Abstract
Background
The high rate of comorbidity between depression and cocaine addiction suggests shared molecular mechanisms and anatomical pathways. Limbic structures, such as the Nucleus Accumbens (NAc), play a crucial role in both disorders, yet how different cell types within these structures contribute to the pathogenesis remains elusive. Downregulation of p11 (S100A10) specifically in the NAc elicits depressive like behaviors in mice but its role in drug addiction is unknown.
Methods
We combine mouse genetics and viral strategies to determine how the titration of p11 levels within the entire NAc affects the rewarding actions of cocaine on behavior (6 to 8 mice per group) and molecular correlates (3 experiments, 5 to 8 mice per group). Finally, the manipulation of p11 expression in distinct NAc dopaminoceptive neuronal subsets distinguished cell type specific effects of p11 on cocaine reward (5 to 8 mice per group).
Results
We demonstrate that p11 knockout mice have enhanced cocaine conditioned place preference (CPP), which is reproduced by the focal downregulation of p11 in the NAc of wild-type mice. In wild-type mice, cocaine reduced p11 expression in the NAc, while p11 overexpression exclusively in the NAc reduced cocaine CPP. Finally, we identify dopamine receptor-1 (D1) expressing medium spiny neurons (MSNs) as key mediators of p11’s effects on cocaine reward.
Conclusions
Our data provide evidence that disruption of p11 homeostasis in the NAc particularly in D1 expressing MSNs may underlie pathophysiological mechanisms of cocaine rewarding action. Treatments to counter maladaptation of p11 levels may provide novel therapeutic opportunities for cocaine addiction.
Keywords: Cocaine reward, p11 (s100a10), Nucleus Accumbens, conditional knock down, striato nigral pathway, striatopallidal pathway
Introduction
Reward-related learning is an essential conserved behavior necessary for survival, and any dysfunction of this system can result in serious pathologic consequences. Perturbations of the natural reward mechanisms can result in both depression and addictive behaviors. Major depressive symptoms influenced by these mechanisms include anhedonia (1), whereas cocaine addiction can promote depressive symptoms while simultaneously increasing responses to drug rewards (2–4). The high prevalence and comorbidity of these pathologies (5–7) emphasizes the need for a better understanding of the anatomical and molecular pathways that they share.
The NAc is an integrative structure essential for reward-related learning (8–10). It receives dopaminergic inputs from the ventral tegmental area (VTA), while NAc GABAergic projecting medium spiny neurons (MSNs) are divided into 2 distinct populations differentiated by their molecular signatures and projections. The striato-nigral neurons, comprising the direct pathway, express predominantly the dopamine D1 receptor, substance P, and dynorphin, whereas the striato-pallidal neurons, comprising the indirect pathway, express predominantly the dopamine D2 receptor and enkephalin. Striatonigral neurons appear to have a major role on the initial rewarding and locomotor-related responses to cocaine (11–13). Moreover, cocaine triggers a distinct molecular response in D1 expressing MSNs, involving the phosphorylation of ERK, histone H3, and MSK1. This is followed by the induction of immediate early genes as c-fos and zif-268 (14, 15). Inactivation of the direct pathway neurons of the NAc interferes with cocaine conditioned-place preference and locomotor responses (CPP) (11), while direct optogenetic activation enhances this response (12).
S100A10 (p11) is a small adaptor protein for several receptors and channels targeted to the plasma membrane (16–20). Mice lacking p11 (p11ko) show evidence of behavioral despair and anhedonia, consistent with a depression-like phenotype (16). Previously we identified the NAc as a key anatomical structure mediating the effects of p11 on depression-like behaviors (17). Focal downregulation of p11 in the NAc of wild type (wt) mice recapitulates the depressive-like symptoms observed in p11Ko mice, whereas focal restoration of p11 with adeno-associated virus (AAV) exclusively within the NAc of p11ko mice normalized behavioral despair (17, 21). Given the manipulation of p11 expression altered the consumption of naturally rewarding substances like sucrose prompted us to investigate the role of p11 on drug-related reward.
Here, we investigate the hypothesis that NAc p11 impacts behavioral responses to cocaine by affecting reward learning. We show that chronic cocaine treatment reduces p11 expression in the NAc. This appears to mediate enhanced behavioral responses to cocaine, as p11ko mice show increased cocaine CPP. This effect is normalized by focal restoration of p11 expression only in the NAc of p11ko. We also show that p11 in the NAc of wild type mice interferes with establishment of cocaine CPP and c-fos induction by cocaine treatment, since these are both decreased following focal overexpression of p11 using viral vectors. Finally, using mice expressing CRE-recombinase in either D1- or D2-receptor neurons in combination with viral delivery of CRE-inducible p11-specific conditional short hairpin RNA (shRNA), we identified dopamine D1 expressing MSN as being critical for the instatement of phenotype.
Methods and Materials
Animals
Mice expressing the CRE recombinase under Drd2 (ER44) and Drd1 (Ey262) were obtained from the MMRRC, wildtype C57/BL6 mice from Charles River, and p11 KO in C57/BL6 genetic background were derived and maintained at The Rockefeller University (16). Eight to 12-wk-old males at the beginning of each experiment were housed two to five per cage with ad libitum access to food and water, and maintained on a reverse 12-h light/12-h dark cycle.. Stereotactic surgical procedures were performed under ketamine-xylazine anesthesia. A total of 2 × 109 (2 µl in PBS) genomic particles of recombinant AAV vectors serotype 2 were injected bilaterally into the Nac (anteroposterior +1.3, mediolateral ±0.9, dorsoventral −4.7 from bregma) over 5 min by using a microinfusion pump (World Precision Instruments, Sarasota, FL). Ten weeks of recovery were allowed prior to performing CPP. Following all behavioral procedures, injection site accuracy was determined by immunohistochemistry; animals with mis-targeted injections were excluded from analysis. All studies followed institutional guidelines for the use and care of animals.
