Abstract
Objective
This study aimed to quantitatively compare findings of emotional functioning across studies of adolescents and adults with congenital heart disease (CHD) through meta-analysis.
Design
The current meta-analysis included 22 studies of adolescent and adult survivors of CHD who completed measures of emotional functioning. Effect sizes were represented by Hedge’s g. Heterogeneity was calculated and possible moderators (i.e., lesion severity, age, study location, study quality) were examined.
Results
Overall, adolescent and adult survivors of CHD did not differ in emotional functioning from healthy controls or normative data. However, significant heterogeneity was found, and there was a trend for degree of lesion severity to moderate emotional functioning. Further analysis of lesion severity indicated that individuals with moderate lesions reported better emotional functioning than controls/normative data. Limitations in existing literature precluded examination of patient age as a moderator. Study location and quality did not explain a significant portion of the variance in effects.
Conclusion
Findings suggest that differences in emotional functioning may exist across lesion severities, and individuals with moderately severe lesions are emotionally thriving. Given the diversity within CHD lesion classifications, future studies should include other indicators of disease severity, including measures of morbidity, to determine how disease may affect emotional functioning among survivors of CHD. Furthermore, authors and journals need to ensure that research is reported in enough detail to facilitate meta-analysis, a critically important tool in answering discrepancies in the literature.
Keywords: Meta-analysis, Emotional functioning, Emotional distress
Introduction
Due to significant medical advances, more than 1,000,000 adults in the U.S. are living with congenital heart disease (CHD), and this number is expected to continue growing by 5% per year.1,2 Adolescents and adults with CHD are challenged by various stressors, including reproductive and body image concerns, insurance coverage, and additional surgeries. Alternatively, as individuals with CHD age, they have more time to accept the condition, practice self-management, and acquire more knowledge about one’s heart condition. These experiences may differentially influence the emotional functioning of individuals with CHD from adolescence into adulthood, which in turn could have significant implications for the timing of intervention to ensure the best medical and psychosocial outcomes.
A meta-analysis of 11 published studies measuring parent-report of emotional functioning among children and adolescents with CHD found that only older children and adolescents with CHD showed an increased risk of internalizing problems (e.g., depressive and anxiety symptoms) and some risk for externalizing behavior problems (e.g., hyperactivity and aggression).3 Severity of the congenital lesion did not explain the variability in emotional functioning amongst the 11 studies. Karsdorp and colleagues3 suggested that an overprotective parenting style and/or hormonal and brain changes that occur in adolescence may be contributing factors to the increased risk for internalizing behaviors among individuals with CHD in this age group. The finding that older children and adolescents experience more symptoms of emotional distress than younger children suggests that age may play a role in the emotional experience of CHD. However, levels of self-reported emotional distress among adolescents remain unclear, as well as the trajectory of psychosocial functioning into adulthood for survivors of CHD.
Research on emotional functioning among adults with CHD has advanced more quickly in Europe than in the United States, and a recent literature review suggested that the variability in findings may be a function of study location, and more specifically, differences in healthcare systems.4 Several U.S. studies indicated worse emotional functioning among adults with CHD as compared to same-age reference norms, which was not found by a majority of the European studies. The literature review by Kovacs and colleagues (2005) also addressed lesion severity as contributing to emotional functioning, though the authors noted that the relationship remains unclear as evidenced by contradictory findings in the literature.
Although the adult clinical population continues to grow and research findings have been contradictory, there have been no meta-analyses conducted to date on the self-reported emotional functioning of adolescent and adult survivors of CHD. The aim of the current study was to synthesize the available published literature to (1) quantify the strength of the differences between individuals with CHD as compared to healthy controls or normative data on measures of emotional functioning, and (2) explore potential moderators of emotional functioning, including lesion severity, age, study location, and study quality.
Methods
Operationalization of Outcomes
For the purposes of this meta-analysis, the outcome variable emotional functioning was defined as psychological symptoms, including symptoms of depression (i.e., feeling down, loss of energy, irritability, etc.) and anxiety (i.e., nervousness, worry, tension, etc.). Therefore, acceptable measures of emotional functioning included specific symptoms of emotional distress and not general psychosocial functioning (e.g., difficulty getting along with others) or emotional quality of life (e.g., interference of psychological symptoms with daily activities of living).
Literature Search
Literature searches were conducted (January 1980 through May 2013, including ePublications) using PubMed (1360 results) and PsychInfo (943 results). Citations in previous meta-analyses3 and literature reviews4 were also screened, as well as citations offered during contact with individual authors. Full details of the search strategies and limitations are available upon request.
