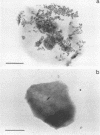
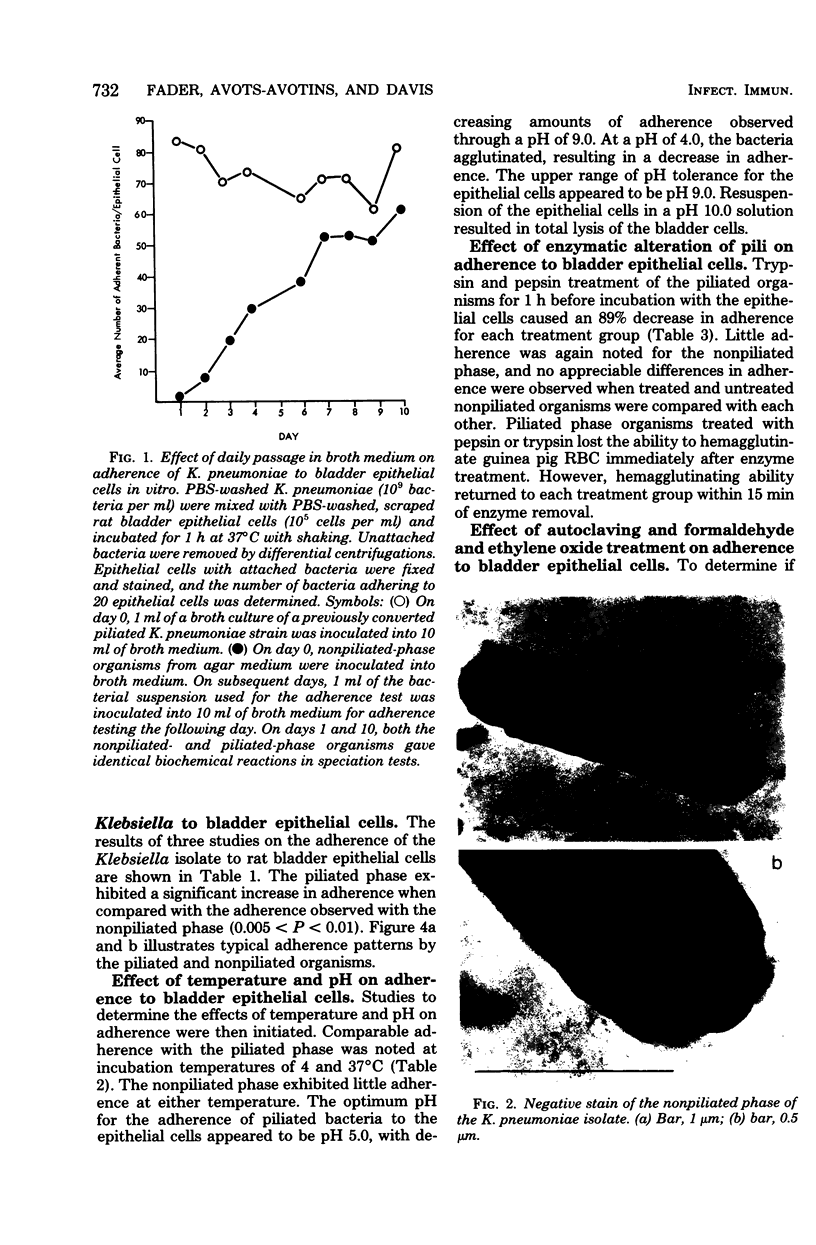
Abstract
The possible role of pili in the pathogenesis of urinary tract infections caused by Klebsiella pneumoniae was studied in an in vitro mixture of a phosphate-buffered saline suspension of rat bladder epithelial cells and phosphate-buffered saline-washed K. pneumoniae. Nonpiliated and piliated populations derived from a single K. pneumoniae strain were obtained by controlling the total time of growth in broth medium. The piliated phase demonstrated a significant increase in adherence when compared to the nonpiliated phase. Incubation of the bacteria and epithelial cell mixture at 4 and 37 degrees C resulted in no differences in adherence; optimal adherence occurred at pH 5. Pretreatment of the bacteria with enzymes to destroy the pili resulted in a decrease in adherence, as did killing the bacteria by various means before adherence testing. Pretreatment of the epithelial cells with certain saccharides inhibited bacterial adherence. Finally, a 96% decrease in adherence was observed after coincubation of bacteria and epithelial cells with papain-treated antipili antibodies. Thus, it appears that pili on the surface of K. pneumoniae mediate attachment of the bacteria to rat bladder epithelial cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- CONSTABLE F. L. Fimbriae and haemagglutinating activity in strains of Bacterium cloacae. J Pathol Bacteriol. 1956 Jul;72(1):133–136. doi: 10.1002/path.1700720117. [DOI] [PubMed] [Google Scholar]
- DUGUID J. P. Fimbriae and adhesive properties in Klebsiella strains. J Gen Microbiol. 1959 Aug;21:271–286. doi: 10.1099/00221287-21-1-271. [DOI] [PubMed] [Google Scholar]
- DUGUID J. P., SMITH I. W., DEMPSTER G., EDMUNDS P. N. Non-flagellar filamentous appendages (fimbriae) and haemagglutinating activity in Bacterium coli. J Pathol Bacteriol. 1955 Oct;70(2):335–348. doi: 10.1002/path.1700700210. [DOI] [PubMed] [Google Scholar]
- Duguid J. P., Darekar M. R., Wheater D. W. Fimbriae and infectivity in Salmonella typhimurium. J Med Microbiol. 1976 Nov;9(4):459–473. doi: 10.1099/00222615-9-4-459. [DOI] [PubMed] [Google Scholar]
