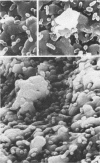
Abstract
A mixture of saliva-coated hydroxyapatite beads and radioactively labeled bacteria has been employed as an in vitro model for the initial phase of dental plaque formation. Adsorption in this model can be expressed by the Langmuir adsorption isotherm, and the adherence of oral streptococci can be expressed as the product of the affinity constant (Ka) and the number of binding sites (N), KaN. With this approach, Streptococcus sanguis serotype 1 strains adhered better (KaN = [187 +/- 72] X 10(-2)) than serotype 2 strains (KaN = [97 +/- 84] X 10(-2)); a t test showed this difference to be statistically significant to the 99.99% confidence level. Strains of S. mitis, S. mutans, and S. salivarius did not appear to adhere as well. To analyze the bacterial receptors involved in adherence, competition studies in which increasing quantities of unlabeled bacteria were added to a fixed quantity (4 X 10(9) cells per ml) of 3H-labeled serotype 1, reference strain S. sanguis G9B, were performed. These studies indicated that the type 1 strains competed for the same, or closely related, binding sites. Competition studies using serotype 2 S. sanguis strains resulted in an increased binding of reference strain G9B to hydroxyapatite. Scanning electron microscopy indicated this effect was due to the formation of localized aggregations of bacteria, presumably representing the two bacterial types. The results of competition studies with S. mitis were variable, and several strains of other oral bacteria showed little or no competition.
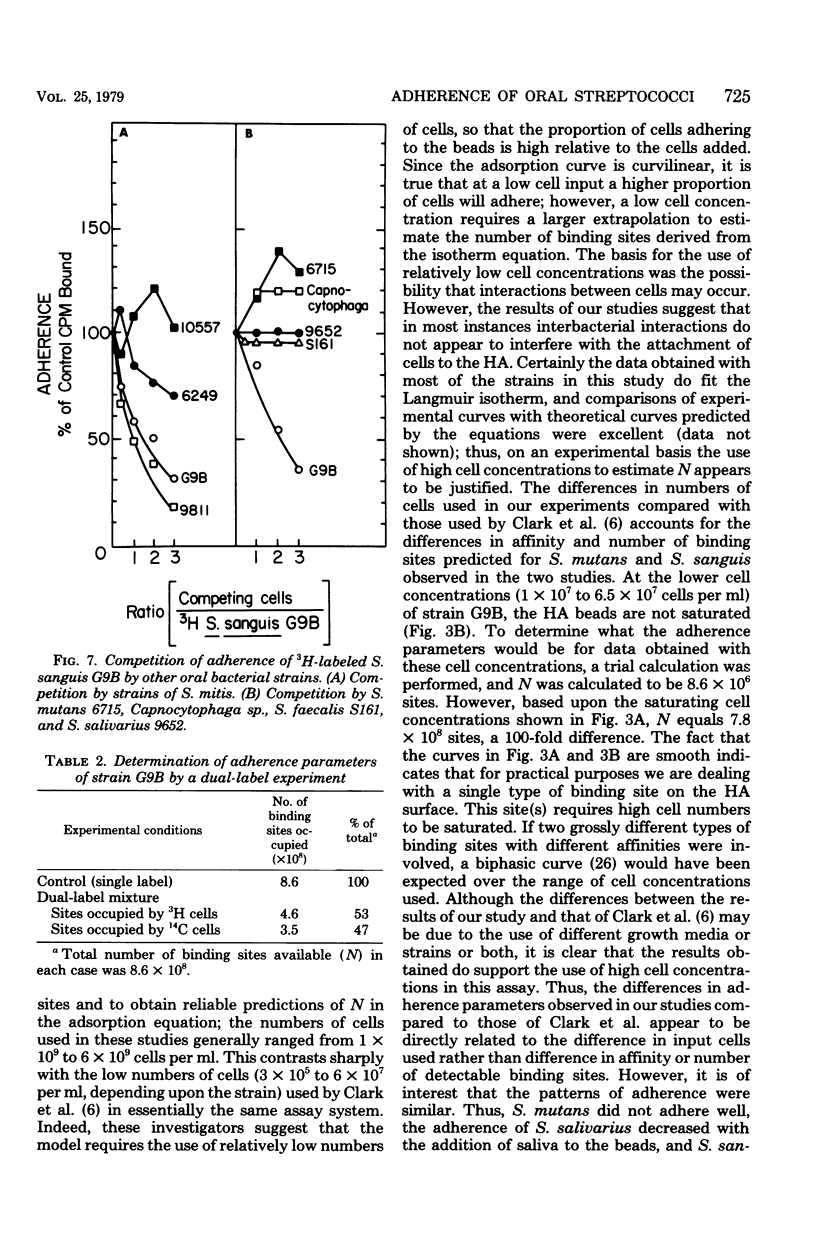
Full text
PDF


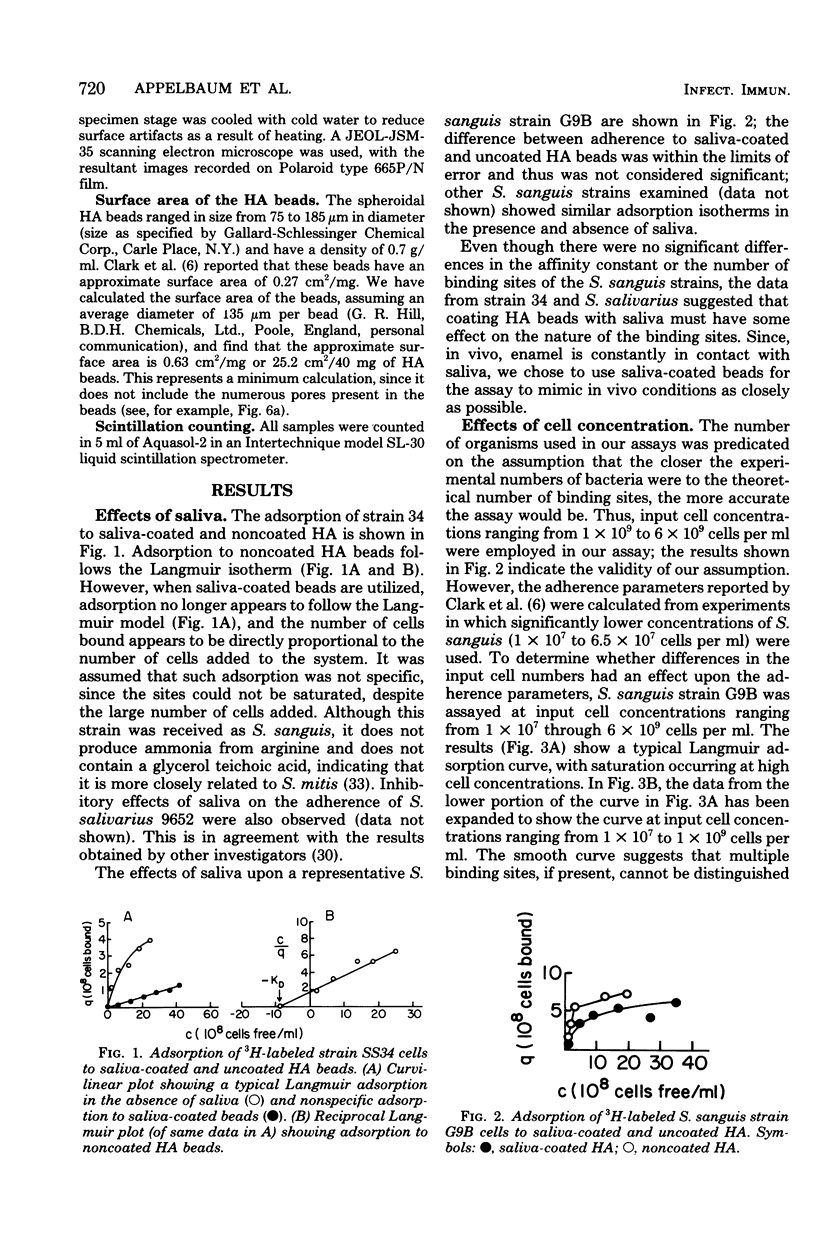
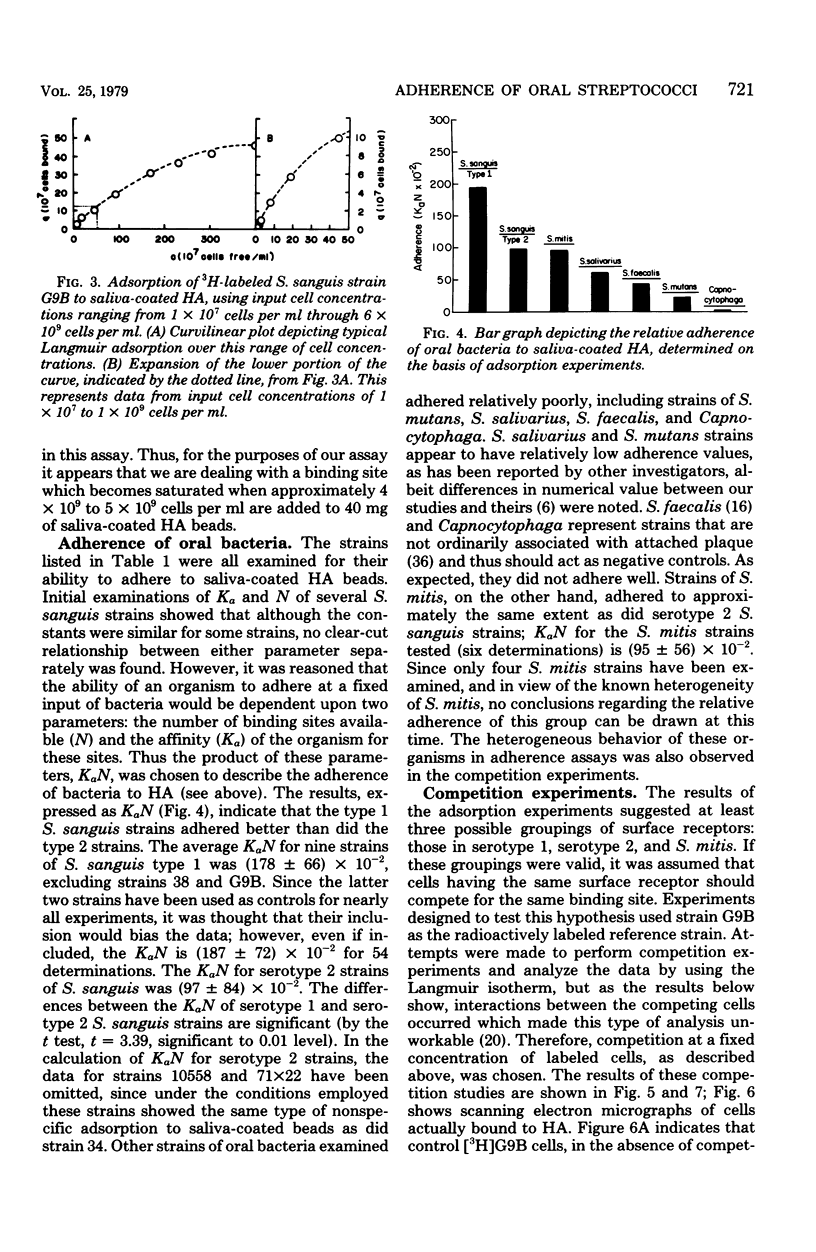
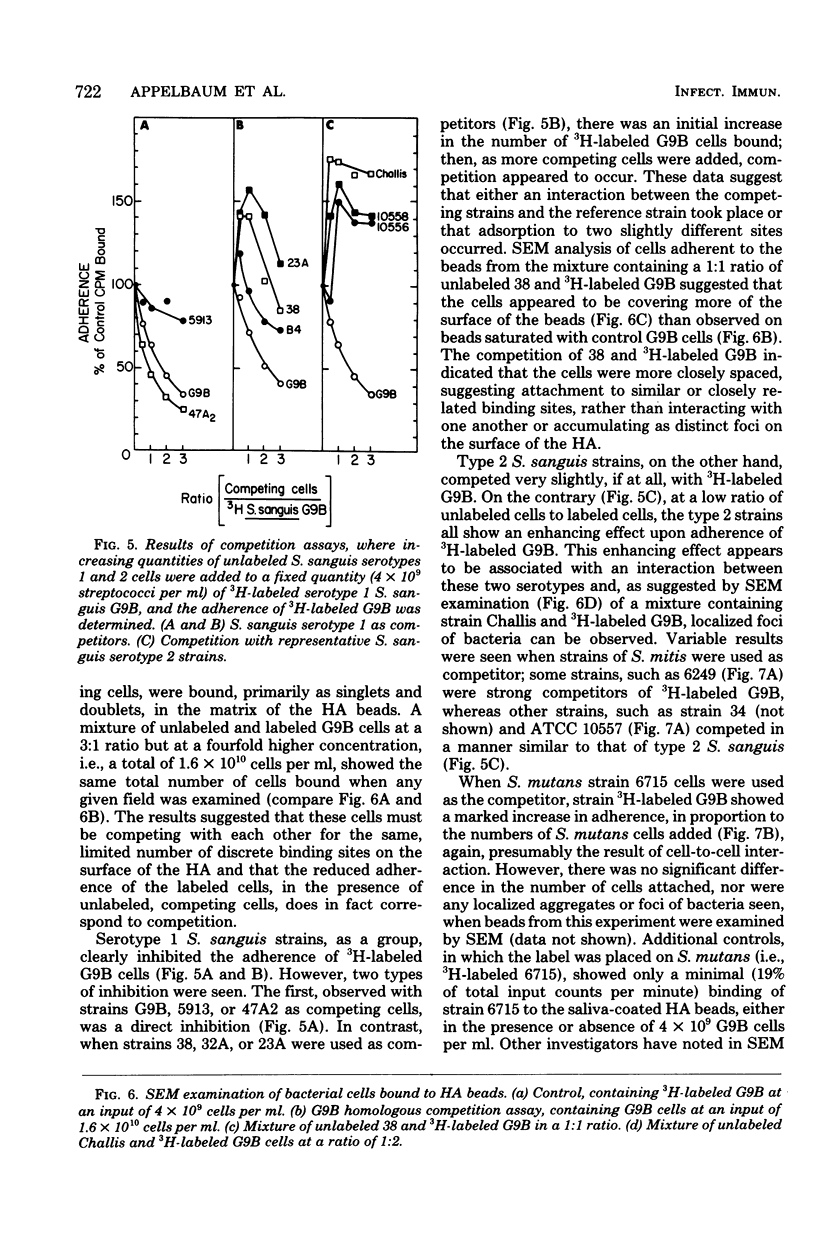





