Abstract
Objectives
To examine neuropsychiatric and neuropsychological predictors of progression from normal to early clinical stages of Alzheimer’s disease (AD).
Design
Longitudinal study
Setting
Massachusetts Alzheimer’s Disease Research Center longitudinal cohort
Participants
From a total sample of 559 older adults, 454 were included in the primary analysis: 283 with clinically normal cognition (CN), 115 with mild cognitive impairment (MCI) and 56 with subjective cognitive concerns (SCC) but no objective impairment, a proposed transitional group between CN and MCI.
Measurements
Two latent cognitive factors (Memory-Semantic, Attention-Executive) and two neuropsychiatric factors (Affective, Psychotic) were derived from the Alzheimer’s Disease Centers’ Uniform Data Set neuropsychological battery and Neuropsychiatric Inventory brief questionnaire. Factors were analyzed as predictors of time to progression to a worse diagnosis using a Cox proportional-hazards regression model with backward elimination. Covariates included baseline diagnosis, gender, age, education, prior depression, antidepressant medication, symptom duration, and interaction terms.
Results
Higher/better Memory-Semantic factor score predicted lower hazard of progression (HR=0.4 for one SD increase, p<0.0001), and higher/worse Affective factor score predicted higher hazard (HR=1.3 for one SD increase, p=0.01). No other predictors were significant in adjusted analyses. Using diagnosis as a sole predictor of transition to MCI, the SCC diagnosis carried a 4-fold risk of progression compared to CN (HR=4.1, p<0.0001).
Conclusions
These results identify affective and memory-semantic factors as significant predictors of more rapid progression from normal to early stages of cognitive decline and highlight the subgroup of cognitively normal elderly with SCC as those with elevated risk of progression to MCI.
Keywords: Neuropsychiatric and Neuropsychological factors, Subjective Cognitive Concerns, Mild Cognitive Impairment, Alzheimer’s disease
INTRODUCTION
Alzheimer’s disease (AD) is currently understood to be a pathophysiological process that begins with a long preclinical phase. Research criteria propose a three-stage model of preclinical AD in which temporally ordered biomarker abnormalities precede and then coincide with newly emerging behavioral and cognitive symptoms in clinically normal individuals (those without significant impairment on cognitive tests). These subtle but detectable symptoms define stage 3 preclinical AD, a transitional state that may be evident prior to the diagnosis of mild cognitive impairment (MCI).(1) It is an urgent public health challenge to identify the earliest cognitive and non-cognitive manifestations of preclinical AD in order to advance detection and diagnosis in clinical settings and in early intervention trials.
Subjective cognitive concerns (SCC) are perceived changes in memory or thinking reported by clinically normal older adults, or those who know them well, that can occur despite normal performance on neuropsychological testing. Prior studies have used “subjective cognitive impairment”, “impaired, not MCI”, or “Pre-MCI” designations to classify older individuals with SCC who demonstrate normal cognitive test performance and do not meet diagnostic criteria for MCI.(2–4) In clinically normal individuals, SCC have been associated with biomarkers of AD pathophysiology (5–7) and also with increased rates of progression to MCI and dementia compared to those without SCC.(4, 8) Thus, SCC are a defining feature of stage 3 preclinical AD and are considered a possible diagnostic entity identifying those at risk for progression to AD dementia. (2)
Subtle neuropsychiatric symptoms may also reflect upstream pathophysiological changes in, at-risk, older adults with or without SCC, and may be among the first detectable symptoms in preclinical AD. Neuropsychiatric symptoms, including major depression, subsyndromal depression and anxiety, have been associated with cognitive, functional and AD biomarker changes in clinical cohorts with MCI and in community-dwelling elderly.(9–14) Nonetheless, the role of neuropsychiatric symptoms in progression from clinically normal status to MCI is unclear. To date, relatively few prospective studies have examined neuropsychiatric symptoms in adjudicated samples of clinically normal elderly (15–17) and neuropsychiatric factors have rarely been studied as predictors of progression to MCI and early AD dementia.(18)
In addition, the relationship of SCC and neuropsychiatric symptoms to each other and to progression in preclinical AD also remain undefined. SCC have been strongly associated with depression, anxiety and neuroticism in cross-sectional studies of community dwelling older adults, with and without objective cognitive impairment (19–22) and have been considered by many to be an indication of poor mental health and unrelated to cognitive decline and AD. There have been very few longitudinal studies that have analyzed both SCC and neuropsychiatric symptoms, such as depression and anxiety, to assess whether these cognitive and non-cognitive symptoms are independent predictors of very early clinical decline and progression to MCI and dementia.(22–24) Prior studies have also rarely controlled for possible confounders such as a history of depression or the use of antidepressant medication.(25)
The objective of this study was to examine neuropsychiatric and neuropsychological factors that predict transition in the early AD pathway with special attention to progression to MCI in clinically normal elderly with and without SCC. The study cohort included older individuals, classified at baseline as CN (clinically normal cognition without SCC), as SCC (clinically normal individuals with self or informant reported cognitive concerns, who did not meet criteria for MCI based on normal neuropsychological test performance) or as amnestic MCI. It was expected that a subset of the CN and SCC groups would progress to early clinical stages of AD based on advanced age, and that some would also experience subtle cognitive and behavioral symptoms at baseline. We hypothesized that greater affective neuropsychiatric symptoms and lower performance on tests of episodic and semantic memory would predict more rapid clinical progression to MCI. We further hypothesized that individuals within the SCC group would demonstrate a higher rate of progression to MCI than the CN group.
