Abstract
Introduction
Disruptions in calcium (Ca) homeostasis during exercise may influence skeletal adaptations to exercise training. In young men, vigorous cycling causes increases in parathyroid hormone (PTH) and bone resorption (C-terminal telopeptides of type I collagen; CTX); responses are attenuated by Ca supplementation. Study aims were to determine whether vigorous walking causes similar increases in PTH and CTX in older women and how the timing of Ca supplementation before and during exercise influences these responses.
Methods
In Experiment 1 (EXP1), 10 women (61±4 y) consumed 125 mL of either a Ca-fortified (1 g/L) or control beverage every 15 minutes during exercise starting 60 minutes before and continuing during 60 minutes of exercise. In EXP2, 23 women (61±4 y) consumed 200 mL of a Ca-fortified (1 g/L) or control beverage every 15 minutes starting 15 minutes before and continuing during 60 minutes of exercise. The exercise was treadmill walking at 75–80% VO2 peak.
Results
In EXP1, serum ionized Ca (iCa) decreased in the control condition (p<0.001), but not with Ca supplementation. PTH increased after exercise on both days (Ca, p=0.05; control, p=0.009), but was attenuated by Ca supplementation (8.3 vs. 26.1 pg/mL; p=0.03). CTX increased only on the control day (p=0.02). In EXP2, serum iCa decreased on Ca and control days (Ca and control, p<0.001), but less so on the Ca day (p=0.04). PTH (Ca and control, p<0.001) and CTX (Ca, p=0.02; control p=0.007) increased on the Ca and control day and there were no differences in the changes.
Conclusion
The timing of Ca supplementation may be a key mediator of Ca homeostasis during acute exercise. Further research is necessary to determine how this influences skeletal adaptations to training.
Keywords: Calcium supplementation, bone metabolism, postmenopausal, parathyroid hormone, exercise
INTRODUCTION
Exercise is generally accepted as having favorable effects on bone health and is recommended for both prevention and treatment of low bone mineral density (BMD)(15). However, endurance athletes, including cyclists and runners, have sometimes been observed to have low BMD (8,14,16,19, 21). Young male road cyclists studied over a year of training and competition were found to have a significant decline in BMD at the hip and lumbar spine. The decline of 1% to 2% per year was similar to the accelerated bone loss that occurs in early postmenopausal women (2). Collegiate male basketball players have also been observed to lose 6% of total bone mineral content (BMC) over a year of training and competition (11). These findings support the concept that, under certain circumstances, vigorous exercise training may cause bone mineral loss.
The mechanisms by which exercise could lead to a loss of BMD are not known. One possibility is that excessive amounts of calcium (Ca) are lost during exercise through dermal and other sources. If serum ionized Ca (iCa) level declines during exercise, this would be expected to trigger an increase parathyroid hormone (PTH), which defends serum Ca levels by enhancing Ca absorption, reducing renal excretion, and mobilizing skeletal Ca (i.e., increasing bone resorption) (4). Indeed, such disruptions in Ca homeostasis have been observed in young men during prolonged or vigorous cycling bouts (1, 3, 6, 14, 21). Further, the ingestion of Ca before and during exercise in these studies attenuated the decline in iCa and increase in PTH (3,6).
Because most of the studies of the disruption in Ca homeostasis during exercise have been done in young male cyclists, it is unknown whether these disruptions in Ca homeostasis are unique to young men, to athletes, or to weight-supported exercise. Specifically, it is not known whether similar disruptions occur during weight-bearing exercise or in older sedentary adults (e.g., postmenopausal women at risk for bone loss). In this context, the aims of the study were to determine whether vigorous walking causes increases in PTH and bone resorption (C-terminal telopeptides of type I collagen; CTX) in older women and how the responses are influenced by the timing of Ca supplementation before and during exercise.
MATERIALS AND METHODS
Study participants
Participants were healthy postmenopausal women (Experiment 1 (EXP 1), n=10; EXP 2, n=23), aged 50 to 75 y. Health status was evaluated by medical history, physical examination, blood chemistries (complete blood count, comprehensive metabolic panel, thyroid stimulating hormone, intact PTH, 25-hydroxy(OH) vitamin D), a spot urine Ca-to-creatinine ratio to estimate 24-hour urinary calcium losses, and a graded exercise stress test. Exclusion criteria included: abnormal thyroid, kidney, or liver function; PTH ≥69 pg/mL; 25(OH) vitamin D ≤20 ng/mL; urine Ca-to-creatinine ratio >0.31; use of teriparatide, calcitonin, oral steroids, sex hormones, selective estrogen receptor modulators (SERMs), bisphosphonates or other drugs known to affect calcium metabolism; evidence of myocardial ischemia, serious arrhythmias, or aortic stenosis during vigorous exercise; and resting blood pressure >150/90 mmHg. The Colorado Multiple Institutional Review Board approved the experiments. All volunteers provided written informed consent to participate.