Viral vectors
Viral vectors used: AAV2.sh.luc.YFP, AAV2.sh.luc.P11, AAV2.sh.p11.YFP, AAV2.lox.sh.luc.mCh, AAV2.lox.sh.p11.mCh. The non-inducible vectors were described before in (17). The cre inducible ShRNA vector was cloned as follows. A loxP (ATAACTTCGTATAGCATACATTATACGAAGTTAT) sequence was inserted at position 25 in the H1 polIII promoter, downstream of the TATAbox. Immediately followed by the sequence ACCGGTTTTTTCGTACG and a second loxP sequence. Stuffer DNA, a fragment of GFP cloned with the primers 5’-ACCGGTCCGCCAAGCTGCAGGTG-3’ and 5’-ACCGGTGCCGTCCTGGGGGTACAGCTTCTC-3’, was inserted between the loxP sites using the Age1 restriction site. The shRNAs were cloned immediately downstream of the second loxP site between a BglII and SpeI restriction sites. ShRNA sequences used in this study were: Shluc: 5’-cccCGCTGGAGAGCAACTGCATcttcctgtcaATGCAGTTGCTCTCCAGCGttttt-3’ Shp11: 5’- cccGGATCCTCTGGCTGTGGACActtcctgtcaTGTCCACAGCCAGAGGATCCttttt-3’
All viral vectors were prepared as described in (22). Briefly, virus stocks were prepared by packaging the vector plasmids into AAV serotype 2 particles with a helper-free plasmid transfection system. The vectors were purified with heparin affinity chromatography and dialyzed against PBS. AAV titers were determined by quantitative polymerase chain reaction (PCR) with primers to a fragment of the AAV backbone.
Conditioned Place Preference
The place conditioning procedure was conducted as previously described (23). Ten weeks after surgery all mice were placed into the conditioning apparatus, which consists of three distinct chambers. Mice that showed any significant preference for either of the two conditioning chambers (<10% of all animals investigated) were excluded from the study. On subsequent days, animals were injected with saline (10 µl/g, i.p.) and confined to one chamber in the morning for 30 min and then injected with cocaine (Sigma-Aldrich, i.p.) and confined for 30 min to the alternate chamber in the afternoon for 2 days (two cocaine and two saline pairings). On the test day, mice were placed back into the apparatus in a drug-free state for 20 min and tested to determine which side they prefer. For the focal p11 silencing experiments, we used a similar paradigm with only one training session (saline or cocaine) per day and four training sessions per chamber.
Locomotor Sensitization
Mice were habituated to the locomotor chamber equipped with an infrared tracking system (16” L × 16” W × 12” H, Med associates, Inc.) for 1 h before the day1 injection. Ambulatory distance on habituation day was used to assign mice to saline or cocaine groups in order to have groups with similar distributions. Mice received a 10 mg/kg daily intraperitoneal cocaine (Sigma-Aldrich) or 0.9% saline injection for 5 days (volume of injection was 10 µl/g). Ambulatory distance was immediately measured for 1 h and the data from the first 10 min were presented. After 1 day of withdrawal from the last cocaine/saline injection (day 7), mice received an injection of cocaine (10 mg/kg) and locomotor activity recorded. On completion, mice were sacrificed to extract total mRNA.
Quantitative Real Time PCR
Immediately after the last behavioral test, mice were sacrificed with a pentobarbital and the brains quickly extracted into ice-cold PBS. Nac (core and shell) was microdissected under the microscope and stored on RNA-later (Ambion). RNA extraction was performed using Trizol reagent (invitrogen) and cDNAs produced from 1 µg of mRNA using iScript reverse trancriptase (Bio-Rad) according to manufacturers instructions. The primers used for p11 were (5’-gcgacaaagaccacttgaca-3’ and 5’-cactggtccaggtccttcat-3’) and for the housekeeping gene glyceraldehydes-3-phosphate dehaydrogenase (GAPDH) were (5’-cctgcaccaccaactgcttag-3’ and 5’-ctgtggtcatgagcccttcc-3’). Real-time PCR mixture contained SYBR-PCR mix (Applied Biosystems), primers to a final concentration of 0.1 pM, and adequate dilutions of cDNA. Reaction was performed in triplicate using a ABI Prism 7000 machine (Applied Biosystems) at universal cycling conditions. Cycle threshold (CT) values were determined by automated threshold analysis with ABI Prism version 1.0 software.
Immunohistochemistry
Mice were deeply anesthetized and perfused transcardially with 0.1 M PBS (pH 7.4) containing 0.1% heparin, followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The brains were removed, postfixed overnight, washed in phosphate buffer at 4°C, and cryoprotected in phosphate buffer containing 30% sucrose. Free floating sections at 20 or 40 µm were generated on a freezing microtome. Primary antibodies used were anti-p11 (1:200; goat antibody; R&D Systems), anti-EGFP (ab290, 1:10,000; Abcam, Inc., Cambridge, MA), and anti-c-Fos (K-25, 1:1000; Santa Cruz Biotech) anti-RFP (1:1000; rabbit antibody, Rockland-inc), anti-CRE (2D8, 1:1000, mouse antibody, Millipore), anti-substance-P (1:400, rabbit antibody; Millipore) and anti-Met-enkepahlin (1:250, rabbit antibody, Abcam, Inc.). Secondary biotinilated antibodies, Vectastain Elite ABC kit, Mouse on Mouse kit and Avidin-Streptavidin blocking reagents were from Vector Laboratories. Blue DAB reagent contained 0.05% DAB, 0.05% Cobalt Chloride, 0.05% Nickel Ammonium Sulfate and 0.015% hydrogen peroxide. Brown DAB reagent contains 0.03% DAB and 0.015% hydrogen peroxide. Fluorescent secondary antibodies (Alexa-conjugated donkey anti-rabbit and donkey anti-mouse) were from Invitrogen.