Study Selection
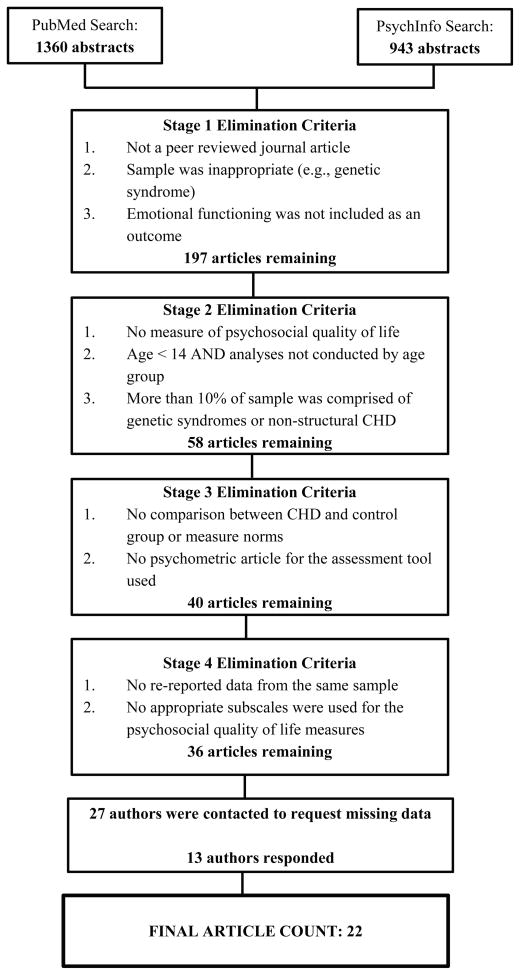
Articles were examined across 4 stages of selection (see Figure 1). Stage 1 included reviewing the abstracts from the PubMed and PsychInfo searches and eliminating those articles that (1) predominantly consisted of participants without structural CHD or had a primary diagnosis of a genetic disorder (e.g., Marfans syndrome) and (2) did not present data on psychosocial functioning. This stage eliminated 2106 of the 2303 abstracts, resulting in a pool of 197 articles. For Stage 2, articles were read in their entirety, and studies were excluded if they (1) did not include a measure of emotional functioning, such as symptom-based assessment tools (e.g., Beck Depression Inventory) or quality of life surveys that had subscales measuring emotional functioning (e.g., The Medical Outcomes Study 36-Item Short-Form Health Survey – Mental Health Subscale), (2) included participants under the age of 14 and did not run analyses separately on adolescents 14 years and older, and (3) had a sample with 10% or more represented by individuals without structural CHD or a genetic disorder that was not identified in Stage 1. Stage 2 eliminated 139 articles from consideration with 58 remaining. During Stage 3, articles were re-reviewed to determine if study analyses compared responses from individuals with CHD to either a control group or measure norms. This process eliminated 18 articles from consideration with 40 articles remaining. Stage 4 included determining what data could be extracted from each of the remaining articles, which resulted in the elimination of 4 additional studies because of data overlap between articles or the desired subscale of a measure was not used in the analyses.
Figure 1.
Identification of studies for inclusion in the meta-analysis.
Of the 36 articles that remained, 29 were missing information that prevented calculation of effect sizes, including sample size, means, and standard deviations. As a result, 27 authors were contacted via email to request the missing information. Each author or designated corresponding author was contacted 3 times over the course of approximately 4 to 8 weeks. Of those contacted, 14 either did not respond or were unable to provide data within the given time frame,5–18 while 13 authors provided the missing information resulting in 22 available studies for inclusion in the meta-analysis19–39 (see Table 1).
Table 1.
Characteristics of Studies Included In the Meta-Analysis.
| Source | Sample Size | Lesion Type | Age Range† | Outcome Measure | Country/Gini Index | Categorical Representation of Gini Index |
|---|---|---|---|---|---|---|
| Berkes et al., 2010 | 63 | S, M, C | 13–18 | PedsQL: Emotional Functioning | Hungary/24.7 | 1 |
| Brandhagen et al., 1991 | 168 | S, M, C | 24–42 | SCL-90: General Severity Index | US/45.0 | 2 |
| Chen et al., 2011 | 289 | S, M, C | 20–59 | BS-RS: General Symptom Index; | Taiwan/32.6 | 2 |
| Cohen et al., 2010 | 27 | S | 60–87 | HADS: Depression; HADS: Anxiety | Isreal/39.2 | 2 |
| Daliento et al., 2005 | 46 | M | 28–36 | SF-36: Mental Health Subscale | Italy/32.0 | 2 |
| Eslami et al., 2013 | 347 | S, M, C | 18–64 | HADS: Depression; HADS: Anxiety | Iran/38.3 | 2 |
| Gratz et al., 2009 | 561 | S, M, C | 17–73 | SF-36: Mental Health Subscale | Germany/27.0 | 1 |
| Irtel et al., 2005 | 67 | M, C | 16–62 | SF-36: Mental Health Subscale | Switzerland/33.7 | 2 |
| Kamphuis et al., 2002 | 79 | C | 18–32 | TAAQOL: Depression; SF-36: Mental Health Subscale | Netherlands/30.9 | 1 |
| Kamphuis et al., 2002 | 79 | S | 17–32 | TAAQOL: Depression; SF-36: Mental Health Subscale | Netherlands/30.9 | 1 |
| Karsdorp et al., 2008 | 66 | S, M, C | 18–56 | STAI: State Anxiety | Netherlands/30.9 | 1 |
| Lane et al., 2002 | 275 | S, M, C | 16–85 | SF-36: Mental Health Subscale | UK/34.0 | 2 |
| Loup et al., 2009 | 153 | S, M, C | 15–37 | SF-36: Mental Health Subscale; HADS: Depression; HADS: Anxiety | Switzerland/33.7 | 2 |
| Lu et al., 2010 | 61 | M | 14–69 | SF-36: Mental Health Subscale | US/45.0 | 2 |
| Müller et al., 2011 | 58 | M, C | 15–55 | SF-36: Mental Health Subscale | Germany/27.0 | 1 |
| Müller et al., 2012 | 767 | S, M, C | 14–65 | SF-36: Mental Health Subscale; CES-D | Germany/27.0 | 1 |
| Rietveld et al., 2004 | 20 | S, M, C | 18–40 | STAI: State Anxiety | Netherlands/30.9 | 1 |
| Riely et al., 2011 | 99 | S, M, C | 17–67 | SF-36: Mental Health Subscale | UK/34.0 | 2 |
| Simko et al., 2003 | 124 | S, M, C | 21–45 | SIP: Emotional Behavior | US/45.0 | 2 |
| Utens et al., 1993 | 85 | S, M, C | 15–17 | YSR: Anxious/Depressed; YSR: Withdrawn | Netherlands/30.9 | 1 |
| van Rijen et al., 2005 | 242 | S, M, C | 20–32 | YASR: Anxious/Depressed; YASR: Withdrawn | Netherlands/30.9 | 1 |
| Winter et al., 2008 | 47 | C | 21–69 | SF-36: Mental Health Subscale | Netherlands/30.9 | 1 |
Abbreviations: BS-RS, Brief Symptom-Rating Scale; C, complex lesion; CES-D, Center for Epidemiologic Studies Depression Scale; HADS, Hospital Anxiety and Depression Scale; M, moderate lesion; PedsQL, Pediatric Quality of Life Inventory; S, simple lesion; SCL-90, Symptom Check List; SF-36, Medical Outcomes Studies Short Form 36-Item; SIP, Sickness Impact Profile; STAI, State-Trait Anxiety Inventory; TAAQOL, Netherlands Organization for Applied Scientific Research-Academic Medical Center; UK, United Kingdom; US, United States; WHOQOL-Bref, the shortened form of the World Health Organization Quality of Life survey; YASR, Young Adult Self-Report; YSR, Youth Self-Report
Age range of observed study data that was included in the meta-analysis. For some studies, this included the entire sample, and for others the included data was a portion of what was reported.