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourgeau G., McBride B. C. Dextran-mediated interbacterial aggregation between dextran-synthesizing streptococci and Actinomyces viscosus. Infect Immun. 1976 Apr;13(4):1228–1234. doi: 10.1128/iai.13.4.1228-1234.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J. A numerical taxonomic study of human oral streptococci. Odontol Revy. 1968;19(2):137–160. [PubMed] [Google Scholar]
- Carlsson J., Grahnén H., Jonsson G. Lactobacilli and streptococci in the mouth of children. Caries Res. 1975;9(5):333–339. doi: 10.1159/000260166. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Grahnén H., Jonsson G., Wikner S. Establishment of Streptococcus sanguis in the mouths of infants. Arch Oral Biol. 1970 Dec;15(12):1143–1148. doi: 10.1016/0003-9969(70)90005-1. [DOI] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Gibbons R. J. Influence of salivary components and extracellular polysaccharide synthesis from sucrose on the attachment of Streptococcus mutans 6715 to hydroxyapatite surfaces. Infect Immun. 1977 Nov;18(2):514–523. doi: 10.1128/iai.18.2.514-523.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Geesey G. G., Cheng K. J. How bacteria stick. Sci Am. 1978 Jan;238(1):86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- Eden C. S., Eriksson B., Hanson L. A. Adhesion of Escherichia coli to human uroepithelial cells in vitro. Infect Immun. 1977 Dec;18(3):767–774. doi: 10.1128/iai.18.3.767-774.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén C. S., Hansson H. A. Escherichia coli pili as possible mediators of attachment to human urinary tract epithelial cells. Infect Immun. 1978 Jul;21(1):229–237. doi: 10.1128/iai.21.1.229-237.