Results
Chronic cocaine administration downregulates p11 expression in the NAc
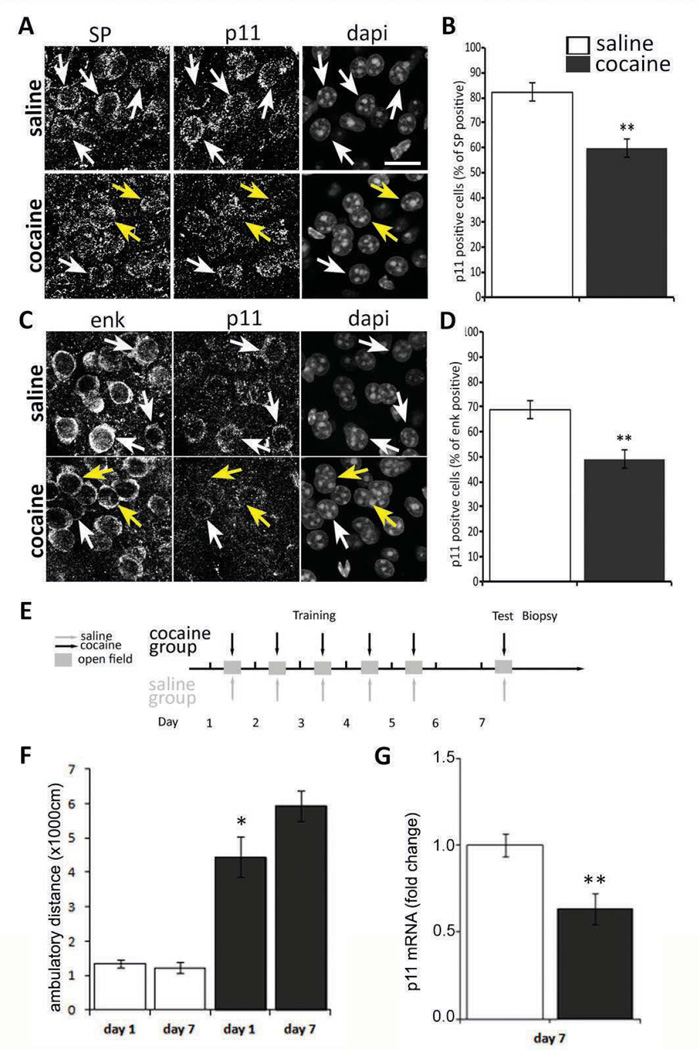
Given our prior work demonstrating the importance of p11 specifically in the NAc for control of depression-like behaviors, we hypothesized that NAc p11 may also influence responses to drugs of abuse, such as cocaine. To this end, we first asked whether chronic cocaine treatment modulates the expression of endogenous p11 within the NAc of wt mice (Figure S1A). Following cocaine administration in an open field (7 days, 10mg/Kg), p11 protein was downregulated in both substance P expressing D1 MSN (Figure 1A, 1B), and in enkepahlin expressing D2 MSN (figure 1C, D), as demonstrated by a reduction in the number of cells co-expressing p11 and enkephalin or substance P after cocaine administration when compared to saline controls (n=5 for both groups). Because the context of cocaine administration can elicit different molecular adaptations, we measured the expression of p11 mRNA following two distinct behavioral paradigms of chronic cocaine administration: locomotor sensitization and CPP. In the locomotor sensitization paradigm, two groups of mice were injected with intraperitoneal saline (n=10) or cocaine (n=10), and immediately placed in an open field where their ambulatory activity was recorded for 10 minutes. This procedure was repeated daily for 5 days. On day 7, mice were challenged with cocaine or saline and placed on the same test environment (Figure 1E). On test day, ambulatory distance was increased by 33% in the cocaine group when compared to day 1, as expected (Figure 1F). Immediately following the last test, the NAc was dissected to analyze RNA transcripts levels. We found that p11 transcripts were robustly downregulated in NAc biopsies from mice exposed to chronic passive administration of cocaine when compared to vehicle controls (Figure 1G). In the CPP paradigm, we tested a higher cocaine concentration (20 mg/kg) to determine whether p11 downregulation was dependent upon cocaine dosage. Mice were injected daily with cocaine or saline before being placed in the corresponding paired chamber (Figure S1B). After 2 days of conditioning, the mice were tested for CPP, preference for the cocaine paired side was confirmed, and each mouse received 3 additional doses of cocaine or saline before the NAc was dissected (Figure S1C). We found the relative p11 mRNA levels were again significantly reduced in the cocaine treated group to the same degree that the one observed in the locomotor sensitization test (Figure S1G). Therefore, chronic cocaine administration reduces p11 mRNA and protein expression in the NAc, particularly in D1- and D2-expressing MSNs.
Figure 1. Chronic cocaine treatment reduces the expression of p11 in the NAc.
Chronic cocaine treatment reduces p11 expression on substance P (SP) expressing D1 MSN (A) Representative immunohistochemistry of SP and p11 expression in NAc of saline and cocaine treated C57BL/6 mice. The white arrows indicate MSN co-expressing SP and p11, the yellow arrows indicate cells expressing SP and no detectable p11. (B) Quantification of cells expressing detectable levels of p11 among SP positive cells. Data are presented as mean ± SEM and analyzed using a two tailed paired T test (p<0.01) scale bar represents 10 µm. Chronic cocaine treatment reduces p11 expression on enk expressing D2 MSN (C) Representative immunohistochemistry of enk and p11 expression in NAc of saline and cocaine treated mice. White arrows indicate MSN co-expressing enk and p11, yellow arrows indicate cells expressing SP and no detectable p11. (D) Quantification of cells expressing detectable levels of p11 among enk positive cells. Data are presented as mean ± SEM and analyzed using a two tailed paired T test (p<0.01). (E) Experimental timeline. Adult C57BL/6 mice (n=10) were treated for 5 days with IP injections of 10 mg/kg of cocaine or saline in an open field arena and challenged on day 7 with cocaine or saline in the same context. (F) Cocaine treated mice display classical locomotor sensitization during the first 10 minutes of exposition to the drug contrary to saline treated mice. Data are presented as mean ± SEM and analyzed using a two tailed paired T test (p<0.05). (G) Cocaine treated mice decrease mRNA expression levels of p11 in the NAc when compared to saline treated mice as measured by qPCR (p<0.01).;