MATERIALS AND METHODS
Participants
The study sample was drawn from the ongoing Massachusetts Alzheimer’s Disease Research Center (MADRC) longitudinal cohort that was established in 2005. Participants, ranging from CN to dementia, were recruited through the community and specialty clinics. Data for these analyses were obtained from MADRC visits from September 2005 through May 2011. The MADRC contributes data to the National Alzheimer’s Coordinating Center (NACC), a central databank registry established by the National Institute on Aging. (26) From a total sample of 559 participants, 454 with longitudinal data were included in the primary analysis and were classified as follows at baseline: 283 CN, 56 SCC, 115 MCI.
All participants provided informed consent in accordance with the Partners Human Research Committee prior to undergoing any study procedures.
Initial and annual assessments included medical evaluation, clinical dementia rating (CDR), (27) Mini-Mental State Exam (MMSE), (28) and the Alzheimer’s Disease Center (ADC) Uniform Data Set (UDS) standard battery of neuropsychological tests and neuropsychiatric assessments.(29) Participants were assigned to diagnostic groups by an experienced clinician or by consensus panel on the basis of clinical history, presentation and formal assessments (CDR and UDS neuropsychological performance). The NACC/UDS protocol does not define neuropsychological test performance cutoffs for the purpose of assigning diagnoses. At the MADRC, determination of normal range is guided by cognitive test performance within 1.5 standard deviation adjusted for age and premorbid intelligence based on the American National Adult Reading Test score.(30)
CN participants were without subjective memory or cognitive concerns, had a CDR Global score of 0 and performed normally on cognitive testing in all domains. Participants with informant-based or self-reported changes in memory or cognitive tasks were classified as SCC if they had a CDR Sum of Boxes (CDR-SB) score greater than 0, specifically with a box score of 0.5 for either Memory or Judgment and Problem Solving, or both, but performed normally on cognitive testing in all domains. We did not further define CDR box scores for the SCC group, therefore participants with SCC could have non-zero ratings in other CDR boxes. Amnestic MCI, single or multiple domain, participants had subjective memory concerns accompanied by objective memory impairment on testing and were not demented. Individuals classified as non-amnestic MCI or with dementia diagnoses at baseline were excluded. On longitudinal follow-up participants transitioned within the 3 diagnostic categories defined at baseline and some progressed to probable AD dementia or dementia unspecified, indicating that the clinician was confident of the diagnosis of dementia but uncertain about the etiology.
There were eight participants in the MADRC cohort who were pre-excluded from the study sample of 559 as follows: three individuals with baseline diagnoses of MCI who reverted to CN or SCC at the second study visit and were then lost to follow up and five participants with baseline diagnoses of SCC who reverted to CN at visit two and discontinued the study. These rates of reversion from baseline diagnoses of SCC or MCI to less impaired diagnoses are similar to those reported elsewhere.(4, 18) Due to the small number of reversions, there was inadequate power to examine this component of diagnosis transition. Data from these subjects were therefore not included in these analyses that examined progression to a more severe diagnosis.
Neuropsychological Measures
Performance measures from the UDS neuropsychological battery were reduced to two cognitive factors via a principal component analysis on the baseline visit data. Two factors explained 67.1% of the variance and were significantly correlated with each other (r= 0.64, p < 0.001). Factor 1 contributed 56.8% of the variance and was defined as a Memory-Semantic factor. Factor 2 contributed 10.3% of the variance and was defined as an Attention-Executive factor. The Memory-Semantic factor included the Wechsler Memory Scale Logical Memory IA- immediate recall and IIA-delayed recall scores (31), Category Fluency (animals, vegetables) (32) and the Boston Naming Test (30 item, odd numbered) (33). Tests corresponding to the Attention-Executive Factor were Digit Span Forward and Backward (34), Trail Making Test Parts A and B (Trails B) (35) and Digit Symbol (36). The items selected to be included in the respective cognitive factors (on the basis of a loading cutoff of 0.3) were transformed to standard Z scores and adjusted such that higher scores indicated better performance. The average of those non-missing was used as the respective factor score (set to missing if more than half of the variables were missing). Out of the total sample, average values were imputed for missing items for one subject for the Memory-Semantic factor and for three subjects for the Attention-Executive factor.
