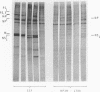
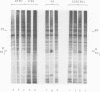
Abstract
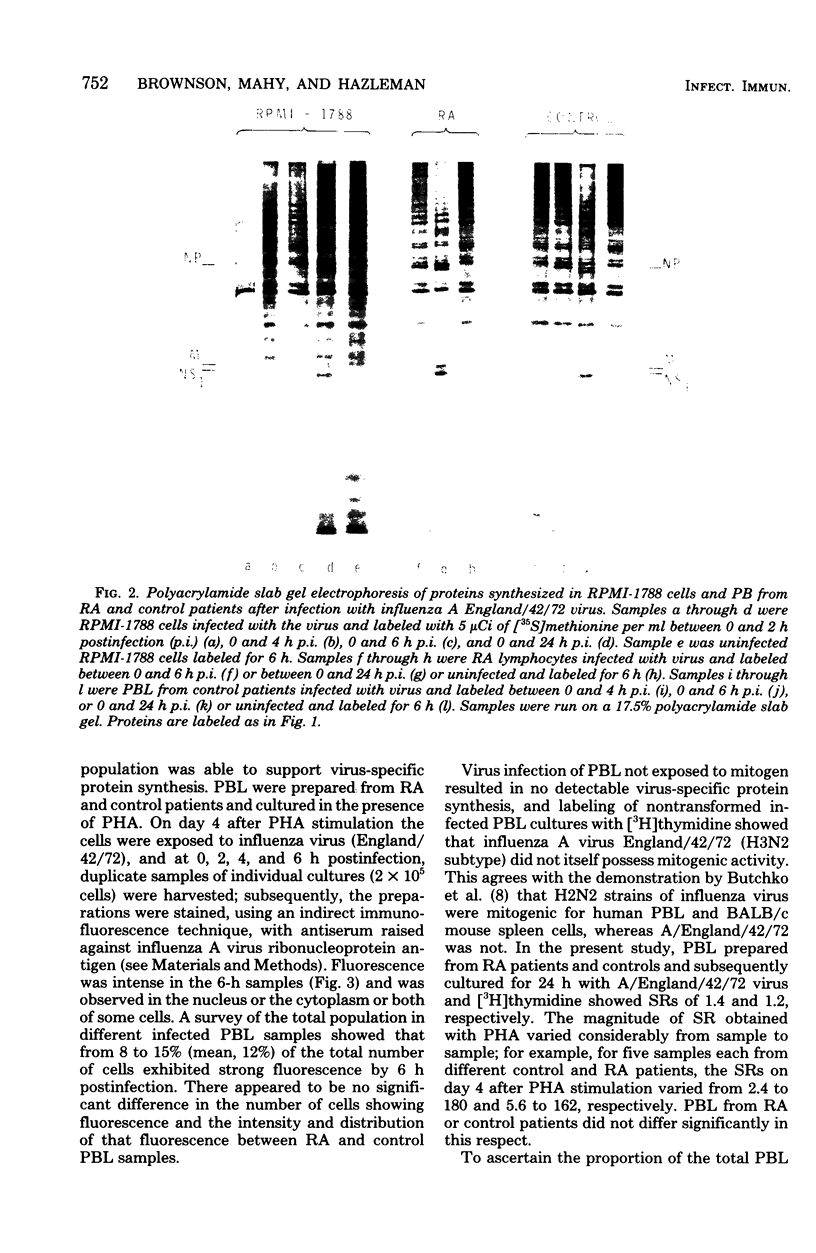
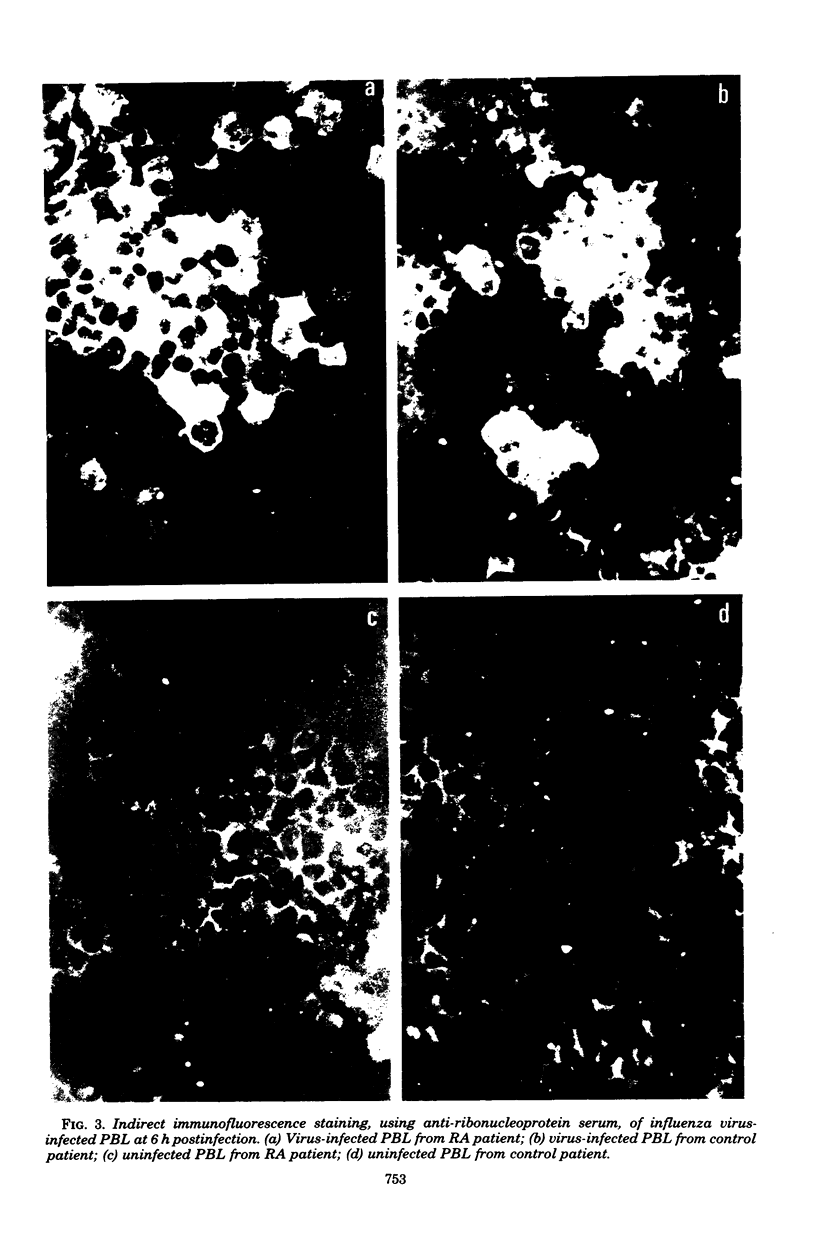
Peripheral blood lymphocytes cultured in the presence of phytohemagglutinin, concanavalin A, or pokeweed mitogen were exposed in vitro to influenza A virus. The synthesis of several virus-specific proteins, including the nucleoprotein, membrane protein, and nonstructural 1 protein were detected, although no infectious virus was produced by the lymphocyte cultures. Evidence was obtained that only a subpopulation of mitogen-transformed cells would support virus protein synthesis. A comparison of the interactions of influenza A virus with lymphocytes from normal individuals and from rheumatoid arthritis patients showed that the same range of virus-specific proteins were made, in similar quantities, regardless of the source of lymphocytes.