Loss of p11 increases the rewarding effects of cocaine
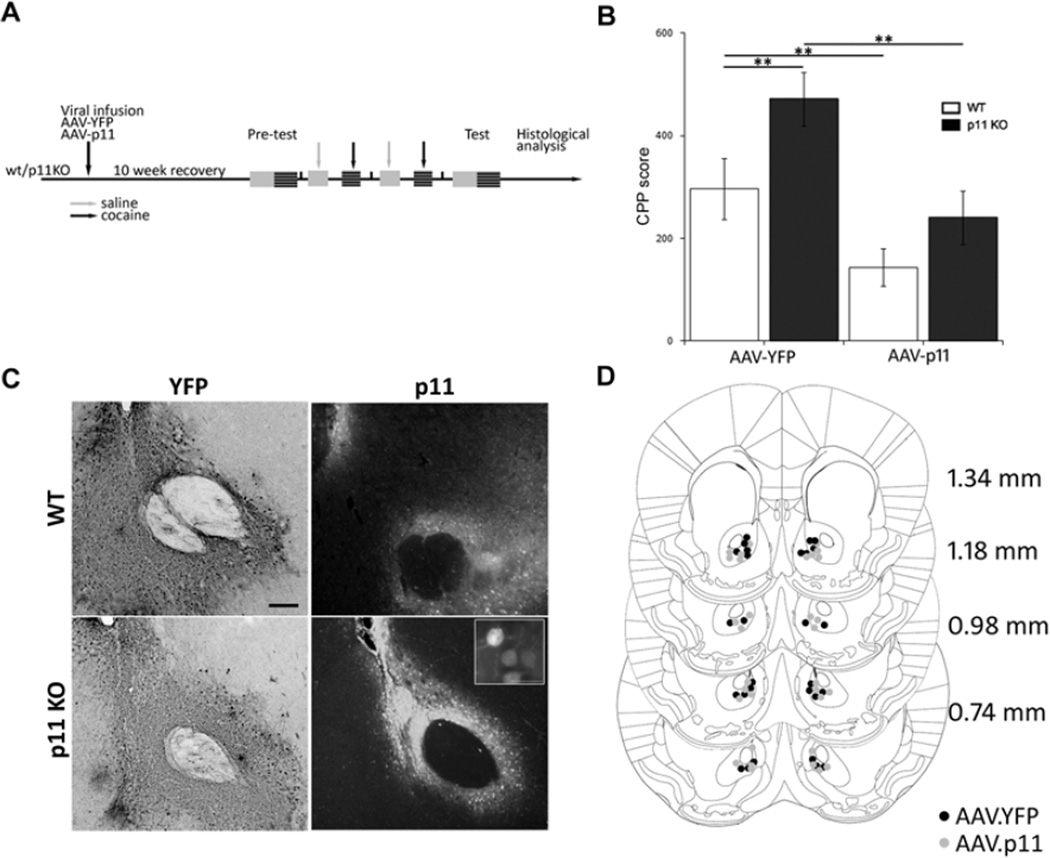
To explore whether downregulation of NAc p11 in wt mice in response to cocaine is a potential mediator of the behavioral response to cocaine administration, we used CPP as a measure of the initial rewarding effect of cocaine in p11ko and wt littermates. Despite being a reliable and widely used behavioral correlate for measuring reward, raw CPP scores can be highly variable between animal cohorts (24, 25) Therefore, we have only compared CPP scores with the internal control for each experiment. Here, we chose a CPP paradigm consisting of 2 days of conditioning with 7.5 mg/kg cocaine, a minimal dose that can produce physiological and biochemical cocaine responses (26). Using this modified procedure, we found that p11ko mice injected in the NAc bilaterally with AAV vectors expressing the control protein yellow fluorescent protein (AAV-YFP) exhibit an increased CPP score when compared to wt littermates (Figure 2B). Therefore, p11ko mice are more sensitive to CPP at this cocaine dose compared to wt littermates.
Figure 2. P11 in NAc modulates cocaine CPP.
(A) Experimental timeline. P11 KO and wt littermates were injected in the NAc with AAV2.p11 or AAV2.YFP, 10 weeks later mice were submitted to cocaine (7.5 mg/kg) CPP as described in the materials and methods section. (B) Control injected p11KO show increased CPP compared to control injected wt littermates. Focal restoration of p11 in the NAc of p11 KO normalizes cocaine CPP scores. Focal overexpression of p11 in the NAc of wt mice reduces their CPP score compared to control injected wt. Data (means ±SEM n=6–8 per group) were analyzed using 2 way ANOVA. Effect of genotype F(1, 27)= 6.87, p=0.015; effect of injected virus F(1, 27)=13.65, p=0.0011; interaction F(1, 27)=0.65, NS. Post hoc comparison (Tukey HSD test) **p<0.01. (C) Representative histological analysis of injection sites. Black scale bar, 1mm. Insert is a magnification showing p11 expression on AAV.p11 injected p11KO. (D) Cartoon shows the location of the viral injection sites at the indicated coordinates from Bregma according to Franklin and Paxinos (2008).
Overexpression of p11 in the NAc reduces cocaine reward
Since cocaine administration reduces p11 expression in the NAc and p11ko mice demonstrated increased cocaine CPP compared with wt controls, we hypothesized that restoring NAc p11 expression might reverse this behavioral response. We have previously shown that depression-like behaviors in p11ko mice can be reversed by focally restoring p11 in the NAc using AAV-mediated gene transfer (17). Using a similar paradigm, p11ko and wt littermates were injected bilaterally in the NAc (Figure 2C,D) with the AAV-p11 viral vectors ten weeks before CPP training (Figure 2A). As predicted, focal restoration of p11 expression in the NAc of p11ko mice reduced cocaine CPP score to wt levels (Figure 2B). This effect is not exclusive to p11ko mice, as wt mice receiving AAV-p11 in the NAc also had significantly lower CPP score relative to AAV-YFP injected wt controls (Figure 2B). Taken together, our data indicate that the reduction in endogenous NAc p11 expression following chronic cocaine contributes to drug-evoked pathological behaviors, which can be overcome by the ectopic expression of p11 directly in the NAc.