- Eden C. S., Eriksson B., Hanson L. A. Adhesion of Escherichia coli to human uroepithelial cells in vitro. Infect Immun. 1977 Dec;18(3):767–774. doi: 10.1128/iai.18.3.767-774.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén C. S., Hansson H. A. Escherichia coli pili as possible mediators of attachment to human urinary tract epithelial cells. Infect Immun. 1978 Jul;21(1):229–237. doi: 10.1128/iai.21.1.229-237.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLIES R. R., DUGUID J. P. The fimbrial antigens of Shigella flexneri. J Hyg (Lond) 1958 Sep;56(3):303–318. doi: 10.1017/s0022172400037803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny C., Carnahan J., Brinton C. C., Jr Mechanical removal of F pili, type I pili, and flagella from Hfr and RTF donor cells and the kinetics of their reappearance. J Bacteriol. 1969 Jun;98(3):1294–1306. doi: 10.1128/jb.98.3.1294-1306.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old D. C. Inhibition of the interaction between fimbrial haemagglutinins and erythrocytes by D-mannose and other carbohydrates. J Gen Microbiol. 1972 Jun;71(1):149–157. doi: 10.1099/00221287-71-1-149. [DOI] [PubMed] [Google Scholar]
- PUTNAM F. W., TAN M., LYNN L. T., EASLEY C. W., MIGITA S. The cleavage of rabbit gamma-globulin by papain. J Biol Chem. 1962 Mar;237:717–726. [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Type I Escherichia coli pili: characterization of binding to monkey kidney cells. J Exp Med. 1977 Nov 1;146(5):1182–1194. doi: 10.1084/jem.146.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J. Host-parasite interaction in the rat renal pelvis: a possible role for pili in the pathogenesis of pyelonephritis. J Exp Med. 1974 Dec 1;140(6):1696–1711. doi: 10.1084/jem.140.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Ofek I. Influence of pili on the virulence of Proteus mirabilis in experimental hematogenous pyelonephritis. J Infect Dis. 1978 Nov;138(5):664–667. doi: 10.1093/infdis/138.5.664. [DOI] [PubMed] [Google Scholar]
- Smith T. W., Jr, Roberts J. A. Chronic pyelonephritis. Electron microscopic study. II. Persistence of variant bacterial forms. Invest Urol. 1978 Sep;16(2):154–162. [PubMed] [Google Scholar]