Full text
PDF

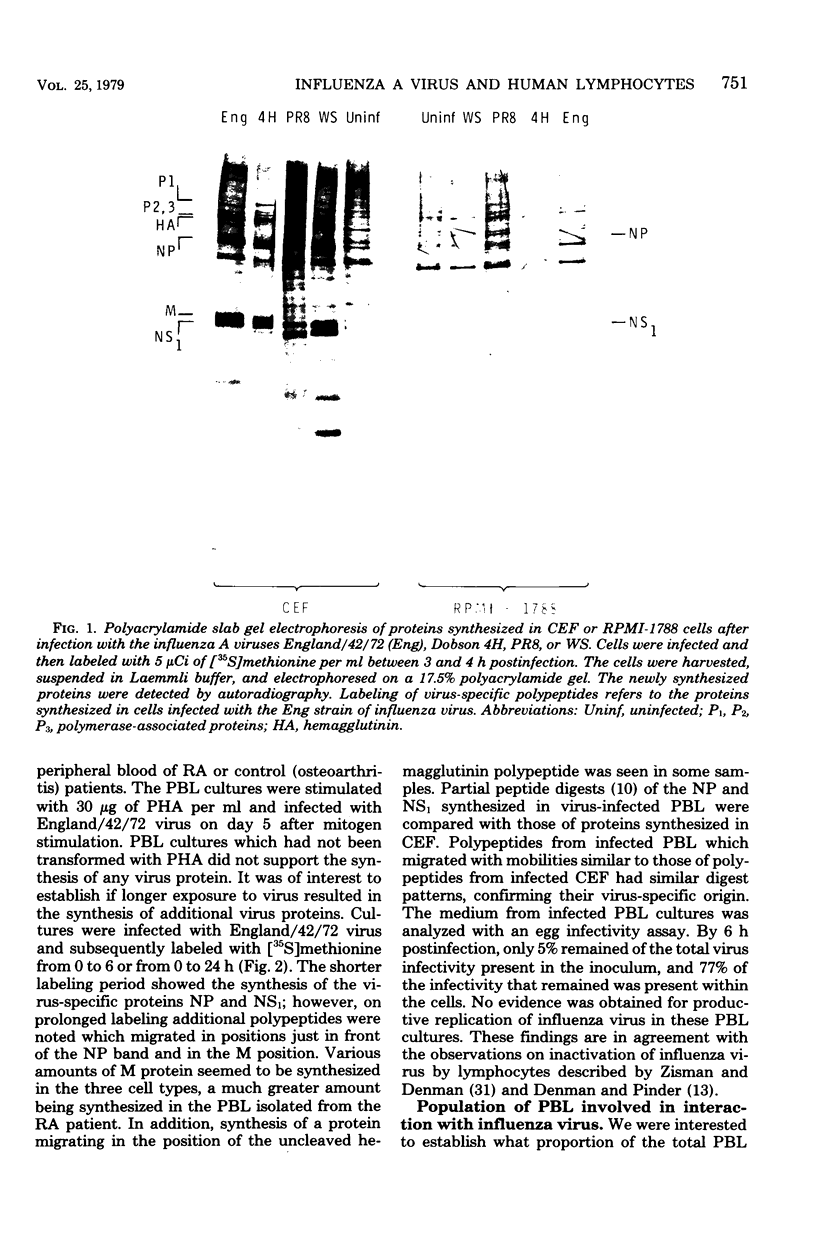



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Interactions of antibodies, complement components and various cell types in immunity against viruses and pyogenic bacteria. Transplant Rev. 1974;19(0):3–55. doi: 10.1111/j.1600-065x.1974.tb00127.x. [DOI] [PubMed] [Google Scholar]
- BARRY R. D., IVES D. R., CRUICKSHANK J. G. Participation of deoxyribonucleic acid in the multiplication of influenza virus. Nature. 1962 Jun 23;194:1139–1140. doi: 10.1038/1941139a0. [DOI] [PubMed] [Google Scholar]
- Biddison W. E., Doherty P. C., Webster R. G. Antibody to influenza virus matrix protein detects a common antigen on the surface of cells infected with type A influenza viruses. J Exp Med. 1977 Sep 1;146(3):690–697. doi: 10.1084/jem.146.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland R., Mahy B. W. Deoxyribonucleic acid-dependent ribonucleic acid polymerase activity in cells infected with influenza virus. J Virol. 1968 Jan;2(1):33–39. doi: 10.1128/jvi.2.1.33-39.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braciale T. J. Immunologic recognition of influenza virus-infected cells. II. Expression of influenza A matrix protein on the infected cell surface and its role in recognition by cross-reactive cytotoxic T cells. J Exp Med. 1977 Sep 1;146(3):673–689. doi: 10.1084/jem.146.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgen D., Hazleman B., Ward M., McCallum M. Immunological studies in frozen shoulder. Ann Rheum Dis. 1978 Apr;37(2):135–138. doi: 10.1136/ard.37.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butchko G. M., Armstrong R. B., Martin W. J., Ennis F. A. Influenza A viruses of the H2N2 subtype are lymphocyte mitogens. Nature. 1978 Jan 5;271(5640):66–67. doi: 10.1038/271066a0. [DOI] [PubMed] [Google Scholar]
- Cambridge G., Mackenzie J. S., Keast D. Cell-mediated immune response to influenza virus infections in mice. Infect Immun. 1976 Jan;13(1):36–43. doi: 10.1128/iai.13.1.36-43.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Denman A. M., Pelton B. K., Appleford D., Kinsley M. Virus infections of lympho-reticular cells and auto-immune diseases. Transplant Rev. 1976;31:79–115. [PubMed] [Google Scholar]
- Denman A. M., Pinder M. Measruement of immunological function in man: interaction between virus and human leukocytes. Proc R Soc Med. 1974 Dec;67(12 Pt 1):1219–1221. doi: 10.1177/003591577406712P109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman A. M. The viral theory of connective tissue diseases: a review. Med Biol. 1975 Apr;53(2):61–84. [PubMed] [Google Scholar]
- Effros R. B., Doherty P. C., Gerhard W., Bennink J. Generation of both cross-reactive and virus-specific T-cell populations after immunization with serologically distinct influenza A viruses. J Exp Med. 1977 Mar 1;145(3):557–568. doi: 10.1084/jem.145.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett E. A., Pringle C. R., Wunner W. H., Skehel J. J. Virus replication in enucleate cells: vesicular stomatitis virus and influenza virus. J Virol. 1974 Feb;13(2):394–399. doi: 10.1128/jvi.13.2.394-399.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackemann M. M., Denman A. M., Tyrrell D. A. Inactivation of influenza virus by human lymphocytes. Clin Exp Immunol. 1974 Apr;16(4):583–591. [PMC free article] [PubMed] [Google Scholar]