Possible Moderators of Variance in Effect Sizes
Lesion severity was determined using classifications outlined by the American College of Cardiology/American Heart Association 2008 guidelines,41 resulting in designations of simple, moderate, and complex. For studies that compared lesion types, data from each lesion was included in both the overall analysis with studies that did not make separate lesion comparisons, as well as analyzed separately so that effect sizes could be examined for each lesion severity classification.
The Gini Index is a rating of inequality of income distribution that has been used to explore the relationship between countries and health-related quality of life,42 as well as implemented as a proxy for access to healthcare in previous meta-analyses.43 The closer the index reaches 100, the more inequitable income distribution is in the country. For the purposes of moderation, Gini Index scores were analyzed both continuously (using meta-regression),44 as well as categorically. For categorical analysis, countries were split into 2 groups based on median split of the Gini Index for the observed studies: 24.7 to 30.9 (most equitable), 32.0 to 45.0 (least equitable).
The quality of each study was rated by 2 coauthors using a modified instrument by Downs and Black45 that included 5 components of quality. The intraclass correlation coefficient was 0.84. Any differences that arose were resolved through discussion between the raters before a final rating was assigned. The 5 components evaluated included (1) whether the sample’s demographics were clearly described, (2a) how patients were approached for enrollment (e.g., identified through clinic roster, approached in a waiting room), (2b) how controls were approached for enrollment, (3) whether the number of declines for participation were reported, (4) the appropriateness of the statistical procedures, and (5) whether the emotional distress measure(s) had been psychometrically validated. Components 1 and 4 were rated 0 to 2, while the other components were rated as either 0 or 1. A composite quality score was created for each study, which was a sum of the 5 components. Points ranged from 0 to 8 for studies without a control group and 0 to 10 for those studies with a control group. Total scores were created by dividing the number of points obtained by the number of points possible. Similar to the Gini Index, study quality was analyzed both continuously and categorically. For categorical analysis, study quality was divided into 3 groups based on the distribution of scores: 40 to 50 (less rigorous), 63 to 80 (moderate rigor), 88 to 90 (greater rigor).
Data Analysis
Means, standard deviations, sample sizes, and exact p values were used to calculate effect sizes, which were represented by Hedge’s g. Effect sizes indicate the magnitude of differences between individuals with CHD and controls or measure norms. Positive effect sizes suggested that individuals with CHD reported better emotional functioning than controls or measure norms, whereas negative values suggested poorer emotional functioning. Effect sizes < 0.2 represent a negligible effect, 0.2 to 0.5 suggest a small effect, 0.5 to 0.8 suggest a medium effect, and > 0.8 indicate a large effect size.46 When multiple measures of emotional functioning were used in the same study, the effect sizes were averaged to produce one Hedge’s g value to represent that study. The Comprehensive Meta-Analysis software program (Version 2)47 was used to compute Hedge’s g, as well as determine the statistical significance and 95% confidence intervals for each mean effect size using a random effects model.
Heterogeneity of the effect size was assessed using the Cochran’s Q statistic and the I2 statistic (a transformation of the Q statistic that indicates the percentage of variation in the effect size estimate attributable to heterogeneity rather than sampling error).48 A non-significant Q statistic suggests that the pooled effect size represents a uniform finding in which there is little variation among study effect sizes and that any variability present is due to random error. On the other hand, a significant Q statistic suggests that there may be moderating factors that can, in part, explain the variability in effect sizes. In the current study, we planned to examine moderators when significant heterogeneity was identified, including lesion severity, age, country of origin as represented by the Gini Index, and study quality.
Moderators were examined by using both meta-regression, as well as subdividing the data set as a function of each moderator. The Comprehensive Meta-Analysis program calculated the meta-regression equations, as well as effect sizes at each level and across all levels of the moderator for categorical analysis.