Neuropsychiatric Measures
Neuropsychiatric symptoms were measured using the Neuropsychiatric Inventory brief questionnaire form (NPI-Q) (37), an informant-based instrument that rates twelve neuropsychiatric symptom items for presence and severity over the past month. Each item is scored as absent (0) or present (1, 2 or 3) using an ordinal scale with higher score indicating greater severity. The factor analysis yielded two neuropsychiatric factors defined as an Affective factor corresponding to NPI-Q items depression, irritability, agitation, disinhibition, anxiety and apathy and a Psychotic factor that included hallucinations, aberrant motor behavior, night-time behaviors, appetite disturbance and delusions (higher factor score denotes greater severity; loading cutoff 0.3). The two factors correlated at r=0.43 (p<0.0001) with 45.4% of the total variance explained by the combined factors. The neuropsychiatric factor scores were computed in a manner analogous to that of the cognitive factors above.
Statistical Analyses
SAS Version 9.3, JMP Pro version 10 and SPSS Version 21 were used for analyses.
We employed Cox proportional hazards regression models with backward elimination (using a conservative p≤0.01 cutoff) to identify predictors of time to transition to a more severe diagnosis with respect to time in the study (years). Participants with stable diagnoses were treated as “censored”, providing partial information. In this cohort, participants with a baseline diagnosis of CN either remained stable or transitioned to SCC or MCI. For the baseline SCC group, participants who were not stable, and did not revert to CN, transitioned to either MCI or probable AD dementia. Participants who progressed from the baseline MCI group developed probable AD dementia or “unspecified dementia”. All varieties of transition were included in the same Cox model to increase sample size and power, but baseline diagnosis and interactions thereof, were predictor terms in the model in order to study any differential transition among these baseline diagnostic groups, adjusted for other covariates.
The original pool of predictors in the Cox model were: the Memory-Semantic and Attention-Executive cognitive factors, the Affective and Psychotic neuropsychiatric factors, the interaction of diagnosis with each of these cognitive and neuropsychiatric factors, baseline diagnosis, sex, the interaction of diagnosis and sex, baseline age, self or informant reported duration of cognitive symptoms at baseline (years; set to mean 0±0.1 SD with random normal deviations for CN), years of education, binary predictors for self or informant reported depressive symptoms occurring greater than two years ago or within the past two years, and current use of antidepressant medications. The proportional hazards assumption of the Cox models was tested with a Kolmogorov simulation test, and all predicators of primary interest in all final backward elimination models were individually found to pass this test. For the Cox models, the significance test for the overall model was a likelihood ratio test, whereas all significance tests for individual predictors were based on a Wald Chi-square test and confidence intervals for hazard ratios were based on a profile likelihood. (See Supplemental Digital Content for additional data analysis methods).
RESULTS
Significant differences were present across diagnostic groups at baseline with respect to age, sex, education, MMSE, duration of cognitive symptoms, depressive symptoms within the past two years, and the two cognitive and two neuropsychiatric factor scores. In contrast, depressive symptoms prior to two years and use of antidepressant medication were not significant (see Table 1) (For further description, see Supplemental Digital content).
Table 1.
Baseline Demographic and Clinical Data
| Group | All subjects | CN | SCC | MCI | Test (df) | p value |
|---|---|---|---|---|---|---|
| N | 559 | 338 | 65 | 156 | ||
| N with 2 or more study visits (% of total within diagnosis) | 454a (81.2) | 283 (83.7) | 56 (86.2) | 115 (73.7) | X2 =8.2 (2) | 0.02 |
| Age (years) | 72.5±9.6b | 69.6±9.8 | 76.4±6.5 | 77.0±8.1 | F=42 (2,556) | <0.0001 |
| Sex (% male) | 35.4c | 28.4 | 47.7 | 45.5 | X2 =19 (2) | <0.0001 |
| Education (years) | 16.2±2.6d | 16.3±2.4 | 17.1±2.3 | 15.6±3.0 | F=8.3 (2,556) | 0.0003 |
| MMSE | 28.6±1.9e | 29.2±1.0 | 29.0±1.4 | 27.0±2.7 | F=94 (2,555) | <0.0001 |
| Duration of cognitive symptoms at baseline (years) | 2.0±3.6f | 0.0±0.1 | 6.0±4.6 | 5.0±3.7 | t=1.4 (219) | 0.162 |
| Depressive symptoms within 2 years (%) | 16.4g | 12.1 | 15.6 | 26.1 | X2 =15 (2) | 0.0005 |
| Depressive symptoms prior to 2 years (%) | 17.8 | 16.6 | 20.3 | 19.6 | X2=1 (2) | 0.6 |
| Antidepressa nt medication use (%) | 13.7 | 12.4 | 15.9 | 15.6 | X2=1.2 (2) | 0.6 |
| Memory- Semantic factor (z score) | 0.267±0.613h | 0.524±0.4 | 0.411±0.5 | −0.353±0.6 | F=183 (2,554) | <0.0001 |
| Attention- Executive factor (z score) | 0.275±0.540i | 0.432±0.4 | 0.442±0.4 | −0.136±0.6 | F=80 (2,553) | <0.0001 |
| Affective factor (z score) | −0.211±0.750j | −0.398±0.5 | −0.098±0.9 | 0.158±1.0 | F=32 (2,525) | <0.0001 |
| Psychotic factors (z score) | −0.179±0.568k | −0.262±0.4 | −0.156±0.6 | −0.003±0.9 | F=11 (2,525) | <0.0001 |
CN (clinically normal cognition), MCI (mild cognitive impairment), MMSE (Mini-Mental State Examination), SCC (subjective cognitive concerns). All values (except n, sex, depression and antidepressant use) represent mean ± standard deviation. Associations between groups were assessed using analysis of variance or t-test for continous variables and the chi-square test for categorical variables. Additional Tukey and chi-square post-hoc pair-wise comparisons were performed (see below).
