K12 Career Development Award (QVH) through Grant Number KL2 TR000081 (National Center for Advancing Translational Sciences, NIH).
Footnotes
The authors of this manuscript do not have a proprietary interest.
References
- 1.Takano M, Kishi S. Foveal retinoschisis and retinal detachment in severely myopic eyes with posterior staphyloma. Am J Ophthalmol. 1999;128:472–476. doi: 10.1016/s0002-9394(99)00186-5. [DOI] [PubMed] [Google Scholar]
- 2.Baba T, Ohno-Matsui K, Futagami S, et al. Prevalence and characteristics of foveal retinal detachment without macular hole in high myopia. Am J Ophthalmol. 2003;135:338–342. doi: 10.1016/s0002-9394(02)01937-2. [DOI] [PubMed] [Google Scholar]
- 3.Gaucher D, Haouchine B, Tadayoni R, et al. Long-term follow-up of high myopic foveoschisis: natural course and surgical outcome. Am J Ophthalmol. 2007;143:455–462. doi: 10.1016/j.ajo.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 4.Wu PC, Chen YJ, Chen YH, et al. Factors associated with foveoschisis and foveal detachment without macular hole in high myopia. Eye. 2009;23:356–361. doi: 10.1038/sj.eye.6703038. [DOI] [PubMed] [Google Scholar]
- 5.Panozzo G, Mercanti A. Optical coherence tomography findings in myopic traction maculopathy. Arch Ophthalmol. 2004;122:1455–1460. doi: 10.1001/archopht.122.10.1455. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi H, Kishi S. Vitreous surgery for highly myopic eyes with foveal detachment and retinoschisis. Ophthalmology. 2003;110:1702–1707. doi: 10.1016/S0161-6420(03)00714-0. [DOI] [PubMed] [Google Scholar]
- 7.Kuhn F. Internal limiting membrane removal for macular detachment in highly myopic eyes. Am J Ophthalmol. 2003;135:547–549. doi: 10.1016/s0002-9394(02)02057-3. [DOI] [PubMed] [Google Scholar]
- 8.Sayanagi K, Ikuno Y, Tano Y. Tractional internal limiting membrane detachment in highly myopic eyes. Am J Ophthalmol. 2006;142:850–852. doi: 10.1016/j.ajo.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Ikuno Y, Gomi F, Tano Y. Potent retinal arteriolar traction as a possible cause of myopic foveoschisis. Am J Ophthalmol. 2005;139:462–467. doi: 10.1016/j.ajo.2004.09.078. [DOI] [PubMed] [Google Scholar]
- 10.Baba T, Tanaka S, Maesawa A, et al. Scleral buckling with macular plombe for eyes with myopic macular retinoschisis and retinal detachment without macular hole. Am J Ophthalmol. 2006;142:483–487. doi: 10.1016/j.ajo.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 11.Ikuno Y, Tano Y. Early macular holes with retinoschisis in highly myopic eyes. Am J Ophthalmol. 2003;136:741–744. doi: 10.1016/s0002-9394(03)00319-2. [DOI] [PubMed] [Google Scholar]
- 12.Matsumura N, Ikuno Y, Tano Y. Posterior vitreous detachment and macular hole formation in myopic foveoschisis. Am J Ophthalmol. 2004;138:1071–1073. doi: 10.1016/j.ajo.2004.06.064. [DOI] [PubMed] [Google Scholar]
- 13.Sayanagi K, Ikuno Y, Tano Y. Spontaneous resolution of retinoschisis and consequent development of retinal detachment in highly myopic eye. Br J Ophthalmol. 2006;90:652–653. doi: 10.1136/bjo.2005.085233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanda S, Uemura A, Sakamoto Y, Kita H. Vitrectomy with internal limiting membrane peeling for macular retinoschisis and retinal detachment without macular hole in highly myopic eyes. Am J Ophthalmol. 2003;136:177–180. doi: 10.1016/s0002-9394(03)00243-5. [DOI] [PubMed] [Google Scholar]