Because studies with statistically significant findings are more likely to be published than those with non-significant results, publication bias was assessed using an adjusted rank correlation test,49 as well as a regression procedure to measure funnel plot asymmetry.50
Results
Individuals with CHD vs. Control/Normative Data
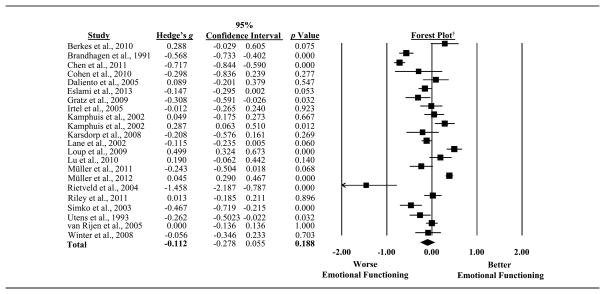
Levels of emotional functioning between individuals with CHD and healthy controls or normative data were compared in 22 studies with the magnitude of effect sizes ranging from −1.46 to 0.50 (See Figure 2). No differences between survivors of CHD and norms/controls in emotional functioning were found in 13 studies. Among the remaining 9 studies that did report differences, the findings from 6 studies suggested that individuals with CHD had greater emotional distress than controls/norms. Overall, the pooled effect size suggested that survivors of CHD did not report greater emotional distress than controls/norms (Hedge’s g = −0.11, 95% CI: −0.28 to 0.06, p = 0.188) (See Figure 2). However, significant heterogeneity across studies was found (Q statistic = 330.14, df = 21, p < 0.001, I2 = 94%), warranting the exploration of possible moderators.
Figure 2.
Forest plot depicting results for emotional distress among all observed studies.
†The boxes on the forest plot vary in size depending on the weight of the study in the meta-analysis. The diamond represents the pooled effect size.
Leave-one-out analyses, a measure of sensitivity, indicated that the removal of any one study did not change the statistical significance of the pooled effect size, suggesting that the meta-analysis results are fairly robust.
Lesion Severity
Data were not available for the individual lesion severities in 11 out of the 22 studies because individuals with “complex,” “moderate,” and “simple” classifications were combined within the studies.19–21, 24, 25, 29, 30, 35–38 This resulted in 11 studies being eligible for inclusion in analyses for lesion severity because the studies were either homogeneous or the lesion types had been analyzed separately (see Figure 3).
Figure 3.
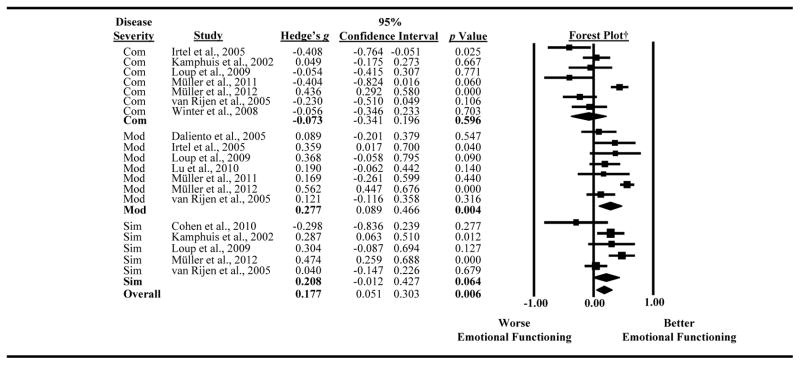
Forest plot depicting results by lesion severity.
†The boxes on the forest plot vary in size depending on the weight of the study in the meta-analysis. The diamond represents the pooled effect size.
Abbreviations: Com, complex; Mod, moderate; Sim, simple.
A trend was found for lesion severity as a moderator of the variance across studies (Q statistic = 4.49, df = 2, p = 0.106). For individuals with complex lesions, 7 of the studies were included in the analyses, and the magnitude of effect sizes ranged from −0.41 to 0.44. Survivors with complex lesions reported similar levels of emotional functioning as control or normative data (Hedges’ g = −0.07, 95% CI: −0.34 to 0.120, p = 0.596). Samples of individuals with moderate lesions were also represented in 7 studies, with the magnitude of effect sizes ranging from 0.09 to 0.58. Those with moderate lesions reported significantly better emotional functioning than controls or measure norms (Hedge’s g = 0.28, 95% CI: 0.09 to 0.47, p = 0.004). Lastly, samples of individuals with simple lesions were represented in 5 studies, and the magnitude of the effect sizes ranged from −0.29 to 0.47. A trend was identified for survivors of CHD with simple lesions to report better emotional functioning than controls/norms (Hedge’s g = 0.21, 95% CI: −0.01 to 0.43, p = 0.064).
Leave-one-out analyses were also conducted on each lesion severity subgroup to examine the stability of the findings. For studies represented in the “simple” classification, removing those by Cohen and colleagues (2010)22 and van Rijen and colleagues (2005)39 resulted in a larger and significant effect size (Hedge’s g = 0.27, p = 0.012; Hedge’s g = 0.27, p = 0.025). However, with the removal of either study, the amount of heterogeneity amongst the studies represented in the “simple” classification remained significant. The removal of any study for those represented in the “complex” and “moderate” classifications did not change the magnitude and significance of the effect size.
Age
Age was unable to be examined as a moderator because most studies did not report findings for adolescents, young adults, and adults separately. Of the studies included in the meta-analysis, 9 combined adolescents (< 18 years of age), young adults (18–25 years of age), and adults (25–64 years of age) with no comparisons across age groups.25, 26, 28, 30–32, 33, 34, 36 Furthermore, a limited number of studies independently represented the 3 age ranges (2 studies only included adolescents19,38 and 1 study only included older adults22), preventing statistical age comparisons.
Study Location
All of the observed studies were included in analyses examining study location as a moderator of heterogeneity in effect sizes (see Table 1 for categorical designations). No differences in effect sizes were found as a function of the Gini Index scores reflecting levels of income distribution equality using random effects models in either meta-regression (β = 0.41, SE = 0.43, p = 0.33) or categorical analysis (Q statistic = 0.20, p = 0.65).