Study design
EXP 1 and 2 were both randomized, double-blinded crossover trials that each involved two 60-minute bouts of treadmill walking under conditions of control or Ca supplementation. Study beverages included a Ca-free sports beverage (control; Marigot Ltd, Cork, Ireland) and a Ca-fortified sports beverage containing 1000 mg of Ca per liter (Aquamin Soluble, Lithothamnion species, marine-derived multi-mineral complex containing calcium carbonate; Marigot Ltd, Cork, Ireland). The control and Ca-fortified beverages were otherwise matched for pH, electrolytes, carbohydrate and taste.
If Ca supplementation before and during exercise attenuates the exercise-induced increases in serum PTH and CTX, as has been observed in young male cyclists, it is possible that such a supplementation strategy could enhance skeletal adaptations to exercise training. However, before experiments can be designed to address that hypothesis, it is important to evaluate timing strategies for Ca supplementation. Accordingly, EXP 1 and 2 used different timing strategies for the ingestion of the Ca-fortified or control drinks relative to exercise. In EXP 1, participants consumed 125 mL of either the Ca-fortified beverage (1 g/L) or the placebo beverage (control) every 15 minutes starting 60 minutes before and continuing during exercise (1 L total). This supplementation approach was selected because it was similar to the approach used in previous studies of young male cyclists. (3, 6) Thus, the results of EXP 1 can be evaluated relative to those observed previously. Because initiating Ca supplementation 1 hour before exercise may not be practical, EXP 2 delivered the same Ca load (1000 mg) that has been found to be effective in attenuating the PTH response to exercise, (3,6) but initiated the administration 15 minutes rather than 60 minutes before exercise. Participants in EXP 2 consumed 200 mL of either a Ca-fortified (1 g/L) or control beverage every 15 minutes starting 15 minutes before and continuing during exercise (1 L total). Participants were allowed to consume only the study beverage during each exercise bout. The order of the tests within each experiment was randomized and counterbalanced. The time interval between exercise bouts was 3 to 10 days.
Aerobic Power (VO2 peak)
Peak aerobic power was measured at baseline using an individualized treadmill protocol with open-circuit spirometry (ParvoMedics; Sandy, UT). Subjects warmed up to determine the level walking speed that elicited a heart rate of ~70% of age-predicted maximal heart rate. During the test, this speed was maintained and treadmill grade was increased by 2% every 2 minutes. Heart rate was monitored continuously using a 12-lead electrocardiogram (Quinton Q4500; Quinton Instruments, Seattle, WA) and blood pressure was measured during each exercise stage. VO2 peak was the average of the two highest consecutive 30-second VO2 measurements.
Dual-energy x-ray absorptiometry (DXA)
Participants had DXA scans performed at baseline using Hologic Delphi-W instrument (Hologic Delphi-W instrument, software v11.2, Waltham, MA). Total body, lumbar spine (L2–L4), and proximal femur (total hip, femoral neck, trochanter, subtrochanteric region) scans were measured by DXA.
60-minute exercise sessions
Participants performed two 60-minute bouts of treadmill walking at a workload corresponding to 75–80% of VO2 peak. Treadmill speed and grade were the same on both test days. To control for diurnal variability in PTH, both tests were conducted at approximately the same time of day for each participant. Dietary Ca and vitamin D intake was controlled by instructing participants to consume the same self-determined meals on the day before both exercise bouts. Subjects were provided with a food diary and asked to record all food intake prior to their visit. This diary was collected to ensure that the same meals were consumed prior to exercise. Participants were asked not to take Ca and/or vitamin D supplements the day of an exercise bout. Sweat loss was estimated during the exercise bouts as previously described (1,22). Nude, dry body weight was measured before and after exercise. Change in weight adjusted for fluid loss and intake was used to estimate sweat volume.
Blood Sampling and Analysis
In EXP 1, serum blood samples were collected 60 minutes before exercise (−60 minutes; before the first dosing with the study beverage), immediately before exercise (0 minutes), and immediately (60 minutes) and 30 minutes after (90 minutes) exercise for the measurement of serum iCa, hematocrit, PTH, and CTX. In EXP 2, serum blood samples were collected immediately before exercise (0 minutes) and immediately (60 minutes) and 30 minutes after (90 minutes) exercise for the measurement of iCa, hematocrit, and PTH; CTX was measured only at 0 and 90 minutes. For both experiments, serum iCa and hematocrit were measured in real-time (i.e., within 5 minutes of blood sample collection) using a cartridge-based point-of-care whole blood analyzer (iSTAT, Abbott, East Windsor, NJ); the reported CVs are 1.1% for iCa and 1.5% for hematocrit. Hematocrit was measured to assess shifts in plasma volume in response to exercise, as previously described (1,3). For PTH and CTX, serum samples were stored at −80°C for subsequent batch analysis. CTX (Nordic Bioscience Diagnostics, Herlev, Denmark) was measured by ELISA. Intra- and inter-assay coefficients of variation (CV) were 2.7–10.3% and 2.5–9.2%, respectively, for CTX. Intact PTH was measured by a two-site chemiluminescent enzyme-labeled immunometric assay on an Immulite 1000 analyzer (Siemens, Tarrytown, NY); intra- and inter-assay CVs were 2.9–3.5% and 4.8–6.8%, respectively.