- 15.Ikuno Y, Sayanagi K, Ohji M, et al. Vitrectomy and internal limiting membrane peeling for myopic foveoschisis. Am J Ophthalmol. 2004;137:719–724. doi: 10.1016/j.ajo.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Sayanagi K, Ikuno Y, Tano Y. Reoperation for persistent myopic foveoschisis after primary vitrectomy. Am J Ophthalmol. 2006;141:414–417. doi: 10.1016/j.ajo.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Kwok AK, Lai TY, Yip WW. Vitrectomy and gas tamponade without internal limiting membrane peeling for myopic foveoschisis. Br J Ophthalmol. 2005;89:1180–1183. doi: 10.1136/bjo.2005.069427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spaide RF, Fisher Y. Removal of adherent cortical vitreous plaques without removing the internal limiting membrane in the repair of macular detachments in highly myopic eyes. Retina. 2005;25:290–295. doi: 10.1097/00006982-200504000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Fang X, Weng Y, Xu S, et al. Optical coherence tomographic characteristics and surgical outcome of eyes with myopic foveoschisis. Eye. 2009;23:1336–1342. doi: 10.1038/eye.2008.291. [DOI] [PubMed] [Google Scholar]
- 20.Shimada N, Ohno-Matsui K, Baba T, et al. Natural course of macular retinoschisis in highly myopic eyes without macular hole or retinal detachment. Am J Ophthalmol. 2006;142:497–500. doi: 10.1016/j.ajo.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 21.Shimada N, Ohno-Matsui K, Yoshida T, et al. Progression from macular retinoschisis to retinal detachment in highly myopic eyes is associated with outer lamellar hole formation. Br J Ophthalmol. 2008;92:762–764. doi: 10.1136/bjo.2007.131359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steven P, Laqua H, Wong D, Hoerauf H. Secondary paracentral retinal holes following internal limiting membrane removal. Br J Ophthalmol. 2006;90:293–295. doi: 10.1136/bjo.2005.078188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang X, Zheng X, Weng Y, et al. Anatomical and visual outcome after vitrectomy with triamcinolone acedonide-assisted epiretinal membrane removal in highly myopic eyes with retinal detachment due to macular hole. Eye. 2009;23:248–54. doi: 10.1038/eye.2008.60. [DOI] [PubMed] [Google Scholar]
- 24.Mochizuki Y, Enaida H, Hisatomi T, et al. The internal limiting membrane peeling with brilliant blue G staining for retinal detachment due to macular hole in high myopia. Br J Ophthalmol. 2008;92:1009–1010. doi: 10.1136/bjo.2007.126300. [DOI] [PubMed] [Google Scholar]
- 25.Kadonosono K, Yazama F, Itoh N, et al. Treatment of retinal detachment resulting from myopic macular hole with internal limiting membrane removal. Am J Ophthalmol. 2001;131:203–207. doi: 10.1016/s0002-9394(00)00728-5. [DOI] [PubMed] [Google Scholar]
- 26.Yooh HS, Brooks HL, Jr, Capone A, et al. Ultrastructural features of tissue removed during idiopathic macular hole surgery. Am J Ophthalmol. 1996;122:67–75. doi: 10.1016/s0002-9394(14)71965-8. [DOI] [PubMed] [Google Scholar]
- 27.Sayanagi K, Ikuno Y, Tano Y. Reoperation for persistent myopic foveoschisis after primary vitrectomy. Am J Ophthalmol. 2006;141:414–417. doi: 10.1016/j.ajo.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Shimada N, Sugamoto Y, Ogawa M, Takase H, Ohno-Matsui K. Fovea-sparing internal limiting membrane peeling for myopic traction maculopathy. Am J Ophthalmol. 2012 Oct;154(4):693–701. doi: 10.1016/j.ajo.2012.04.013. [DOI] [PubMed] [Google Scholar]