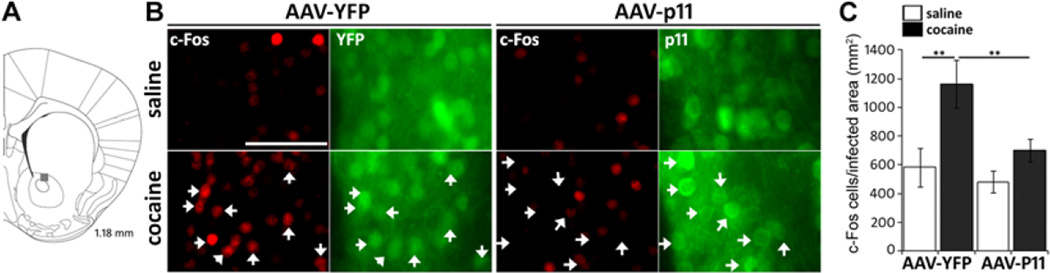
p11 overexpression reduces c Fos induction in response to cocaine
Chronic or acute treatment with psychostimulants have been reported to induce the expression of the immediate early gene c-fos, a well-characterized biomarker of neuronal and behavioral changes elicited by cocaine administration (27, 28). Therefore, we examined whether p11 overexpression alters cocaine induced c-Fos expression (Figure 3). To this end, wt mice were injected in the NAc (Figure 3A) with either AAV-YFP or AAV-p11 overexpressing vectors, treated for 10 days with cocaine (10mg/kg) or saline, and analyzed 2 hrs after the last injection. As expected, mice injected with the control AAV-YFP virus responded to cocaine administration with a robust c-Fos induction in YFP-positive neurons (Figure 3). In contrast, NAc neurons infected with the AAV-p11 failed to upregulate c-Fos expression in response to cocaine administration (Figure 3B, C). This set of data is consistent with our observations that overexpressing p11 in the NAc reduces sensitivity to cocaine reward.
Figure 3. P11 overexpression prevents cocaine induced c Fos activation in the NAc.
(A) The position of the NAc region used for quantification is indicated by grey square at 1.18 mm from Bregma according to Franklin and Paxinos (2008). (B) c-Fos immunoreactivity in the NAc core of control (AAV-YFP) or AAV-p11 infected mice 2 hrs after the last saline or cocaine injection of a 10 day treatment. White arrows indicate infected cells, note strong c-Fos induction after cocaine treatment in YFP infected cells, and decreased c-Fos induction by cocaine in p11 infected neurons. Scale bar 50 µm. (C) quantification of c-Fos positive cells within the infected area. Data (means ±SEM n=6–10 per group) were analyzed using two way ANOVA. Effect of injected virus F(1, 28)= 9.23, p=0.0055; effect of drug treatment F(1, 28)= 11.46, p=0.0024; interaction F(1, 28)= 0.16, NS. Post hoc comparison (Tukey HSD test) **p<0.01.
P11 effect on cocaine CPP is mediated by D1 receptor containing neurons in the NAc
Specific neuronal subtypes within the NAc may discriminate the behavioral responses to drugs of abuse. For instance, optogenetic activation of NAc D1-expressing MSNs enhances cocaine CPP, whereas optogenetic activation of D2-expressing MSNs attenuates cocaine CPP (12). Furthermore, optogenetic inhibition of ChAT-expressing neurons blocks cocaine CPP (29). Within the NAc, p11 is highly expressed in cholinergic (ChAT) interneurons, and it is also present at lower levels in MSN (Figure S1A), with roughly equal expression levels in dopamine D1 or dopamine D2 containing neurons (Figure 1) (21). Consistently, cocaine administration produces a strong activation of D1-expressing MSNs in the NAc, as demonstrated by the induction of c-fos and other immediate early genes (12, 14), an effect that we found to be dependent upon p11 expression (Figure 3).
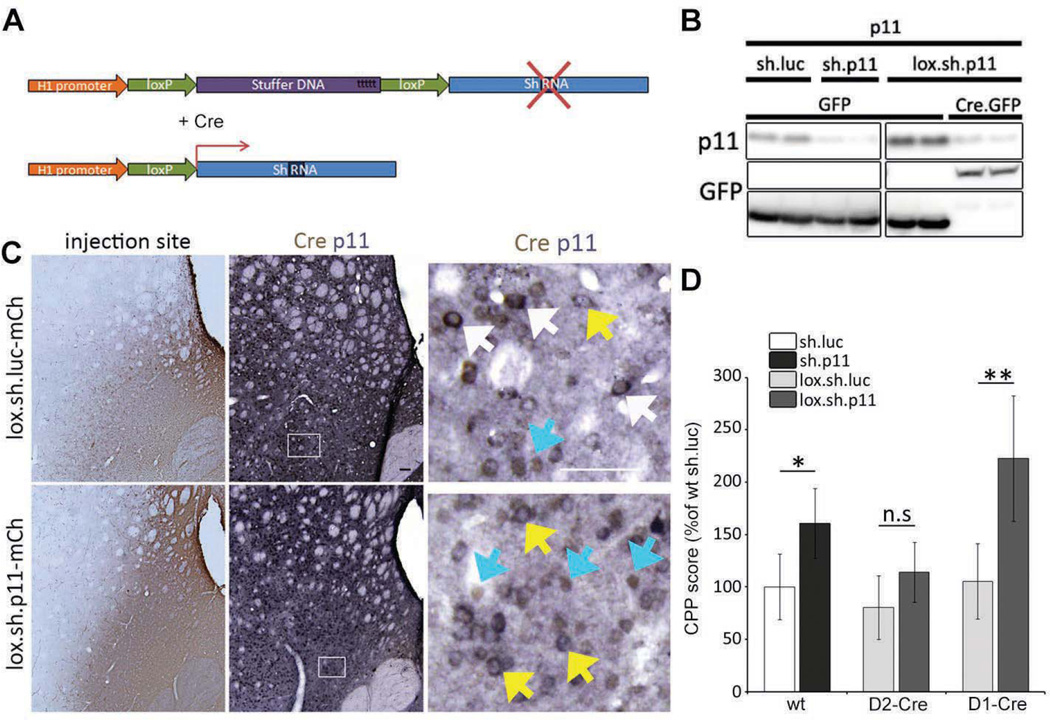
In order to determine if the effect of Nac p11 on cocaine CPP is mostly driven by either neuronal subsets, we developed a novel Cre inducible AAV vector, lox.sh.p11, to selectively express small hairpin RNAs downstream of a floxed STOP cassette (Figure 4A, Figure S2a). Previous reports have demonstrated efficient transcription of small RNAs from the Pol III H1-promoter following alteration of the sequence of the 24 nucleotides following the TATA box element, as long as the proper distance is maintained (30). Therefore, we replaced the 16 bp immediately downstream of the TATA box element by a 34 bp loxP element, followed by 300 bp of stuffer DNA (STOP cassette), a second loxP, and finally the sequence for the shRNA (Figure 4A, Figure S2a). In the absence of Cre recombinase the STOP cassette prevents the transcription of the shRNA. After recombination of the loxP elements, the stuffer DNA is eliminated and the shRNA can be transcribed (Figure 4A, B). The resulting sequence after recombination contains 16 extra nucleotides between the TATA box element and the shRNA sequence but the additional distance does not appear to affect the efficiency of the transcription of the shRNA as demonstrated by a comparable silencing efficiency of the lox.sh.p11 vector and the original sh.p11 vector (Figure 4B). This new AAV vector allows for conditional p11 gene downregulation when combining the cell type specificity of BAC-Cre transgenic lines with the anatomical precision of stereotactic AAV delivery.