Statistical Methods
The primary outcome was the change in PTH from immediately before to immediately after exercise; changes in iCa and CTX were secondary outcomes. Because these experiments were considered pilot studies to inform future research on whether Ca supplementation before exercise influences skeletal adaptations to exercise training, the primary tests of interest were the within-condition changes in the outcomes. However, we also evaluated whether the changes in outcomes from before to after exercise were different under control versus Ca conditions. Both types of comparisons were based on a paired t test. Because changes in CTX in response to acute exercise have been found to peak 30 minutes after exercise (6), the change in CTX was evaluated from before to 30 minutes after exercise. To avoid the probability of Type I and II errors for the comparisons of secondary outcomes, we relied on the consistency across measures rather than adjusting for multiple comparisons. Unless otherwise stated, data are reported as mean ± SD or mean with 95% confidence interval (CI). All analyses were performed using SAS 9.2 (SAS Institute Inc., Cary NC).
RESULTS
EXP 1
Participants were aged 61 ± 4 years, with BMI 27.2 ± 4.4 kg/m2, VO2 peak 24.5 ± 3.6 mL/min/kg, lumbar spine t-score −0.62 ± 1.50, total hip t-score −0.75 ± 1.07 and 25OH vitamin D level 36.6 ± 14.7 ng/mL.
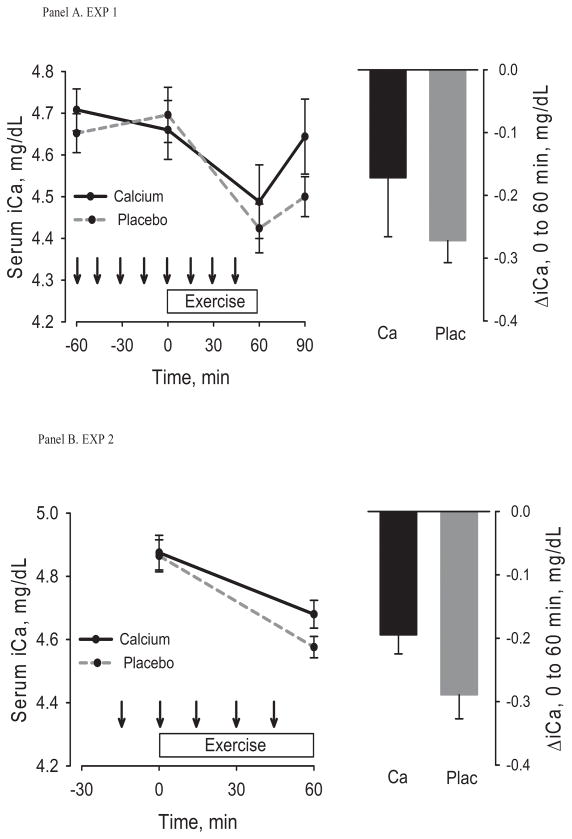
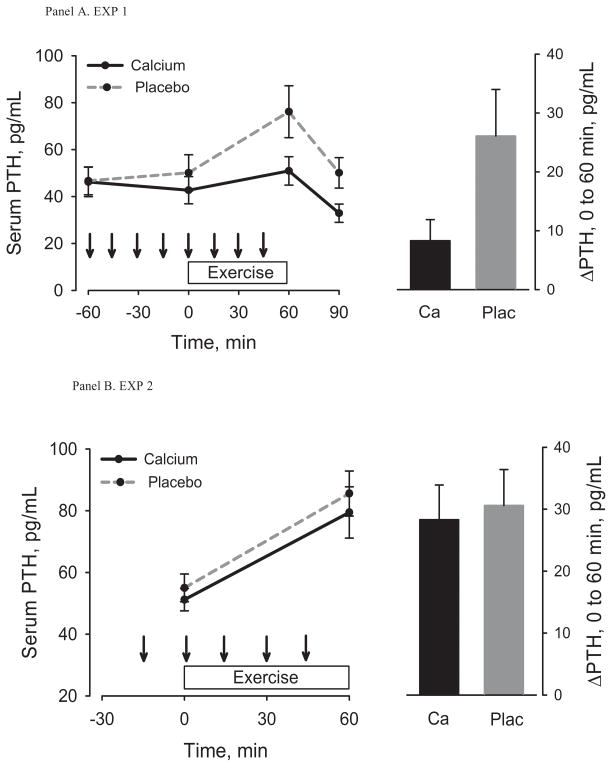
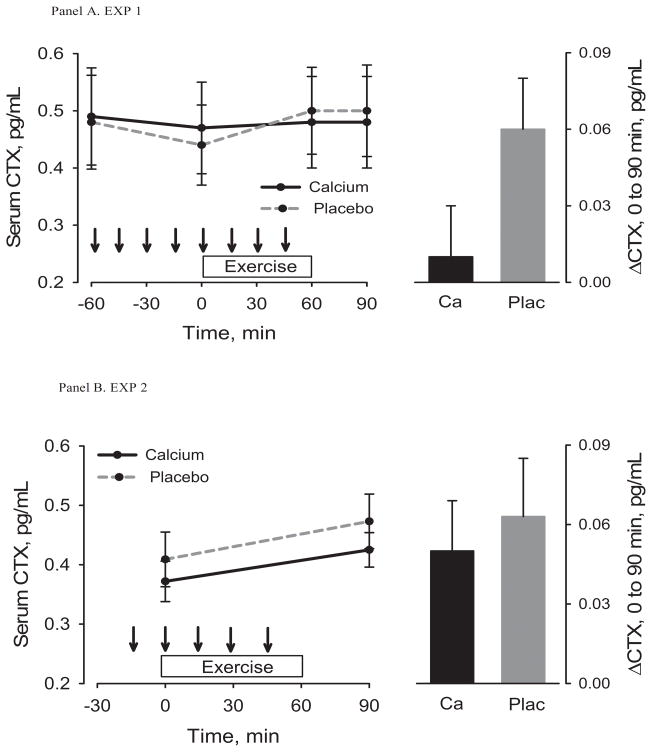
The effects of pre-exercise Ca dosing on serum iCa, PTH and CTX from −60 minutes to immediately before exercise were included in the Figures (1a, 2a, 3a) for descriptive purposes only. Statistical analyses focused on changes from immediately before to after exercise. Serum iCa decreased from immediately before to immediately after exercise on the control day (p<0.001) but not on the Ca day (p=0.10) (Table 1, Figure 1a); the change was not statistically different between conditions (p=0.37). Exercise caused an increase in PTH under both conditions (Ca, p=0.05; control, p=0.009), but it was attenuated by Ca (8.3 (0.0,16.5) vs. 26.1 (8.2, 44.0) pg/mL, p=0.03) (Table 1, Figure 2a). CTX increased (p=0.02) 30 minutes after exercise on the control day only (Table 1, Figure 3a); the increase was smaller on the control day, but not statistically different from the Ca day (p=0.08). There were no significant changes in hematocrit (Table 1) in response to exercise and no significant differences in the changes between the two test conditions. Therefore, the post-exercise parameters were not adjusted for shifts in plasma volume. The estimated sweat loss was 0.51 ± 0.15 kg and 0.55 ± 0.21 kg (p=0.23) for the control and Ca conditions.
Figure 1.
Serum ionized calcium (iCa) before, during, and after exercise (left) under control and Ca supplementation conditions and the change from immediately before to immediately after exercise (right). Arrows indicate consumption of placebo or Ca-enriched beverage. The top panel is for Experiment 1 and the bottom panel for Experiment 2. Within-group change, * p < 0.05, ** p< 0.001; Between-group difference, ǂ p <0.05.
Figure 2.
Serum PTH before, during, and after exercise (left) under control and Ca supplementation conditions and the change from immediately before to immediately after exercise (right). Arrows indicate consumption of placebo or Ca-enriched beverage. The top panel is for Experiment 1 and the bottom panel is for Experiment 2. Within-group change, * p < 0.05, ** p< 0.001; Between-group difference, ǂ p <0.05.
Figure 3.
Serum CTX before, during, and after exercise (left) under control and Ca supplementation conditions and the change from immediately before to 30 minutes after exercise (right). Arrows indicate consumption of placebo or Ca-enriched beverage. The top panel is for Experiment 1 and the bottom panel is for Experiment 2.
Within-group change, * p < 0.05, ** p< 0.001; Between-group difference, ǂ p <0.05.
Table 1.