- Inglis S. C., Carroll A. R., Lamb R. A., Mahy B. W. Polypeptides specified by the influenza virus genome I. Evidence for eight distinct gene products specified by fowl plague virus. Virology. 1976 Oct 15;74(2):489–503. doi: 10.1016/0042-6822(76)90355-x. [DOI] [PubMed] [Google Scholar]
- Israel A., Semmel M., Huppert J. Host-range mutant of fowl plague virus (FPV): comparison of the genome and virus proteins. Virology. 1975 Dec;68(2):503–509. doi: 10.1016/0042-6822(75)90290-1. [DOI] [PubMed] [Google Scholar]
- Jao R. L., Wheelock E. F., Jackson G. G. Production of interferon in volunteers infected with Asian influenza. J Infect Dis. 1970 Apr;121(4):419–426. doi: 10.1093/infdis/121.4.419. [DOI] [PubMed] [Google Scholar]
- Mahy B. W., Hastie N. D., Armstrong S. J. Inhibition of influenza virus replication by -amanitin: mode of action. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1421–1424. doi: 10.1073/pnas.69.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor P. D., Dimmock N. J. Inhibition of synthesis of influenza virus proteins: evidence of two host-cell-dependent events during multiplication. Virology. 1975 Sep;67(1):114–123. doi: 10.1016/0042-6822(75)90409-2. [DOI] [PubMed] [Google Scholar]
- Virelizier J. L., Postlethwaite R., Schild G. C., Allison A. C. Antibody responses to antigenic determinants of influenza virus hemagglutinin. I. Thymus dependence of antibody formation and thymus independence of immunological memory. J Exp Med. 1974 Dec 1;140(6):1559–1570. doi: 10.1084/jem.140.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock E. F., Toy S. T. Participation of lymphocytes in viral infections. Adv Immunol. 1973;16:123–184. doi: 10.1016/s0065-2776(08)60297-7. [DOI] [PubMed] [Google Scholar]
- Wilson A. B., Planterose D. N., Nagington J., Park J. R., Barry R. D., Coombs R. R. Influenza A antigens on human lymphocytes in vitro and probably in vivo. Nature. 1976 Feb 19;259(5544):582–584. doi: 10.1038/259582a0. [DOI] [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. Lymphocyte receptors for myxoviruses and paramyxoviruses. J Immunol. 1974 Jun;112(6):2176–2183. [PubMed] [Google Scholar]
- Yap K. L., Ada G. L., McKenzie I. F. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature. 1978 May 18;273(5659):238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]
- Zisman B., Denman A. M. Inactivation of myxoviruses by lymphoid cells. J Gen Virol. 1973 Aug;20(2):211–223. doi: 10.1099/0022-1317-20-2-211. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Courtneidge S. A., Skehel J. J., Crumpton M. J., Askonas B. A. Cytotoxic T cells kill influenza virus infected cells but do not distinguish between serologically distinct type A viruses. Nature. 1977 May 26;267(5609):354–356. doi: 10.1038/267354a0. [DOI] [PubMed] [Google Scholar]