Figure 4. D1 receptor expressing MSNs mediate the effect of NAc p11 on cocaine CPP.
(A) Scheme of the Cre inducible shRNA expression system used to selectively silence p11 expression. (B) HEK 293 cells were transfected with the indicated constructs and probed for p11 expression 72hrs later. In the presence of Cre lox.sh.p11 construct silences p11 with a similar efficiency than the constitutively active sh.p11. (C) D1-Cre mice injected in the NAc with Cre inducible AAV2-lox.sh.p11 or control AAV2-lox.sh.luc, viral spread at the site of the injection was determined by RFP staining, an adjacent slice was stained for Cre in brown, and p11 in blue. Right panel is a magnification of the double staining in the infected area (white square), white arrows indicate cells expressing Cre and p11 in control injected mice, yellow arrows indicate cells expressing p11 alone and blue arrows indicate cells expressing Cre alone. Scale bar is 50 µm. (d) Ten week old D1-Cre or D2-Cre mice were injected in the NAc with Cre inducible AAV2-lox.sh.p11 or control AAV2-lox.shluc, wt littermates were injected with AAV2-sh.p11 or AAV2-sh.luc 10 weeks later mice were submitted to cocaine (7.5mg/kg) place conditioning as described in the materials and methods section. Data (means ±SEM n=5–10 per group) were analyzed using two way ANOVA. Effect of injected virus F(1, 45)= 5.06, p=0.0301; effect of genotype F(2, 45)= 1.45, NS; interaction F(2, 45)= 0.42, NS. Post hoc comparison (Tukey HSD test) *p<0.05; **p<0.01.
To specifically silence p11 in different neuronal populations of the NAc, we injected either lox.sh.p11 or lox.sh.luc AAV vectors into the NAc of two different transgenic mouse lines which express Cre-recombinase from two different promoters. Dopamine D2-receptor BAC-Cre mice (D2-Cre) target striatopallidal, enkephalin expressing MSNs (Figure S3) and ChAT neurons (14, 31) and dopamine D1-receptor BAC-Cre mice which target striatonigral, substance P expressing MSNs as previously characterized (Figure S3) (31). First, we controlled that a similar proportion of Cre positive cells can be infected in both D1 and D2 Cre lines (43,8% and 44,3% respectively) (figure S2b,c). Then, we confirmed in vivo the selective downregulation of p11 in Cre-expressing cells in D1-Cre (Figure 4C, Figure S2d) and D2-Cre (Figure S2e) injected mice. Importantly, both BAC-Cre lines were able to develop reliable cocaine CPP, confirming normal striatal function as previously reported (32). Therefore, we analyzed cocaine CPP (8 days conditioning cocaine dose: 7.5mg/kg) in these BAC-Cre mice engineered to produce cell type-specific loss of p11 in the NAc. We compared their behavior to wt littermates injected with the previously characterized sh.p11 vector (17) to globally downregulate p11 in the entire NAc. As predicted by the increase in cocaine CPP observed in p11ko mice (Figure 2B), global silencing of p11 in the NAc increased cocaine CPP (Figure 4D). In clear contrast, specific silencing of p11 in D2-expressing NAc MSNs and cholinergic interneurons did not have an effect on cocaine CPP (Figure 4D). Only the specific silencing of p11 in D1-expressing MSNs of the NAc increased robustly cocaine CPP compared with control mice (Figure 4D), indicating that p11 within this cell type is a major molecular determinant of biochemical and behavioral responses to chronic cocaine. It should be noted that the Cre-mediated induction achieved with these vectors was specific to activation of the shRNA, while the second cassette produced the reporter gene (mCherry) that was independent of Cre-expression. Therefore, AAV transduction and reporter expression were equivalent in all animals, regardless of Cre expression, with only activation of the shRNA limited to the Cre expressing cells (Figure S1), further confirming that the effect on cocaine CPP was specific to p11 inhibition in D1-Cre labeled neurons and not due to a non-specific effect of viral transduction or marker gene expression.
Discussion
The NAc integrates signals from different regions of the limbic system in order to fine-tune the response to affective and reward-related stimuli. As a result, the NAc is at the center of sustained research efforts to identify common molecular pathways to psychiatric disorders such as drug addiction and depression. The small adaptor protein p11 has been previously described as an important modulator of depressive-like behaviors (16, 17, 21). Here, we characterize a cell-type specific role of p11 in dopamine D1 expressing MSNs in the NAc for the initial reward-related response to cocaine. In addition to their depressive-like phenotypes, we show that p11ko mice also have an increased sensitivity to the rewarding effects of cocaine. Using viral vectors, we identify the NAc as the core brain region mediating this effect. In conjunction with promoter specific-Cre transgenic mice, we developed a lox.shRNA viral strategy that allowed us to target p11 knock-down in specific cell-types within the NAc. We then demonstrated that p11 action on cocaine behaviors was largely due to modulation of striato-nigral neurons expressing dopamine D1 receptors, but not D2 receptors.