Metabolic responses before and after 60 minutes of exercise under placebo and calcium (Ca) supplementation conditions in experiment 1 (n=10). Results are mean±SD or mean change (95% CI).
| Condition | Before | After* | Change (95% CI) | p value | |
|---|---|---|---|---|---|
| iCa, mg/dL | Calcium | 4.66 ± 0.22 | 4.49 ± 0.28 | −0.17 (−0.38, 0.04) | 0.10 |
| Control | 4.70 ± 0.21 | 4.42 ± 0.18 | −0.27 (−0.35, −0.19) | <0.001 | |
| Ca vs. Control | −0.10 (−0.34, 0.14) | 0.37 | |||
| PTH, pg/mL | Calcium | 42.7 ± 18.2 | 50.9 ± 19.1 | 8.3 (0.0,16.5) | 0.05 |
| Control | 50.1 ± 24.4 | 76.1 ± 35.0 | 26.1 (8.1, 44.0) | 0.009 | |
| Ca vs. Control | 17.8 (2.3, 33.3) | 0.03 | |||
| CTX, ng/mL | Calcium | 0.47 ± 0.24 | 0.48 ± 0.26 | 0.01 (−0.04, 0.05) | 0.73 |
| Control | 0.44 ± 0.22 | 0.50 ± 0.24 | 0.06 (0.01, 0.11) | 0.02 | |
| Ca vs. Control | 0.05 (−0.01, 0.12) | 0.08 | |||
| Hct, % | Calcium | 40.9 ± 3.2 | 41.4 ± 3.5 | 0.5 (−1.2, 2.2) | 0.53 |
| Control | 40.8 ± 2.3 | 41.6 ± 2.7 | 0.8 (−0.2, 1.8) | 0.10 | |
| Ca vs. Control | 0.3 (−1.3, 1.9) | 0.69 |
iCa, ionized calcium; PTH, parathyroid hormone; Hct; hematocrit; CTX, C-terminal telopeptides of type I collagen
samples were obtained immediately after exercise for iCa, PTH, and HCT and 30 minutes after exercise for CTX
EXP 2
Participants were aged 61 ± 4 years, with BMI 26.5 ± 4.5 kg/m2, VO2peak 24.0 ± 4.4 mL/min/kg, lumbar spine t-score −0.67 ± 1.23, total hip t-score −0.42 ± 1.13 and 25OH vitamin D level 34.0 ± 9.3 ng/mL.
Serum iCa decreased from immediately before to after exercise (Ca, p<0.001; control, p<0.001) (Table 2, Figure 1b) and PTH (Ca, p<0.001; control p<0.001) and CTX (Ca, p=0.02; control, p=0.007) increased in both the Ca and control conditions (Table 2, Figure 2b and 3b). The decrease in iCa on the Ca day was attenuated when compared with the decrease on the control day (p=0.04). The changes in PTH (p=0.16) and CTX (p=0.37) were not statistically different between conditions (Table 2). There were no significant changes in hematocrit (Table 2) in response to exercise and no differences in the change between the two test conditions. Therefore, the post-exercise parameters were not adjusted for shifts in plasma volume. The estimated sweat loss was 0.58 ± 0.23 kg and 0.52 ± 0.22 kg (p=0.30) for the control and Ca conditions.
Table 2.
Metabolic responses before and after exercise under control and Ca supplementation conditions in experiment 2 (n = 23). Results are mean±SD or mean change (95% CI).
| Condition | Before | After* | Change (95% CI) | p value | |
|---|---|---|---|---|---|
| iCa, mg/dL | Calcium | 4.87 ± 0.26 | 4.68 ± 0.21 | −0.19 (−0.26, −0.13) | <.001 |
| Control | 4.87 ± 0.23 | 4.58 ± 0.16 | −0.29 (−0.36, −0.21) | <.001 | |
| Ca vs. Control | −0.09 (−0.18, −0.01) | 0.04 | |||
| PTH, pg/mL | Calcium | 51.2 ± 17.1 | 79.4 ± 38.9 | 28.3 (14.9, 41.6) | <.001 |
| Control | 55.0 ± 21.2 | 85.6 ± 34.5 | 30.58 (18.5, 42.6) | <.001 | |
| Ca vs. Control | 6.0 (−2.6, 14.5) | 0.16 | |||
| CTX, ng/mL | Calcium | 0.37 ± 0.16 | 0.42 ± 0.14 | 0.05 (0.01, 0.10) | 0.02 |
| Control | 0.41 ± 0.22 | 0.47 ± 0.21 | 0.06 (0.02, 0.11) | 0.007 | |
| Ca vs. Control | 0.02 (−0.03, 0.07) | 0.37 | |||
| Hct, % | Calcium | 40.73 ± 6.39 | 42.73 ± 2.03 | 0.50 (−1.23, 2.23) | 0.53 |
| Control | 41.74 ± 3.12 | 42.22 ± 2.66 | 0.80 (−0.20, 1.80) | 0.10 | |
| Ca vs. Control | 0.30 (−1.35, 1.95) | 0.69 |
iCa, ionized calcium; PTH, parathyroid hormone; Hct; hematocrit; CTX, C-terminal telopeptides of type I collagen
samples were obtained immediately after exercise for iCa, PTH, and HCT and 30 minutes after exercise for CTX
DISCUSSION
The aims of the study were to determine whether 1 hour of vigorous walking disrupts Ca homeostasis in older women and whether disruptions are mitigated by the timing Ca supplementation before and during exercise. In EXP 1, the major findings were that vigorous walking on the control day caused a decrease in serum iCa and an increase in PTH and CTX. All of these responses were attenuated (albeit not all significantly) when Ca ingestion started an hour before and continued during exercise. When Ca ingestion was initiated only 15 minutes before the start of exercise (EXP 2), the decrease in serum iCa was attenuated but the increases in PTH and CTX were not. This suggests that the timing of Ca supplementation prior to exercise plays an important role in regulating changes in Ca homeostasis during exercise. These results in postmenopausal women during vigorous treadmill walking are consistent with those observed previously in young male road cyclists during prolonged or intensive cycling (1,3).