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Balcerzak-Raczkowski I. B. Interbacterial aggregation of Actinomyces naeslundii and dental plaque streptococci. J Periodontal Res. 1977 Jan;12(1):11–20. doi: 10.1111/j.1600-0765.1977.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Facklam R. R. Physiological differentiation of viridans streptococci. J Clin Microbiol. 1977 Feb;5(2):184–201. doi: 10.1128/jcm.5.2.184-201.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost A. J., Wanasinghe D. D., Woolcock J. B. Some factors affecting selective adherence of microorganisms in the bovine mammary gland. Infect Immun. 1977 Jan;15(1):245–253. doi: 10.1128/iai.15.1.245-253.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Moreno E. C., Spinell D. M. Model delineating the effects of a salivary pellicle on the adsorption of Streptococcus miteor onto hydroxyapatite. Infect Immun. 1976 Oct;14(4):1109–1112. doi: 10.1128/iai.14.4.1109-1112.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Spinell D. M., Skobe Z. Selective adherence as a determinant of the host tropisms of certain indigenous and pathogenic bacteria. Infect Immun. 1976 Jan;13(1):238–246. doi: 10.1128/iai.13.1.238-246.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. On the formation of dental plaques. J Periodontol. 1973 Jun;44(6):347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- Gold O. G., Jordan H. V., van Houte J. The prevalence of enterococci in the human mouth and their pathogenicity in animal models. Arch Oral Biol. 1975 Jul;20(7):473–477. doi: 10.1016/0003-9969(75)90236-8. [DOI] [PubMed] [Google Scholar]