for CN vs. MCI (df=1), p=0.009; for and SCC vs. MCI (df=1), p=0.04
for CN vs. SCC and MCI (df=556), p<0.0001
for CN vs. SCC (df=1), p=0.002; for CN vs. MCI (df=1), p=0.0002
for CN vs. MCI (df=556), p=0.01; for SCC vs. MCI, p=0.0003
for CN vs. MCI and SCC vs. MCI (df=555), p<0.0001
CN values randomly drawn from a normal distribution with mean of zero and standard deviation of 0.1 (to avoid an attenuated bias for their error variance); t-test for SCC vs. MCI.
for CN vs. MCI (df=1), p=0.0001
for CN and SCC vs. MCI (df=554), p<0.0001
for CN and SCC vs. MCI (df=553), p<0.0001
for CN vs. SCC (df=525), p=0.01; for CN vs. MCI, p<0.0001
for CN vs. MCI (df=525), p<0.0001
Out of a total of 559 participants, 454 had a minimum of at least 2 visits required for the primary longitudinal model examining predictors of time to progression in diagnosis. A significantly larger proportion of those with MCI had only one study visit, and were thereby excluded from primary analyses, as compared to CN and SCC. However, there was no significant difference between the one visit versus multiple visit group on any predictor or other baseline demographic or clinical variable, nor did these groups significantly interact with baseline diagnosis in their relations to any of these variables. All 559 participants were included in a secondary model examining age at time of transition in diagnosis (see Supplemental Digital Content).
For the primary analysis, the mean time to event or censoring was 2.43±1.0 years (range=0.87–5.49), which did not differ significantly between baseline diagnostic groups. Final diagnoses at transition or censoring are shown in Table 2.
Table 2.
Final diagnosis for 454 subjects with at least 1 follow-up visit
| Baseline Diagnosis | Baselinen | Final diagnosis at transition or censoring† | ||||
|---|---|---|---|---|---|---|
| CN | SCC | MCI | Probable AD dementia | Dementia unspecified | ||
| CN n (%) | 283 | 242 (85.5) | 19 (6.7) | 22 (7.8) | 0 | 0 |
| SCC n (%) | 56 | 0 | 39 (69.6) | 16 (28.6) | 1 (1.8) | 0 |
| aMCI n (%) | 115 | 0 | 0 | 79 (68.7) | 28 (24.3) | 8 (7.0) |
CN (clinically normal cognition), MCI (mild cognitive impairment), SCC (subjective cognitive concerns).
Mean time to event or censoring for all participants was 2.43±1.0 years with no significant group-wise differences.
Cox Analysis of Time to Progression in Diagnosis
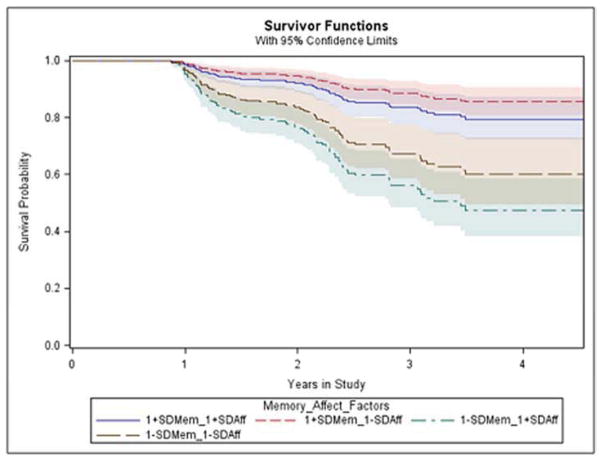
The backward elimination Cox analysis of time in the study to progression in diagnosis revealed only two significant terms: the Memory-Semantic cognitive factor and the Affective neuropsychiatric factor, with the model as a whole being highly significant (see Table 3). More specifically, a higher (better) Memory-Semantic factor score predicted less hazard (hazard ratio, HR=0.4 for a one SD increase in Memory-Semantic factor score) and a higher (worse) Affective factor score predicted greater hazard (HR=1.3 for a one SD increase in Affective factor score; (see Figure 1). No interaction terms were significant indicating no difference among the diagnostic groups in how the Affective factor or Memory-Semantic factor related to hazard beyond variation considered to be chance. (See Supplemental Digital Content for similar results with non-parametric survival analysis and age at time of diagnosis transition).