Baseline PTH values for participants in both EXP 1 and EXP 2 were normal and similar to those of young male cyclists (2,3). Previous studies of young male cyclists in our laboratory found increases in PTH of 32.9 ± 24.6 pg/mL in response to 2 hours of moderate-intensity cycling and 74.0 ± 14.2 pg/mL in response to 1 hour of high-intensity cycling (1,3). In those studies, the magnitude of increase in PTH appeared to be related to crude estimates of the magnitude of dermal Ca loss (1,3). This supports the notion that acute exercise can cause a decrease in serum Ca, perhaps as a result of dermal Ca loss through sweating, that requires activation of counter-regulatory mechanisms to maintain serum Ca (i.e., PTH). Sweat Ca concentration was not measured in the current study. However, sweat volume was considerably lower in postmenopausal women performing relatively vigorous walking (~0.5 L/h) than in young men performing moderate- (~1 L/h) or high-intensity (~1.5 L/h) cycling. (1,3). Despite the lower sweat volume, women in EXP 1 and EXP 2 still had increases in PTH during exercise on the control days that were similar in magnitude (~28 pg/mL) to increases in young male cyclists during moderate-intensity exercise. Sweat production is affected not only by exercise intensity, ambient conditions, and hydration status (17), but also by age. With advancing age there is lower sweat gland output and alterations in the sweat gland itself (10). It is not known whether aging affects sweat Ca concentration.
It seems plausible that the attenuation of the PTH response to exercise by supplemental Ca in EXP 1 was mediated by the better preservation of serum iCa levels on the Ca day compared to the control day. However, the changes in serum iCa in both experiments must be interpreted cautiously because the sampling of blood before and after exercise may not accurately reflect the dynamic relation between serum iCa and PTH during exercise. As an example of the responsiveness of PTH to change in iCa, raising serum iCa by only ~0.1 mmol/L over 10 minutes via intravenous infusion resulted in a decrease in PTH of ~80% over the same time interval (9). Similarly, decreasing serum iCa by only 8% over 30 minutes via infusion of disodium ethylenediaminetetraacetic acid generated a 300% increase in PTH (26). Previous studies have been equivocal regarding whether exercise causes a decline in serum iCa or total Ca (6,12,13,23,24). However, measurements were typically performed before and after exercise. Isolating the change in serum iCa as the primary determinant of the PTH response to exercise will require either more frequent measurements of iCa and PTH during exercise or experiments that control serum Ca levels during exercise (e.g., via intravenous Ca infusion).
Increases in PTH and CTX immediately after exercise have been demonstrated in studies of intense exercise lasting approximately one hour (6,13). A study that measured these parameters during recovery after exercise found that CTX peaked 1 hour after exercise and was still elevated at 2 hours of recovery (6). In that study, Ca-enriched water ingested before and during exercise diminished the exercise-induced increase in CTX that occurred under a control condition (6). The finding that Ca supplementation during exercise attenuated increases in CTX suggests that the disruption in Ca homeostasis can be minimized when Ca is available to be absorbed from the gut (6). Our study revealed a significant increase in CTX during the control condition in EXP 1. Although not significant in the current study, the increase in CTX was attenuated in the Ca-supplemented condition. It is possible that the differences in the change in CTX between conditions would have been more apparent if measurements had been obtained more than 1 hour after exercise.
It is difficult to judge how increases in PTH and bone resorption in response to acute exercise may influence the BMD adaptations to exercise. Recombinant PTH is one of the most effective therapies for osteoporosis and the only pharmacologic therapy that acts by stimulating bone formation rather than suppressing bone resorption. This seems paradoxical, given that clinical conditions of excess PTH, such as primary or secondary hyperparathyroidism, causes bone loss (18). However, whereas chronic elevation of PTH (i.e., hyperparathyroidism) leads to bone loss, transient increases in PTH are anabolic for bone (7,20). It is currently unknown if acute increases in endogenous PTH in response to exercise influence bone metabolism in a similar manner as exogenous teriparatide and lead to favorable bone remodeling. Although both exercise and pharmacologic PTH administration generate transient increases in PTH, the former appears to be a response to declining serum iCa whereas the latter is not (26). Accordingly, the bone formation and resorption responses to PTH under these two conditions may be different. In support of this concept, the acute increase in CTX in the current study under conditions of elevated PTH does not appear to occur with pharmacologic PTH therapy (25). Daily administration of exogenous teriparatide over 12 months resulted in an early and sustained increase in bone formation but bone resorption did not increase until after 1 month of treatment (25). Further research will be needed to understand whether repeated exercise-induced increases in PTH, and possibly CTX, have favorable or unfavorable effects on skeletal adaptations to exercise.