Study Quality
Study quality did not moderate the effect sizes reported by the observed studies as confirmed using both meta-regression (β = −0.25, SE = 0.31, p = 0.43) and categorical analysis (Q statistic = 0.14, p = 0.93).
Publication Bias
Formal testing using adjusted rank correlation tests (p = 0.43) and the regression intercept approach (p = 0.30) confirmed no publication bias. Data are available upon request.
Discussion
This paper is the first to quantitatively compare studies of emotional functioning among adolescent and adult survivors of CHD. The meta-analysis included 22 empirical papers and found significant heterogeneity in the results of studies. However, analyses of factors that may account for variability in results, such as differences in study populations and methodological rigor, were not conclusive. Lesion severity neared statistical significance as a moderator, and examination of simple effects indicated that individuals with moderate lesions reported better emotional functioning than controls or measure norms.
The trend identified for lesion severity suggests that disease severity may explain some of the variability in findings for emotional functioning among CHD survivors across studies. However, the current classification system of “complex,” “moderate” and “simple” may not be sufficient to capture the true impact of disease on patients’ experiences. For example, individuals with transposition of the great arteries or tricuspid atresia, both of which are considered complex lesions, have a 90% survival rate within 10 years after surgery. 41 Yet, the presence of other long-term risk factors for some lesion types, such as protein-losing enteropathy among individuals with tricuspid atresia, can contribute to significant morbidity and increased risk for mortality that may not be typical of all individuals considered to have complex disease. Therefore, alternative classification systems that take into account surgical outcomes, such as the Risk Adjustment for Congenital Heart Surgery 1 (RASCH-1),51 and/or other clinical outcomes of disease (e.g., New York Heart Association Functional Class, number of hospitalizations, comorbid conditions) may explain more of the heterogeneity seen in the literature for emotional functioning among survivors of CHD.
Examination of simple effects from the moderation analyses suggested that individuals with moderate lesions reported better emotional functioning than healthy controls or measure norms. This is surprising because many individuals with moderate lesions may need to undergo future surgeries, experience cardiac symptoms, and are at risk for future health problems, including congestive heart failure and arrhythmias.4 However, variability in lesion types under the classification of “moderate” means that many individuals may also experience few, if any, physical limitations, and may not need future interventions. Therefore, individuals with moderate lesions may demonstrate resiliency, a concept that broadly defined means successful adaptation in the context of significant threats to development.52 Resiliency is optimal in circumstances in which individuals are faced with some adversity, but are not experiencing more persistent reminders of their disease, such as significant physical limitations or reduced life expectancy.53 Similar explanations have been proposed for survivors of pediatric and adult-onset cancer who reported equivalent levels of emotional functioning as controls, as well as better perceptions of general health.53,54 This explanation may also address the statistical trend identified for individuals with simple lesions reporting better emotional functioning than controls/norms, who similar to those with moderate lesions, are unlikely to experience functional limitations and will likely have a normal life expectancy.
Contrary to expectations, individuals with complex lesions did not differ in emotional functioning from controls/norms, despite the likelihood that many of these individuals have encountered significant medical complications, have physical limitations, and may face a poor or uncertain prognosis. The studies included in the “complex” lesion analyses identified various factors that may have contributed to poorer functioning in their samples, such as persistent cyanosis,34 the presence of arrhythmias,26 time since surgery31 and cognitive limitations.33 While Müller and colleagues (2012)34 did not report overall worse emotional functioning among their sample of individuals with transposition, the authors noted that when isolating participants with persistent cyanosis, higher levels of depression were reported. Other studies included in the “complex” lesion analyses primarily included one type of complex lesion and identified the presence of arrhythmias,26 time since surgery31 and cognitive limitations33 as factors that may have contributed to poorer emotional functioning among the study samples. Taken together with the findings for those with moderate lesions, these results call for a more nuanced approach to measuring disease severity using clinical indicators (e.g., New York Heart Association Functional Classification system, number of hospitalizations, and number of comorbid conditions), as well as examining other psychosocial predictors of adjustment (e.g., coping and family support) using multiple sources and methodologies.
Study location also was examined as a possible moderator of the significant heterogeneity. Kovacs and colleagues highlighted that sociocultural factors may explain some of the discrepancy in research findings on emotional distress among survivors of CHD who reside in the United States and other countries,4 especially due to greater economic inequality and less access to healthcare in the U.S.55 Posthoc analyses were conducted comparing the U.S. studies to the other observed studies in the meta-analysis, which did not identify differences in effect sizes. Similarly, study results did not vary as a function of the Gini Index, a measure of inequality of income distribution within a country. This index may not, however, be the optimal indicator of sociocultural factors influencing patient access to healthcare. Other possible measures of sociocultural factors, such as attempting to categorize the type of healthcare system in each country, may be more optimal, but are challenging to classify because of the diversity in healthcare systems. Also, there were only 3 studies from the U.S. represented in the meta-analysis, making comparisons between the U.S. and other countries difficult. Therefore, having more studies representing the U.S. is crucial for identifying sociocultural factors that may help explain the discrepancy among international research findings.
The only previously published meta-analysis on emotional functioning among individuals with CHD found that parents of adolescents reported greater emotional distress than parents of children.3 Therefore, age was another important moderator to explore. However, because all but 4 studies combined data from adolescents, adults, and older adults, age analyses were unable to be conducted. The question still remains of what happens to these adolescents as they age and enter adulthood, at which time they are both faced with new life experiences (e.g., moving out of the home, getting a first job), as well as possibly starting to develop significant health complications (e.g., heart failure, arrhythmia). Age, as well as other important demographic and socio-ecological variables, which are difficult to assess within the realm of meta-analysis, should be examined using multivariate analyses in future studies. This would allow for identification of what factors are associated with resiliency versus risk for poor emotional functioning.