Table 3.
Cox proportional hazards regression analysis showing significant predictors of time to progression to a more severe diagnosis retained in the final model
| Model: X2 =44.2, df=2; p<0.0001 | ||||
|---|---|---|---|---|
| Predictor | Hazard Ratio | 95% Confidence Interval | X2 (df=1) | p value |
| Memory-Semantic Factor | 0.40† | 0.30–0.56 | 31.6 | <0.0001 |
| Affective Factor | 1.29† | 1.05–1.56 | 6.5 | 0.01 |
CN (clinically normal cognition), MCI (mild cognitive impairment), SCC (subjective cognitive concerns), X2 (Likelihood Ratio for the model test and Wald for the individual predictors.)
For Memory-Semantic factor and Affective factor, the hazard ratio is based on one standard deviation increase.
Figure 1.
Survival Curves Predicted by the Cox Model for Progression to More Severe Diagnosis versus Time in the Study for Selected Illustrative Strata Showing One Standard Deviation Above and Below the Mean for the Memory-Semantic Factor and for the Affective Factor. Survival denotes not having progressed. Abbreviations: Standard deviation (SD), Affective (Aff), Memory (Mem)
To further delineate the specific nature of these findings, we reran a Cox analysis of time in the study on the same final derived set of predictors, but this time replacing the Affective factor with each of the six constituent NPI-Q items in its own separate analysis. The depression (HR=1.6, 95% CI (1.1, 2.3); X2=6.7, df= 1, p<0.01), agitation (HR=1.5, 95% CI (1.06, 2.1); X2=6.1, df=1, p=0.01), and irritability (HR=1.4, 95% CI (1.02, 1.8); X2=5.1, df=1, p=0.02) items were each found to be significant, with higher (worse) scores predicting greater hazard of transition to worse diagnosis.
In order to directly compare hazard of transition to MCI in the CN and SCC groups, we ran a final Cox model of time in the study to analyze baseline diagnosis (CN or SCC) as a sole predictor of progression to MCI, unadjusted for neuropsychiatric or cognitive factors. For the purpose of this analysis we defined CN who transitioned to SCC, but not further to MCI, as equivalent to stable CN to the end of their study (censored). We found that those with a SCC diagnosis at baseline had a 4-fold greater risk of progression to MCI compared to those who were CN (HR= 4.1, 95% CI (2.1, 7.7); X2=18.9, df=1, p<0.0001).
DISCUSSION
The purpose of this study was to identify neuropsychiatric and cognitive predictors of clinical progression across early stages of AD within a cohort of older adults initially classified as CN, SCC and MCI. We found that greater symptoms of depression, irritability and agitation as well as lower memory and semantic processing at baseline predicted more rapid progression to a worse diagnosis across all groups. These findings support a model of AD in which cognitive and neuropsychiatric alterations are measureable prior to the stage of MCI, and offer potential to enhance early detection and intervention.
Our results build on prior work that has identified affective symptoms such as anxiety and depression as predictors of progression from MCI to AD dementia.(13, 18, 38–40) For example, in Alzheimer’s Disease Neuroimaging Initiative (ADNI) analyses, Lee et al. demonstrated that adults with MCI and persistent depression over 2 years showed more atrophy in AD related regions, greater declines in cognitive testing, and higher rates of conversion to AD dementia compared to those with no neuropsychiatric symptoms.(13)
We add to prior research by evaluating affective symptoms, not only in MCI, but also in earlier phases of the AD continuum. We found no interaction of affective factor score with baseline diagnosis in our analysis, indicating that these affective symptoms predict disease progression in MCI and also in CN and SCC, two groups hypothesized to include those with preclinical AD. This finding highlights affective symptoms as potential behavioral markers of preclinical AD in CN and SCC and adds to a small number of studies that have examined depression as a predictor of MCI in carefully defined samples of clinically normal, community dwelling elderly. In four studies that compared depressed to non-depressed clinically normal elderly, there was a doubling of the risk of progression to MCI in those participants with depression as defined by the Geriatric Depression Scale, the Center for Epidemiological Studies Depression Scale and the Comprehensive Psychopathological Rating Scale.(15, 16, 25, 38) We add further to this literature by demonstrating that depression and associated symptoms also predict more rapid progression in the novel diagnostic group of clinically normal elderly with SCC.
It has been proposed that affective neuropsychiatric symptoms such as depression and anxiety, occurring as preclinical or early clinical features of AD, may arise from a number of possible mechanisms. Affective symptoms may represent primary manifestations of AD pathophysiology and be associated with AD biomarker abnormalities;(12–14) they may derive from distinct pathological processes, such as vascular lesions or stress-related neurotoxicity, that may secondarily interact with and accelerate AD progression;(41, 42) or these symptoms may indicate psychological responses to newly perceived and distressing subjective cognitive changes (22) that may, in turn, interact with changes in emotional regulation,(43) among other possible mechanisms.(44)
While we found that current affective symptoms predicted clinical progression in all groups, a self-reported history of previous depression within 2 years, or 2 years prior to baseline did not. Based on these data, it is possible that newly emerging affective symptoms or changes in affective regulation, rather than prior or chronic syndromal depression, may be most informative of risk of clinical progression in clinically normal adults, with or without SCC, to MCI. Further research is necessary to delineate the overlapping constructs of late-life depression, subsyndromal depression and affective symptoms in AD to elucidate potential interacting disease mechanisms that are currently not well understood.