If disruptions in Ca homeostasis offset the potential benefits of exercise on bone metabolism, this suggests that the timing of Ca supplementation with exercise may enhance the skeletal benefits of exercise training. The optimal dose and timing of Ca ingestion relative to exercise to mitigate the PTH response are not known. However, our study suggests that 1000 mg of liquid Ca supplementation started 60 minutes before exercise attenuates the decrease in serum iCa and increases in PTH and CTX. Similar effects were not observed when the same Ca dose was started 15 minutes before exercise. Other studies that demonstrated an attenuation of PTH during exercise used a total Ca dose of 1000 mg started 60 minutes before exercise (3,6). Further studies will be needed to better understand the kinetics of calcium flux during exercise.
In summary, this proof-of-concept study demonstrated that the effect of vigorous or prolonged exercise to trigger increases in PTH and CTX, which had previously been observed only in young male cyclists, also occurs in postmenopausal women during brisk walking. The findings in the current study and by others (1,36) that Ca supplementation started 1 hour before and continued during exercise attenuates the increases in PTH and CTX strongly suggests that it is the loss of Ca during exercise that is the mechanistic trigger for the increase in PTH. The long-term effects on BMD of repeated disruptions in Ca homeostasis during exercise training are not currently known.
Acknowledgments
Grant Support: This research was supported by the NIH research grants R01 AG108857, NCATS Colorado CTSI UL1 TR000154, NIDDK P30 DK048520, Hartford/Jahnigen Center of Excellence in Geriatrics Award and Marigot LTD (Cork, Ireland).
We are grateful to the nursing, core laboratory, information systems, and administrative staffs of the Clinical and Translational Research Center and Energy Balance Core of the Nutrition and Obesity Research Center for their support of the study. We also acknowledge the members of our research group who carried out the day-to-day activities for the project. Finally, we thank the women who volunteered to participate in the study for their time and efforts. This research was supported by the NIH research grants R01 AG108857, UL1 TR000154, P30 DK048520, Hartford/Jahnigen Center of Excellence in Geriatrics Award and Marigot LTD (Cork, Ireland). The results of the present study do not constitute endorsement by ACSM.
Footnotes
AUTHOR DISCLOSURE: All authors state that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Study design: DB, KS, PW, WK. Study conduct and data collection: DB, KH, KS. Data analysis: PW. Data interpretation: KS, PW, WK. Drafting manuscript: KS. Revising manuscript content: KS, VS, PW, KH, DB, WK. Approving final version of manuscript: KS, DB, VS, KH, PW, WK.
References
- 1.Barry DW, Kohrt WM. Acute effects of 2 hours of moderate-intensity cycling on serum parathyroid hormone and calcium. Calcif Tissue Int. 2007;80:359–365. doi: 10.1007/s00223-007-9028-y. [DOI] [PubMed] [Google Scholar]
- 2.Barry DW, Kohrt WM. BMD decreases over the course of a year in competitive male cyclists. J Bone Miner Res. 2008;23(4):484–91. doi: 10.1359/jbmr.071203. [DOI] [PubMed] [Google Scholar]
- 3.Barry DW, Hansen KC, Van Pelt RE, Witten M, Wolfe P, Kohrt WM. Acute calcium ingestion attenuates exercise-induced disruption of calcium homeostasis. MedSciSports Exerc. 2011;43(4):617–23. doi: 10.1249/MSS.0b013e3181f79fa8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouassida A, Zalleg D, Zaouali AM, Gharbi N, Duclos M, Richalet JP, Tabka Z. Parathyroid hormone concentrations during and after two periods of high intensity exercise with and without an intervening recovery period. Eur J Appl Physiol. 2003;88:339–344. doi: 10.1007/s00421-002-0721-2. [DOI] [PubMed] [Google Scholar]
- 5.Brown EM. Calcium receptor and regulation of parathyroid hormone secretion. Rev Endocrinol Metab Disord. 2000;1:307–315. doi: 10.1023/a:1026570518919. [DOI] [PubMed] [Google Scholar]
- 6.Guillemant J, Accarie C, Peres G, Guillemant S. Acute effects of an oral calcium load on markers of bone metabolism during endurance cycling exercise in male athletes. Calcif Tissue Int. 2004;74(5):407–14. doi: 10.1007/s00223-003-0070-0. [DOI] [PubMed] [Google Scholar]