Our finding that p11 specifically in NAc D1 receptor-expressing striatonigral MSNs regulate responses to cocaine reward is consistent with a number of studies that identified these same neurons as primary mediators of the reinforcing effects of cocaine (11, 12, 33). Using the same transgenic line to express Cre inducible channelrhodopsin-2 in cell type specific MSNs, Lobo and colleagues demonstrated that direct optogenetic activation of striatonigral neurons in the NAc during cocaine conditioning enhances cocaine reward measured by CPP (12). Using the substance P promoter, a different marker for striatonigral neurons, in addition to conditional tetanus toxin transgenic mice, Hikida and colleagues demonstrated that inhibiting synaptic transmission in striatonigral neurons inhibits cocaine CPP (11). Nonetheless, the consequences to cocaine-induced behaviors from altering either dopamine receptor or downstream signaling molecule function in these neurons remain complex. Dopamine D1 receptor is necessary for other behavioral responses to cocaine, such as locomotor sensitization (13), but mice lacking D1 receptors develop CPP in response to cocaine (34, 35). In a similar manner, blocking c-fos induction in D1-receptor MSNs affects locomotor sensitization but not the establishment of CPP (28). Our findings reinforce the hypothesis that striatonigral neurons are necessary for developing cocaine CPP, and that titration of p11 expression is sufficient to modulate this effect. A variety of functions have been attributed to p11, which could influence activity of striatonigral neurons in response to cocaine. P11 is best known for its role as a regulator of surface expression of membrane proteins such as serotonin receptors 5HT1b and 5HT4 (16, 18), ion channels (19, 20, 36, 37), and its protein partner Annexin A2 (38). More recently, p11 was described to have a nuclear function and regulate gene transcription through SMARCA3 (39). Future anatomical, biochemical and electrophysiological studies of p11 function within the D1-expressing cells are necessary to fully understand the signaling mechanisms leading to the conditioning behavior.
Here we demonstrate that cocaine administration reduces endogenous p11 within the NAc. We have previously shown that p11 expression is also reduced in the NAc of human patients with major depression and that p11ko mice show depression-like phenotypes (16, 17). Moreover we identified the NAc as the key brain region sensitive to p11 homeostasis in which p11 loss exerts pro-depressive effect, most specifically, through the cholinergic (ChAT positive) interneuron population that are the only source of acetylcholine for MSNs of the ventral striatum (17, 21). The role of ChAT cells in cocaine CPP is complex and appears to be dependent on the time and behavioral context. Long-term inactivation of ChAT neurons in the NAc using a targeted toxin strategy increases cocaine CPP dramatically (40), whereas short-term light inactivation of ChAT interneurons during the training sessions produces a stark decrease in cocaine CPP (29). Up to this point, we do not know how the loss of p11 affects the excitability of ChAT cells, but the models we use in this study (transgenic mice and shRNA injections) have long term consequences that are not reversible. Here, we show that downregulation of p11 in D2-containing neurons has no apparent effect on cocaine CPP, and since ChAT neurons express the dopamine D2 receptor (41, 42), this suggests that p11 in NAc ChAT cells is less crucial for the cocaine CPP phenotype than for depression-like behaviors. However, it is possible that alteration of p11 only in ChAT neurons may yield a demonstrable phenotype not observed when p11 is downregulated in all D2 expressing neurons. Further studies to inactivate p11 exclusively in ChAT neurons may clarify this question.
The identification of molecular and anatomical pathways common to addiction and depressive disorders could be useful for the development of new therapeutic strategies for these illnesses. However, therapies that improve addictive behaviors may also promote depressive symptoms and vice versa. Important questions remain to be addressed before understanding the molecular underpinnings of these two highly co-morbid disorders. We previously suggested p11 as a therapeutic target to treat major depressive disorders (MDD). Our present results suggest that reduced NAc p11 is not only associated with MDD but also with enhanced cocaine reward, and that strategies to increase p11 expression and/or activity of downstream binding partners in the NAc may decrease the susceptibility to cocaine abuse in addition to the previously observed therapeutic benefits for depression-like behaviors.
Supplementary Material
Acknowledgments
This work was supported by the JPB Foundation for Medical Research (MGK), the Department of Defense (W81XWH-09-1-0381) (MGK), and the the National Institute on Drug Abuse (P01 DA008227) (EJN).We thank Dr. Vincent Vialou from the Nestler lab (Friedman Brain Institute, Icahn School of Medicine at Mount Sinai, New York, NY 10029) for insightful suggestions on the CPP procedure. Financial disclosures
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors report no biomedical financial interest or potential conflict of interest.