Lower Memory-Semantic factor score, reflecting poorer performance on tests of episodic memory, semantic fluency and naming, also significantly predicted more rapid progression to a worse diagnosis across all groups in our study. Our findings are compatible with other studies in which lower performance on episodic or verbal memory tests predicted clinical progression from clinically normal to MCI to AD dementia.(45–47)
We did not find an association between Attention-Executive factor scores and clinical progression in our study sample in contrast to other reports in which lower performance on Trails B predicted progression from very early mild cognitive impairment (46) or mild cognitive impairment (47) to AD dementia. In ADNI analyses, Johnson et al. found that individuals with MCI experienced executive function declines whereas the clinically normal group did not, suggesting that declines in executive function such as in Trails B, may be particularly useful as a marker of MCI onset and subsequent progression.(47, 48) As only one-quarter of our sample had a baseline diagnosis of MCI, it is possible that there was insufficient power in our analysis to detect this association that may be specific to MCI but not present in earlier stages such as CN or SCC groups.
In a second longitudinal model, we analyzed baseline diagnosis as a sole predictor of progression to MCI and found that individuals in the SCC group, compared to the CN group, were four times more likely to progress to MCI over a mean follow-up period of 2.4 years. Our methods and results are similar to those of Duara et al. who reported that 28.6% of “Pre-MCI” subjects progressed to MCI or dementia over 2–3 years, comparable to 30% of SCC who progressed to MCI or dementia in our study.(4) These findings add to a growing literature supporting SCC as a late stage of preclinical AD, occurring in transition from CN to MCI and dementia. Jessen et al. found that subject-reported memory impairment with, but not without, worry or concern was associated with clinical progression in older subjects who performed normally on cognitive tests, emphasizing the relevance of subject appraisal of cognitive changes in assessing risk prior to onset of MCI.(49) Complimentary work by Gifford and colleagues showed that mutual complaint by both subject and informant had cumulative predictive value and together were associated with a 4-fold hazard of progression from SCC to MCI compared to CN adults with no SCC.(8) In utilizing CDR scores in the current study, we capitalized on both subject and informant subjective complaints, interpreted by skilled clinical raters, which may have enhanced our ability to capture these predictive features of subjective complaints.
There are a number of limitations to our study. We used the CDR, a widely implemented cognitive battery and standardized clinical criteria to classify subjects into diagnostic groups but we did not include AD biomarker data in this study. Therefore AD etiology was inferred from clinical research data. Furthermore, we defined the SCC group using specific findings on research instruments that are not routinely used in the clinic. Additional research is warranted to examine this operational definition of SCC in relation to recently defined, early MCI, and in comparison to other approaches to detecting and measuring SCC in older adults.
While we had available data from the informant-rated NPI-Q that were sufficient to establish significant findings in our analyses, this instrument may not be optimal to detect neurobehavioral symptoms in preclinical AD. By analogy to SCC, subject-rated neuropsychiatric scales may be an important and sensitive component of neuropsychiatric assessment in late preclinical stage AD. It is possible that the detection of neuropsychiatric change in the earliest stages of AD may require new, specialized instruments to probe and assess more subtle changes in perception, behavior and emotional regulation.
In conclusion, we studied a cohort of older adults comprised of CN, SCC, and MCI, and found that greater affective symptoms and lower performance on tests of memory, naming and semantic fluency predicted more rapid progression across all groups. These results identify select neuropsychiatric and neuropsychological factors associated with disease progression across preclinical and early stages of cognitive decline and support a model of clinically normal individuals with SCC as a transitional group between CN and MCI with increased risk of progression to MCI. Close examination of affective changes and subjective cognitive concerns may improve risk assessment for secondary prevention trials in AD and ultimately, for clinical screening, particularly in combination with other sensitive clinical, neuropsychological and biological markers of AD.
Supplementary Material
Acknowledgments
This study was supported by R03 AG045080, R01 AG027435, K23 AG033634, K24 AG035007, the Dupont-Warren/Livingston Fellowships, Harvard Medical School, the Massachusetts Alzheimer’s Disease Research Center (P50 AG005134) and the Harvard Aging Brain Study (P01 AGO36694). The National Alzheimer’s Coordinating Center database is funded by NIA grant U01 AG016976.