- 7.Harada S, Rodan G. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349–355. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- 8.Hind K, Truscott JG, Evans JA. Low lumbar spine bone mineral density in both male and female endurance runners. Bone. 2006;39:880–885. doi: 10.1016/j.bone.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Kamycheva E, Jorde R, Haug E, Sager G, Sundsfjord J. Effects of acute hypercalcaemia on blood pressure in subjects with and without parathyroid hormone secretion. Acta Physiol Scand. 2005;184:113–119. doi: 10.1111/j.1365-201X.2005.01436.x. [DOI] [PubMed] [Google Scholar]
- 10.Kenney W, Fowler S. Methylcholine-activated eccrine sweat gland density and output as a function of age. J Appl Physiol. 1988;65:1082–1086. doi: 10.1152/jappl.1988.65.3.1082. [DOI] [PubMed] [Google Scholar]
- 11.Klesges RC, Ward KD, Shelton ML, Applegate WB, Cantler ED, Palmieri GM, Harmon K, Davis J. Changes in bone mineral content in male athletes. Mechanisms of action and intervention effects. JAMA. 1996;276(3):226–30. Epub 1996/07/17. [PubMed] [Google Scholar]
- 12.Maimoun L, Simar D, Malatesta D, Caillaud C, Peruchon E, Couret I, Rossi M, Mariano-Goulart D. Response of bone metabolism related hormones to a single session of strenuous exercise in active elderly subjects. Br J Sports Med. 2005;39:497–502. doi: 10.1136/bjsm.2004.013151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maimoun L, Manetta J, Couret I, Dupuy AM, Mariano-Goulart D, Rossi M. The intensity level of physical exercise and the bone metabolism response. Int J Sports Med. 2006;27:105–111. doi: 10.1055/s-2005-837621. [DOI] [PubMed] [Google Scholar]
- 14.Mussolino ME, Looker AC, Orwoll ES. Jogging and bone mineral density in men: results from NHANES III. Am J Public Health. 2001;91:1056–1059. doi: 10.2105/ajph.91.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Osteoporosis Foundation. Physician’s guide to prevention and treatment of osteoporosis. Nov 24, 2007. [Google Scholar]
- 16.Nichols JF, Palmer JE, Levy SS. Low bone mineral density in highly trained male master cyclists. Osteoporosis Int. 2003;14:644–649. doi: 10.1007/s00198-003-1418-z. [DOI] [PubMed] [Google Scholar]
- 17.Patterson M, Galloway S, Nimmo M. Variations in regional sweat composition in normal human males. Experimental Physiology. 2000;85.6:869–875. doi: 10.1111/j.1469-445x.2000.02058.x. [DOI] [PubMed] [Google Scholar]
- 18.Potts J, Gardella T. Progress, Paradox and Potential: Parathyroid Hormone Research over Five Decades. Ann NY Acad Sci. 2007;1117:196–208. doi: 10.1196/annals.1402.088. [DOI] [PubMed] [Google Scholar]
- 19.Rico H, Revilla M, Villa LF, Gómez-Castresana F, Alvarez del Buergo M. Body composition in postpubertal boy cyclists. J Sports Med Phys Fitness. 1993;33:278–281. [PubMed] [Google Scholar]
- 20.Rodan G, Martin T. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 21.Sabo D, Bernd L, Pfeil J, Rieiter A. Bone quaility in the lumbar spine in high-performance athletes. Eur Spine J. 1996;5:258–263. doi: 10.1007/BF00301329. [DOI] [PubMed] [Google Scholar]
- 22.Shirreffs SM, Maughan RJ. Whole body sweat collection in humans: an improved method with preliminary data on electrolyte content. J Appl Physiol. 1997;82:336–341. doi: 10.1152/jappl.1997.82.1.336. [DOI] [PubMed] [Google Scholar]
- 23.Takada H, Washino K, Nagashima M, Iwata H. Response of parathyroid hormone to anaerobic exercise in adolescent female athletes. Acta Paediatr Jpn. 1998;40:73–77. doi: 10.1111/j.1442-200x.1998.tb01407.x. [DOI] [PubMed] [Google Scholar]
- 24.Thorsen K, Kristoffersson A, Hultdin J, Lorentzon R. Effects of moderate endurance exercise on calcium, parathyroid hormone, and markers of bone metabolism in young women. Calcif Tissue Int. 1997;60:16–20. doi: 10.1007/s002239900179. [DOI] [PubMed] [Google Scholar]
- 25.Tsujimoto M, Chen P, Miyauchi A, Sowa H, Krege J. PINP as an aid for monitoring patients treated with teriparatide. Bone. 2011;48:798–803. doi: 10.1016/j.bone.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Zikan V, Stepan JJ. Marker of Bone Resorption in Acute Response to Exogenous or Endogenous Parathyroid Hormone. Biomarker Insights. 2008;3:19–24. [PMC free article] [PubMed] [Google Scholar]