The primary limitation of the current meta-analysis was the inability to include 14 studies that exist in the literature. Of the 36 studies identified as eligible for the analysis, 28 articles did not contain information needed for calculating effect sizes, including means, standard deviations, or exact p values. Information was received on 50% of the articles for which inquiries were made. . The International Committee of Medical Journal Editors discourages authors from solely using significance testing that would fail to provide effect size information.56 Therefore, data should also be presented in such a way as to facilitate the extraction of effect sizes. Effect sizes allow readers to determine the magnitude of differences within a sample and provide information for inclusion in meta-analysis, an invaluable tool in medicine.57 For the current study, a publication bias analysis did not identify bias from potential missing studies, but these analyses primarily guard against the lack of non-significant published findings. While the results of the meta-analysis may be altered if the 14 studies were added, the sensitivity analysis suggested that the overall pooled effect size was fairly stable and would not change if any of the included studies were omitted from the analysis. This was further evidenced by examining the results of the 14 studies not included in the current meta-analysis, which revealed that a majority of the studies found no differences between those with CHD and controls/norms,6,7,10,11,14–17 with only a few studies reporting better8,13,18 or worse5,12 emotional functioning than control or normative data.
In conclusion, the current meta-analysis is the first to examine the discrepancy in findings of emotional distress among samples of adolescents and adults with CHD. Disease severity may explain a significant amount of the heterogeneity among published studies, However, the current classification system of “complex,” “moderate,” and “simple” does not accurately describe important distinctions across lesion types or between individuals with the same lesion who may have very different disease outcomes, and it is important for future prospective studies to continue investigating medical factors that may explain differences among lesion types. Meta-analysis is an important tool for synthesizing the literature to make informed conclusions about the status of research in the field of CHD. The current study highlights the vital need for authors to include descriptive information in their manuscripts to facilitate the meta-analytic process and for journals to require such information prior to publication, including means, standard deviations, and exact p values. The number of adults living with CHD will continue to increase, and understanding the factors that contribute to not only their physical well-being, but also their emotional health, is vital for providing optimal care for these individuals.
Acknowledgments
This work was supported by National Institutes of Health Grants T32 HL-630075 (to J.L. Jackson).
We would like to thank those who contributed to this meta-analysis by making considerable efforts to answer our inquiries, including Robert Colligan, Ph.D., Luciano Daliento, M.D., Alfred Hager, M.D., Alexander Kadner, M.D., Mascha Kamphuis, M.D. Ph.D., Deirdre Lane, Ph.D., Jimmy Lu, M.D., Jan Müller, Ph.D., Lynn Simko Ph.D. R.N., Elisabeth Utens, Ph.D., and Michiel Winter, M.D.
Footnotes
The authors do not have any conflicts of interest to report.
Author Contributions
J.L. Jackson: Data collection, data analysis/interpretation; B. Misiti: data collection and critical revision of article; J.A. Bridge: statistics and critical revision of article; C.J. Daniels: interpretation of data and critical revision of article; K. Vannatta: concept/design, interpretation of the data, and critical revision of article.
References
- 1.Williams RG, Pearson GD, Barst RJ, et al. Report of the National Heart, Lung, and Blood Institute Working Group on research in adult congenital heart disease. J Am Coll Cardiol. 2006;47:701–707. doi: 10.1016/j.jacc.2005.08.074. [DOI] [PubMed] [Google Scholar]
- 2.Brickner ME, Hills LD, Lange RA. Congenital heart disease in adults. First of two parts. N Engl J Med. 2000;342:256–263. doi: 10.1056/NEJM200001273420407. [DOI] [PubMed] [Google Scholar]
- 3.Karsdorp PA, Everaerd W, Kindt M, Mulder BJ. Psychological and cognitive functioning in children and adolescents with congenital heart disease: a meta-analysis. J Pediatr Psychol. 2007;32:527–541. doi: 10.1093/jpepsy/jsl047. [DOI] [PubMed] [Google Scholar]
- 4.Kovacs AH, Sears SF, Saidi AS. Biopsychosocial experiences of adults with congenital heart disease: review of the literature. Am Heart J. 2005;150:193–201. doi: 10.1016/j.ahj.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 5.Aldén BT, Gilljam T, Gillberg C. Long-term psychological outcome of children after surgery for transposition of the great arteries. Acta Paediatr. 1998;87:405–410. doi: 10.1080/08035259850156995. [DOI] [PubMed] [Google Scholar]
- 6.Bol Raap G, Meijboom FJ, Kappetein AP, Galema TW, Yap SC, Bogers AJ. Long-term follow-up and quality of life after closure of ventricular septal defect in adults. Eur J Cardiothorac Surg. 2007;322:215–219. doi: 10.1016/j.ejcts.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Bruto VC, Harrison DA, Fedak PW, Rockert W, Siu SC. Determinants of health-related quality of life in adults with congenital heart disease. Congenit Heart Dis. 2007;2:301–313. doi: 10.1111/j.1747-0803.2007.00117.x. [DOI] [PubMed] [Google Scholar]