Footnotes
PREVIOUS PRESENTATION
This was presented as an abstract at the Alzheimer’s Association International Conference 2013: Donovan NJ, Zoller AS, Rudel RK, Amariglio, RE, Locascio JJ, Johnson KA, Sperling RA, Marshall GA, Rentz DM. Neuropsychiatric and cognitive factors predict progression to mild cognitive impairment and dementia. Alzheimer’s Association International Conference (AAIC) 2013, Boston, Massachusetts, USA, July 14-18, 2013 (abstract)
CONFLICTS OF INTEREST AND SOURCES OF FUNDING
No disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reisberg B, Gauthier S. Current evidence for subjective cognitive impairment (SCI) as the pre-mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer’s disease. Int Psychogeriatr. 2008;20(1):1–16. doi: 10.1017/S1041610207006412. [DOI] [PubMed] [Google Scholar]
- 3.Storandt M, Grant EA, Miller JP, et al. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology. 2006;67(3):467–73. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- 4.Duara R, Loewenstein DA, Greig MT, et al. Pre-MCI and MCI: neuropsychological, clinical, and imaging features and progression rates. Am J Geriatr Psychiatry. 2011;19(11):951–60. doi: 10.1097/JGP.0b013e3182107c69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amariglio RE, Becker JA, Carmasin J, et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50(12):2880–6. doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peter J, Scheef L, Abdulkadir A, et al. Gray matter atrophy pattern in elderly with subjective memory impairment. Alzheimers Dement. 2013 doi: 10.1016/j.jalz.2013.05.1764. [DOI] [PubMed] [Google Scholar]
- 7.van Harten AC, Visser PJ, Pijnenburg YA, et al. Cerebrospinal fluid Abeta42 is the best predictor of clinical progression in patients with subjective complaints. Alzheimers Dement. 2012 doi: 10.1016/j.jalz.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Gifford KA, Liu D, Lu Z, et al. The source of cognitive complaints predicts diagnostic conversion differentially among nondemented older adults. Alzheimers Dement. 2013 doi: 10.1016/j.jalz.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson RS, Barnes LL, Mendes de Leon CF, et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59(3):364–70. doi: 10.1212/wnl.59.3.364. [DOI] [PubMed] [Google Scholar]
- 10.Yaffe K, Blackwell T, Gore R, et al. Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry. 1999;56(5):425–30. doi: 10.1001/archpsyc.56.5.425. [DOI] [PubMed] [Google Scholar]
- 11.Lyness JM, Kim J, Tang W, et al. The clinical significance of subsyndromal depression in older primary care patients. Am J Geriatr Psychiatry. 2007;15(3):214–23. doi: 10.1097/01.JGP.0000235763.50230.83. [DOI] [PubMed] [Google Scholar]
- 12.Pomara N, Bruno D, Sarreal AS, et al. Lower CSF amyloid beta peptides and higher F2-isoprostanes in cognitively intact elderly individuals with major depressive disorder. Am J Psychiatry. 2012;169(5):523–30. doi: 10.1176/appi.ajp.2011.11081153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee GJ, Lu PH, Hua X, et al. Depressive symptoms in mild cognitive impairment predict greater atrophy in Alzheimer’s disease-related regions. Biol Psychiatry. 2012;71(9):814–21. doi: 10.1016/j.biopsych.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Potter GG, Krishnan RK, et al. Neural correlates associated with cognitive decline in late-life depression. Am J Geriatr Psychiatry. 2012;20(8):653–63. doi: 10.1097/JGP.0b013e31823e2cc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geda YE, Knopman DS, Mrazek DA, et al. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch Neurol. 2006;63(3):435–40. doi: 10.1001/archneur.63.3.435. [DOI] [PubMed] [Google Scholar]
- 16.Barnes DE, Alexopoulos GS, Lopez OL, et al. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Arch Gen Psychiatry. 2006;63(3):273–9. doi: 10.1001/archpsyc.63.3.273. [DOI] [PubMed] [Google Scholar]
- 17.Becker JT, Chang YF, Lopez OL, et al. Depressed mood is not a risk factor for incident dementia in a community-based cohort. Am J Geriatr Psychiatry. 2009;17(8):653–63. doi: 10.1097/jgp.0b013e3181aad1fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wadsworth LP, Lorius N, Donovan NJ, et al. Neuropsychiatric symptoms and global functional impairment along the Alzheimer’s continuum. Dement Geriatr Cogn Disord. 2012;34(2):96–111. doi: 10.1159/000342119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slavin MJ, Brodaty H, Kochan NA, et al. Prevalence and predictors of “subjective cognitive complaints” in the Sydney Memory and Ageing Study. Am J Geriatr Psychiatry. 2010;18(8):701–10. doi: 10.1097/jgp.0b013e3181df49fb. [DOI] [PubMed] [Google Scholar]
- 20.Mewton L, Sachdev P, Anderson T, et al. Demographic, Clinical, and Lifestyle Correlates of Subjective Memory Complaints in the Australian Population. Am J Geriatr Psychiatry. 2013 doi: 10.1016/j.jagp.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Merema MR, Speelman CP, Foster JK, et al. Neuroticism (not depressive symptoms) predicts memory complaints in some community-dwelling older adults. Am J Geriatr Psychiatry. 2013;21(8):729–36. doi: 10.1016/j.jagp.2013.01.059. [DOI] [PubMed] [Google Scholar]
- 22.Jorm AF, Christensen H, Korten AE, et al. Memory complaints as a precursor of memory impairment in older people: a longitudinal analysis over 7–8 years. Psychol Med. 2001;31(3):441–9. [PubMed] [Google Scholar]
- 23.Snitz BE, Morrow LA, Rodriguez EG, et al. Subjective memory complaints and concurrent memory performance in older patients of primary care providers. J Int Neuropsychol Soc. 2008;14(6):1004–13. doi: 10.1017/S1355617708081332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luck T, Riedel-Heller SG, Luppa M, et al. Risk factors for incident mild cognitive impairment--results from the German Study on Ageing, Cognition and Dementia in Primary Care Patients (AgeCoDe) Acta Psychiatr Scand. 2010;121(4):260–72. doi: 10.1111/j.1600-0447.2009.01481.x. [DOI] [PubMed] [Google Scholar]
- 25.Steenland K, Karnes C, Seals R, et al. Late-life depression as a risk factor for mild cognitive impairment or Alzheimer’s disease in 30 US Alzheimer’s disease centers. J Alzheimers Dis. 2012;31(2):265–75. doi: 10.3233/JAD-2012-111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Alzheimer’s Coordinating Center (NACC) http://www.alz.washington.edu.