- Isaacson R. E., Fusco P. C., Brinton C. C., Moon H. W. In vitro adhesion of Escherichia coli to porcine small intestinal epithelial cells: pili as adhesive factors. Infect Immun. 1978 Aug;21(2):392–397. doi: 10.1128/iai.21.2.392-397.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelstrup J., Funder-Nielsen T. D. Aggregation of oral streptococci with Fusobacterium and Actinomyces. J Biol Buccale. 1974 Dec;2(4):347–362. [PubMed] [Google Scholar]
- Kimura L. H., Pearsall N. N. Adherence of Candida albicans to human buccal epithelial cells. Infect Immun. 1978 Jul;21(1):64–68. doi: 10.1128/iai.21.1.64-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine M. J., Herzberg M. C., Levine M. S., Ellison S. A., Stinson M. W., Li H. C., van Dyke T. Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect Immun. 1978 Jan;19(1):107–115. doi: 10.1128/iai.19.1.107-115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Gibbons R. J. Proportional distribution and relative adherence of Streptococcus miteor (mitis) on various surfaces in the human oral cavity. Infect Immun. 1972 Nov;6(5):852–859. doi: 10.1128/iai.6.5.852-859.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Schauer S. V. Competitive binding among oral strptococci to hydroxyapatite. J Dent Res. 1977 Feb;56(2):157–165. doi: 10.1177/00220345770560021001. [DOI] [PubMed] [Google Scholar]
- Liljemark W. F., Schauer S. V. Studies on the bacterial components which bind Streptococcus sanguis and Streptococcus mutans to hydroxyapatite. Arch Oral Biol. 1975 Sep;20(9):609–615. doi: 10.1016/0003-9969(75)90082-5. [DOI] [PubMed] [Google Scholar]
- McBride B. C., Gisslow M. T. Role of sialic acid in saliva-induced aggregation of Streptococcus sanguis. Infect Immun. 1977 Oct;18(1):35–40. doi: 10.1128/iai.18.1.35-40.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton C., Reynolds H., Genco R. J. Combined micromanipulation, culture and immunofluorescent techniques for isolation of the coccal organisms comprising the "corn cob" configuration of human dental plaque. J Biol Buccale. 1977 Dec;5(4):321–332. [PubMed] [Google Scholar]