- 8.Cox D, Lewis G, Stuart G, Murphy K. A cross-sectional study of the prevalence of psychopathology in adults with congenital heart disease. J Psychosom Res. 2002;52:65–68. doi: 10.1016/s0022-3999(01)00294-x. [DOI] [PubMed] [Google Scholar]
- 9.Dua JS, Cooper AR, Fox KR, Graham Stuart A. Exercise training in adults with congenital heart disease: feasibility and benefits. Int J Cardiol. 2010;138:196–205. doi: 10.1016/j.ijcard.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 10.Fekkes M, Kamphuis RP, Ottenkamp J, et al. Health-related quality of life in young adults with minor congenital heart disease. Psychol Health. 2001;16:239–250. [Google Scholar]
- 11.Immer FF, Althaus SM, Berdat PA, Saner H, Carrel TP. Quality of life and specific problems after cardiac surgery in adolescents and adults with congenital heart diseases. Eur J Cardiovasc Prev Rehabil. 2005;12:138–143. doi: 10.1097/01.hjr.0000159318.62466.dc. [DOI] [PubMed] [Google Scholar]
- 12.Khan AA, Tan JL, Li W, et al. The impact of transcatheter atrial septal defect closure in the older population: a prospective study. JACC Cardiovasc Interv. 2010;3:276–281. doi: 10.1016/j.jcin.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Lemmer J, Heise G, Rentzsch A, et al. Right ventricular function in grown-up patients after correction of congenital right heart disease. Clin Res Cardiol. 2011;100:289–296. doi: 10.1007/s00392-010-0241-8. [DOI] [PubMed] [Google Scholar]
- 14.Mokhles MM, van de Woestijne PC, de Jong PL, et al. Clinical outcome and health-related quality of life after right-ventricular-outflow-tract reconstruction with an allograft conduit. European Journal of Cardio-thoracic Surgery. 2011;40:571–578. doi: 10.1016/j.ejcts.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 15.Saliba Z, Butera G, Bonnet D, et al. Quality of life and perceived health status in surviving adults with univentricular heart. Heart. 2001;86:69–73. doi: 10.1136/heart.86.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Utens EM, Versluis-Den Bieman HJ, Verhulst FC, Meijboom FJ, Erdman RA, Hess J. Psychopathology in young adults with congenital heart disease. Follow-up results. Eur Heart J. 1998;19:647–651. doi: 10.1053/euhj.1997.0824. [DOI] [PubMed] [Google Scholar]
- 17.Vigl M, Niggemeyer E, Hager A, Schwedler G, Kropf S, Bauer U. The importance of socio-demographic factors for the quality of life of adults with congenital heart disease. Qual Life Res. 2011;20:169–177. doi: 10.1007/s11136-010-9741-2. [DOI] [PubMed] [Google Scholar]
- 18.Vandekerckhove KD, Blom NA, Lalezari S, Koolbergen DR, Rijlaarsdam ME, Hazekamp MG. Long-term follow-up of arterial switch operation with an emphasis on function and dimensions of left ventricle and aorta. Eur J Cardiothorac Surg. 2009;35:582–587. doi: 10.1016/j.ejcts.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 19.Berkes A, Varni JW, Pataki I, Kardos L, Kemeny C, Mogyorósy G. Measuring health-related quality of life in Hungarian children attending a cardiology clinic with the Pediatric Quality of Life Inventory. Eur J Pediatr. 2010;169:333–347. doi: 10.1007/s00431-009-1059-0. [DOI] [PubMed] [Google Scholar]
- 20.Brandhagen DJ, Feldt RH, Williams DE. Long-term psychologic implications of congenital heart disease: a 25-year follow-up. Mayo Clin Proc. 1991;66:474–479. doi: 10.1016/s0025-6196(12)62387-8. [DOI] [PubMed] [Google Scholar]
- 21.Chen CA, Liao SC, Wang JK, et al. Quality of life in adults with congenital heart disease: biopsychosocial determinants and sex-related differences. Heart. 2011;97:38–43. doi: 10.1136/hrt.2010.200709. [DOI] [PubMed] [Google Scholar]
- 22.Cohen M, Daniela M, Yalonetsky S, Gagin R, Lorber A. Psychological functioning and health-related quality of life (HRQoL) in older patients following percutaneous closure of the secundum atrial septal defect (ASD) Arch Gerontol Geriatr. 2010;50:e5–e8. doi: 10.1016/j.archger.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Daliento L, Mapelli D, Russo G, et al. Health related quality of life in adults with repaired tetralogy of Fallot: psychosocial and cognitive outcomes. Heart. 2005;91:213–218. doi: 10.1136/hrt.2003.029280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eslami B, Sundin Ö, Macassa G, Khankeh HR, Soares JJF. Anxiety, depressive and somatic symptoms in adults with congenital heart disease. Journal of Psychosomatic Research. 2013;74:49–56. doi: 10.1016/j.jpsychores.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Gratz A, Hess J, Hager A. Self-estimated physical functioning poorly predicts actual exercise capacity in adolescents and adults with congenital heart disease. Eur Heart J. 2009;30:497–504. doi: 10.1093/eurheartj/ehn531. [DOI] [PubMed] [Google Scholar]
- 26.Irtel TA, Vetter C, Stuber T, et al. Impact of arrhythmias on health-related quality of life in adults with congenital cardiac disease. Cardiol Young. 2005;15:627–631. doi: 10.1017/S1047951105001812. [DOI] [PubMed] [Google Scholar]
- 27.Kamphuis MJ, Ottenkamp J, Vliegen HW, et al. Health related quality of life and health status in adult survivors with previously operated complex congenital heart disease. Heart. 2002;87:356–362. doi: 10.1136/heart.87.4.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamphuis M, Vliegen HW, Vogels T, et al. The need for cardiac follow-up in adults with mild congenital cardiac disease. Cardiol Young. 2002;12:474–478. doi: 10.1017/s1047951102000811. [DOI] [PubMed] [Google Scholar]
- 29.Karsdorp PA, Kindt M, Rietveld S, Everaerd W, Mulder B. Interpretation bias for heart sensations in congenital heart disease and its relation to quality of life. Int J Behav Med. 2008;15:232–240. doi: 10.1080/10705500802212916. [DOI] [PubMed] [Google Scholar]