- 27.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20(4):210–6. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 30.Ryan J, Paolo A. A screening procedure for estimating premorbid intelligence in the elderly. The Clinical Neuropsychologist. 1992;6:53–62. [Google Scholar]
- 31.Weschler D. WMS-R Weschler Memory Scale Revised Manual. New York: Ths Psychological Corporation, Harcourt Brace Jovanovich, Inc; 1987. [Google Scholar]
- 32.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–65. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 33.Goodglass HKE. The Assessment of Aphasia and Related Disorders. Philadelphia: Lee & Ferbiger; 1983. [Google Scholar]
- 34.Wechsler DSC. Manual: Wechsler Memory Scale. New York: Psychological Corporation; 1973. [Google Scholar]
- 35.Reitan RM. Validity of the trail making test as a indicator of organic brain damage: Percept Mot Skills. 1958. [Google Scholar]
- 36.Weschler D. WAIS-R manual. New York: The Psychological Corporation; 1981. [Google Scholar]
- 37.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233–9. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 38.Palmer K, Berger AK, Monastero R, et al. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology. 2007;68(19):1596–602. doi: 10.1212/01.wnl.0000260968.92345.3f. [DOI] [PubMed] [Google Scholar]
- 39.Palmer K, Di Iulio F, Varsi AE, et al. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: the role of depression and apathy. J Alzheimers Dis. 2010;20(1):175–83. doi: 10.3233/JAD-2010-1352. [DOI] [PubMed] [Google Scholar]
- 40.Rosenberg PB, Mielke MM, Appleby BS, et al. The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am J Geriatr Psychiatry. 2013;21(7):685–95. doi: 10.1016/j.jagp.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheline YI, Pieper CF, Barch DM, et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67(3):277–85. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheline YI, Wang PW, Gado MH, et al. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93(9):3908–13. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sturm VE, Yokoyama JS, Seeley WW, et al. Heightened emotional contagion in mild cognitive impairment and Alzheimer’s disease is associated with temporal lobe degeneration. Proc Natl Acad Sci U S A. 2013;110(24):9944–9. doi: 10.1073/pnas.1301119110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323–31. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blacker D, Lee H, Muzikansky A, et al. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Arch Neurol. 2007;64(6):862–71. doi: 10.1001/archneur.64.6.862. [DOI] [PubMed] [Google Scholar]
- 46.Dickerson BC, Sperling RA, Hyman BT, et al. Clinical prediction of Alzheimer disease dementia across the spectrum of mild cognitive impairment. Arch Gen Psychiatry. 2007;64(12):1443–50. doi: 10.1001/archpsyc.64.12.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomar JJ, Bobes-Bascaran MT, Conejero-Goldberg C, et al. Utility of combinations of biomarkers, cognitive markers, and risk factors to predict conversion from mild cognitive impairment to Alzheimer disease in patients in the Alzheimer’s disease neuroimaging initiative. Arch Gen Psychiatry. 2011;68(9):961–9. doi: 10.1001/archgenpsychiatry.2011.96. [DOI] [PubMed] [Google Scholar]
- 48.Johnson JK, Gross AL, Pa J, et al. Longitudinal change in neuropsychological performance using latent growth models: a study of mild cognitive impairment. Brain Imaging Behav. 2012;6(4):540–50. doi: 10.1007/s11682-012-9161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jessen F, Wolfsgruber S, Wiese B, et al. AD dementia risk in late MCI, in early MCI, and in subjective memory impairment. Alzheimers Dement. 2013 doi: 10.1016/j.jalz.2012.09.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.