- Orstavik D., Kraus F. W., Henshaw L. C. In vitro attachment of streptococci to the tooth surface. Infect Immun. 1974 May;9(5):794–800. doi: 10.1128/iai.9.5.794-800.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosan B. Absence of glycerol teichoic acids in certain oral streptococci. Science. 1978 Sep 8;201(4359):918–920. doi: 10.1126/science.684416. [DOI] [PubMed] [Google Scholar]
- Rosan B. Antigens of Streptococcus sanguis. Infect Immun. 1973 Feb;7(2):205–211. doi: 10.1128/iai.7.2.205-211.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosan B. Relationship of the cell wall composition of group H streptococci and Streptococcus sanguis to their serological properties. Infect Immun. 1976 Apr;13(4):1144–1153. doi: 10.1128/iai.13.4.1144-1153.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S. S. Microbiology of periodontal disease -- present status and future considerations. J Periodontol. 1977 Sep;48(9):497–504. doi: 10.1902/jop.1977.48.9.497. [DOI] [PubMed] [Google Scholar]
- Takazoe I., Matsukubo T., Katow T. Experimental formation of "corn cob" in vitro. J Dent Res. 1978 Feb;57(2):384–387. doi: 10.1177/00220345780570024101. [DOI] [PubMed] [Google Scholar]
- Tramont E. C., Wilson C. Variations in buccal cell adhesion of Neisseria gonorrhoeae. Infect Immun. 1977 May;16(2):709–711. doi: 10.1128/iai.16.2.709-711.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Pulkkinen A. J. Adherence as an ecological determinant for streptococci in the human mouth. Arch Oral Biol. 1971 Oct;16(10):1131–1141. doi: 10.1016/0003-9969(71)90042-2. [DOI] [PubMed] [Google Scholar]