- 30.Lane DA, Lip GY, Millane TA. Quality of life in adults with congenital heart disease. Heart. 2002;88:71–75. doi: 10.1136/heart.88.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loup O, von Weissenfluh C, Gahl B, Schwerzmann M, Carrel T, Kadner A. Quality of life of grown-up congenital heart disease patients after congenital cardiac surgery. Eur J Cardiothorac Surg. 2009;36:105–111. doi: 10.1016/j.ejcts.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 32.Lu JC, Cotts TB, Agarwal PP, Attili AK, Dorfman AL. Relation of right ventricular dilation, age of repair, and restrictive right ventricular physiology with patient-reported quality of life in adolescents and adults with repaired tetralogy of fallot. Am J Cardiol. 2010;106:1798–1802. doi: 10.1016/j.amjcard.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 33.Müller J, Hess J, Hager A. Exercise performance and quality of life is more impaired in Eisenmenger syndrome than in complex cyanotic congenital heart disease with pulmonary stenosis. International Journal of Cardiology. 2011;150:177–181. doi: 10.1016/j.ijcard.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Müller J, Hess J, Hager A. Minor symptoms of depression in patients with congenital heart disease have a larger impact on quality of life than limited exercise capacity. International Journal of Cardiology. 2012;154:265–269. doi: 10.1016/j.ijcard.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 35.Rietveld S, Mulder BJ, van Beest I, et al. Negative thoughts in adults with congenital heart disease. Int J Cardiol. 2002;86:19–26. doi: 10.1016/s0167-5273(02)00152-3. [DOI] [PubMed] [Google Scholar]
- 36.Riley JP, Habibi H, Banya W, Gatzoulis MA, Lau-Walker M, Cowie MR. Education and support needs of the older adult with congenital heart disease. Journal of Advanced Nursing. 2011;68:1050–1060. doi: 10.1111/j.1365-2648.2011.05809.x. [DOI] [PubMed] [Google Scholar]
- 37.Simko LC, McGinnis KA. Quality of life experienced by adults with congenital heart disease. AACN Clin Issues. 2003;14:42–53. doi: 10.1097/00044067-200302000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Utens EM, Verhulst FC, Meijboom FJ, et al. Behavioural and emotional problems in children and adolescents with congenital heart disease. Psychol Med. 1993;23:415–424. doi: 10.1017/s0033291700028518. [DOI] [PubMed] [Google Scholar]
- 39.van Rijen EH, Utens EM, Roos-Hesselink JW, et al. Longitudinal development of psychopathology in an adult congenital heart disease cohort. Int J Cardiol. 2005;99:315–323. doi: 10.1016/j.ijcard.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Winter MM, Bouma BJ, van Dijk AP, et al. Relation of physical activity, cardiac function, exercise capacity, and quality of life in patients with a systemic right ventricle. Am J Cardiol. 2008;102:1258–1262. doi: 10.1016/j.amjcard.2008.06.053. [DOI] [PubMed] [Google Scholar]
- 41.Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;52:e143–e263. doi: 10.1016/j.jacc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Cifuentes M, Sembajwe G, Tak S, Gore R, Kriebel D, Punnett L. The association of major depressive episodes with income inequality and the human development index. Soc Sci Med. 2008;67:529–539. doi: 10.1016/j.socscimed.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012–1024. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Houwelingen HC, Arenda LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med. 2002;21:589–624. doi: 10.1002/sim.1040. [DOI] [PubMed] [Google Scholar]
- 45.Downs SH, Black N. The feasibility of creating a checklist for the assessment of methodological quality both of randomized and non-randomized studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale: L. Erlbaum Associates; 1988. [Google Scholar]
- 47.Borenstein M, Rothstein H, Cohen J. Comprehensive Meta-Analysis Computer Program and Manual. Englewood Cliffs: Biostat, Inc; 2005. [Google Scholar]
- 48.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 49.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 50.Egger M, Davey Smith G, Scheider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123:110–118. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 52.Masten AS. Resilience in individual development: successful adaptation despite risk and adversity. In: Wang MC, Gordon E, editors. Educational resilience in inner city America: challenges and prospects. Hilldale, NJ: Erlbaum; 1994. pp. 2–25. [Google Scholar]
- 53.Aspinwall LG, MacNamara A. Taking positive changes seriously. Cancer. 2005;104:2549–56. doi: 10.1002/cncr.21244. [DOI] [PubMed] [Google Scholar]
- 54.Langeveld NE, Grootenhuis MA, Voûte PA, de Haan RJ, van den Bos C. Quality of life, self-esteem and worries in young adult survivors of childhood cancer. Psychooncology. 2004;13:867–881. doi: 10.1002/pon.800. [DOI] [PubMed] [Google Scholar]
- 55.Starfield B. Primary care and health. A cross-national comparison. JAMA. 1991;266:2268–227. [PubMed] [Google Scholar]
- 56.International Committee of Medical Journal Editors. Publishing and Editorial Issues Related to Publication in Medical Journals: Preparing a Manuscript for Submission to a Medical Journal. [Accessed January 29, 2014];Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals. http://www.icmje.org/manuscript_a.html. Published 2013.
- 57.Egger M, Smith GD. Meta-Analysis: Potentials and promise. BMJ. 1997;315:1371–1374. doi: 10.1136/bmj.315.7119.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]