References
- 1.Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 3.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 4.Covington HE, 3rd, Maze I, Sun H, Bomze HM, DeMaio KD, Wu EY, et al. A role for repressive histone methylation in cocaine-induced vulnerability to stress. Neuron. 2011;71:656–670. doi: 10.1016/j.neuron.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Jama. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 6.Swendsen JD, Merikangas KR. The comorbidity of depression and substance use disorders. Clin Psychol Rev. 2000;20:173–189. doi: 10.1016/s0272-7358(99)00026-4. [DOI] [PubMed] [Google Scholar]
- 7.Grant BF. Comorbidity between DSM-IV drug use disorders and major depression: results of a national survey of adults. J Subst Abuse. 1995;7:481–497. doi: 10.1016/0899-3289(95)90017-9. [DOI] [PubMed] [Google Scholar]
- 8.Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 9.Martin M, Chen BT, Hopf FW, Bowers MS, Bonci A. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nature neuroscience. 2006;9:868–869. doi: 10.1038/nn1713. [DOI] [PubMed] [Google Scholar]
- 10.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. Cell type specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu M, Guo Y, Vorhees CV, Zhang J. Behavioral responses to cocaine and amphetamine administration in mice lacking the dopamine D1 receptor. Brain Res. 2000;852:198–207. doi: 10.1016/s0006-8993(99)02258-1. [DOI] [PubMed] [Google Scholar]
- 14.Bertran Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y, et al. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:3344–3354. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, et al. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- 17.Alexander B, Warner Schmidt J, Eriksson T, Tamminga C, Arango-Lievano M, Ghose S, et al. Reversal of depressed behaviors in mice by p11 gene therapy in the nucleus accumbens. Sci Transl Med. 2010;2:54ra–76ra. doi: 10.1126/scitranslmed.3001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warner Schmidt JL, Flajolet M, Maller A, Chen EY, Qi H, Svenningsson P, et al. Role of p11 in cellular and behavioral effects of 5-HT4 receptor stimulation. J Neurosci. 2009;29:1937–1946. doi: 10.1523/JNEUROSCI.5343-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renigunta V, Yuan H, Zuzarte M, Rinne S, Koch A, Wischmeyer E, et al. The retention factor p11 confers an endoplasmic reticulum localization signal to the potassium channel TASK 1. Traffic. 2006;7:168–181. doi: 10.1111/j.1600-0854.2005.00375.x. [DOI] [PubMed] [Google Scholar]
- 20.Foulkes T, Nassar MA, Lane T, Matthews EA, Baker MD, Gerke V, et al. Deletion of annexin 2 light chain p11 in nociceptors causes deficits in somatosensory coding and pain behavior. J Neurosci. 2006;26:10499–10507. doi: 10.1523/JNEUROSCI.1997-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warner Schmidt JL, Schmidt EF, Marshall JJ, Rubin AJ, Arango Lievano M, Kaplitt MG, et al. Cholinergic interneurons in the nucleus accumbens regulate depression-like behavior. Proc Natl Acad Sci U S A. 2012;109:11360–11365. doi: 10.1073/pnas.1209293109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgenstern PF, Marongiu R, Musatov SA, Kaplitt MG. Adeno-associated viral gene delivery in neurodegenerative disease. Methods Mol Biol. 2011;793:443–455. doi: 10.1007/978-1-61779-328-8_29. [DOI] [PubMed] [Google Scholar]
- 23.Russo SJ, Wilkinson MB, Mazei-Robison MS, Dietz DM, Maze I, Krishnan V, et al. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 25.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 26.Russo SJ, Bolanos CA, Theobald DE, DeCarolis NA, Renthal W, Kumar A, et al. IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nature neuroscience. 2007;10:93–99. doi: 10.1038/nn1812. [DOI] [PubMed] [Google Scholar]
- 27.Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, et al. Targeted disruption of cocaine activated nucleus accumbens neurons prevents context-specific sensitization. Nature neuroscience. 2009;12:1069–1073. doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Zhang L, Jiao H, Zhang Q, Zhang D, Lou D, et al. c-Fos facilitates the acquisition and extinction of cocaine-induced persistent changes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:13287–13296. doi: 10.1523/JNEUROSCI.3795-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, et al. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010;330:1677–1681. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myslinski E, Ame JC, Krol A, Carbon P. An unusually compact external promoter for RNA polymerase III transcription of the human H1RNA gene. Nucleic Acids Res. 2001;29:2502–2509. doi: 10.1093/nar/29.12.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson AB, Hang GB, Grueter BA, Pascoli V, Luscher C, Malenka RC, et al. A comparison of striatal-dependent behaviors in wild type and hemizygous Drd1a and Drd2 BAC transgenic mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:9119–9123. doi: 10.1523/JNEUROSCI.0224-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hummel M, Unterwald EM. D1 dopamine receptor: a putative neurochemical and behavioral link to cocaine action. Journal of cellular physiology. 2002;191:17–27. doi: 10.1002/jcp.10078. [DOI] [PubMed] [Google Scholar]
- 34.Miner LL, Drago J, Chamberlain PM, Donovan D, Uhl GR. Retained cocaine conditioned place preference in D1 receptor deficient mice. Neuroreport. 1995;6:2314–2316. doi: 10.1097/00001756-199511270-00011. [DOI] [PubMed] [Google Scholar]
- 35.Karasinska JM, George SR, Cheng R, O'Dowd BF. Deletion of dopamine D1 and D3 receptors differentially affects spontaneous behaviour and cocaine-induced locomotor activity, reward and CREB phosphorylation. The European journal of neuroscience. 2005;22:1741–1750. doi: 10.1111/j.1460-9568.2005.04353.x. [DOI] [PubMed] [Google Scholar]
- 36.Mathie A, Rees KA, El Hachmane MF, Veale EL. Trafficking of neuronal two pore domain potassium channels. Curr Neuropharmacol. 2010;8:276–286. doi: 10.2174/157015910792246146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okuse K, Malik-Hall M, Baker MD, Poon WY, Kong H, Chao MV, et al. Annexin II light chain regulates sensory neuron-specific sodium channel expression. Nature. 2002;417:653–656. doi: 10.1038/nature00781. [DOI] [PubMed] [Google Scholar]
- 38.Deora AB, Kreitzer G, Jacovina AT, Hajjar KA. An annexin 2 phosphorylation switch mediates p11-dependent translocation of annexin 2 to the cell surface. J Biol Chem. 2004;279:43411–43418. doi: 10.1074/jbc.M408078200. [DOI] [PubMed] [Google Scholar]
- 39.Oh YS, Gao P, Lee KW, Ceglia I, Seo JS, Zhang X, et al. SMARCA3, a chromatin-remodeling factor, is required for p11-dependent antidepressant action. Cell. 2013;152:831–843. doi: 10.1016/j.cell.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hikida T, Kitabatake Y, Pastan I, Nakanishi S. Acetylcholine enhancement in the nucleus accumbens prevents addictive behaviors of cocaine and morphine. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6169–6173. doi: 10.1073/pnas.0631749100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Moine C, Tison F, Bloch B. D2 dopamine receptor gene expression by cholinergic neurons in the rat striatum. Neurosci Lett. 1990;117:248–252. doi: 10.1016/0304-3940(90)90671-u. [DOI] [PubMed] [Google Scholar]
- 42.Aubry JM, Schulz MF, Pagliusi S, Schulz P, Kiss JZ. Coexpression of dopamine D2 and substance P (neurokinin-1) receptor messenger RNAs by a subpopulation of cholinergic neurons in the rat striatum. Neuroscience. 1993;53:417–424. doi: 10.1016/0306-4522(93)90205-t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.