Abstract
Purpose
The purpose of this study was to evaluate the prognostic value of metabolic tumor volume (MTV) measured by 18F-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT) in patients with diffuse large B-cell lymphoma (DLBCL) treated with rituximab-containing immunochemotherapy.
Methods
Patients with newly diagnosed DLBCL who underwent pre-treatment torso FDG-PET/CT scan taken within 10 days before treatment were included. MTV was defined as the volume of hypermetabolic tissue with a standardized uptake value (SUV) greater than a threshold value of 2.5 and calculated using volume viewer software. Association of MTV with patient characteristics and survival were compared.
Results
A total of 96 patients were evaluated. During a median follow-up period of 27.8 months, 3-year event-free survival (EFS) and overall survival was 69.5 % and 72.9 %, respectively. The Ann Arbor staging showed a limitation of prognosis because there was no difference of EFS between patients with Ann Arbor stage II and those with stage III. On the contrary, among patients with Ann Arbor stage II or III disease (n = 53), the higher MTV group showed significantly inferior EFS compared with the lower MTV group.
Conclusions
In the current study, we identified the pre-treatment MTV measured by FDG-PET/CT as a potential predictor of survival in patients with DLBCL treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP), at least in Ann Arbor stage II and III disease.
Keywords: Diffuse large cell lymphoma, Metabolic tumor volume, Tumor burden, Prognosis, Positron-emission tomography
Introduction
Diffuse large B-cell lymphoma (DLBCL), the most common type of lymphoma, comprises approximately one-third of non-Hodgkin lymphoma (NHL) in adults [1, 2]. DLBCL is a potentially curable disease; therefore, accurate diagnosis and staging of the disease is very important for administration of optimal and tailored treatment, based on rituximab-containing immunochemotherapy, such as rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) [3, 4].
Most high-grade NHLs, including DLBCL, have been staged according to the Ann Arbor staging system, which was initially developed for Hodgkin’s lymphoma (HL) [5]. The Ann Arbor staging system is known to have limitations in application to NHLs, including DLBCL. Ann Arbor staging was originally designed for HL, which spreads mainly contiguously from nodal areas, rather than hematogeneously, as does NHL. In addition, separation of patients into stage II and III according to limitation or extension to one side of the diaphragm stems from consideration of the radiation field in previous years, which is invalid to the era of modern systemic chemotherapy. Despite these disadvantages, Ann Arbor staging has been regarded as a “gold standard” for patients with both HL and NHL, due to: (1) its convenience of use; it is a simple and concise staging system, which only requires information on anatomic distribution of the disease and basic clinical symptoms or signs, such as B symptoms [6]; (2) its usefulness when making decisions regarding incorporation of radiotherapy; (3) it having some prognostic value [7].
However, Ann Arbor stage itself is not an absolute prognostic factor in high-grade NHLs, including DLBCL, and, usually, only one of five components of the international prognostic index (IPI), a crucial clinical prognostic model in patients with NHLs, particularly DLBCL [3]: even a patient with Ann Arbor stage III or IV can only have an IPI score of 1 or 2, if the patient is of younger age (<60 years), has a normal value of serum lactate dehydrogenase (LDH), and favorable Eastern Cooperative Oncology Group (ECOG) performance status (PS). In contrast, a patient with Ann Arbor stage I or II can have three or four components of the IPI factors and can be a candidate for a dismal clinical course. In addition, the Ann Arbor staging system does not perfectly reflect tumor burden; even a patient with numerous lesions and extremely bulky disease will be classified as stage II disease only if tumors are confined to one side of the diaphragm. On the contrary, a patient with two very tiny lesions located above and below the diaphragm, respectively, will be considered as stage III disease. Therefore, it is difficult to conclude that the Ann Arbor staging system is an optimal tool for assessment of the exact tumor burden and predictive of outcomes in patients with DLBCL.
18F-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT) has now become a component of routine pre-treatment evaluation in patients with FDG-avid lymphomas for better delineation of the extent of disease [8]. Metabolic tumor volume (MTV) is a volumetric measurement of tumor tissues with increased FDG uptake [9–12]. The total burden of tumor can be measured semi-quantitatively by MTV and a larger tumor volume showed an association with a poorer survival rate in several solid tumors such as non-small cell lung cancer, uterine cervical cancer, and salivary gland carcinoma [13–15]. Some recent studies have reported the pretreatment MTV as a strong predictor in malignant lymphomas: Song et al. [16–19] found MTV as a prognostic factor in HL nodal site-only DLBCL, extranodal natural killer/T cell lymphoma, nasal type, and primary gastric DLBCL. Sharma et al. [20] studied pediatric patients, diagnosed both Hodgkin and non-Hodgkin lymphoma, and showed significant difference between MTV of partial response and complete response at the pretreatment and interim PET/CT. Similarly, total lesion glycolysis (TLG), one of other volumetric parameters, was also suggested as a potential prognostic indicator in malignant lymphomas [21, 22].
Consequently, we conducted a retrospective analysis under the hypothesis that MTV calculated by FDG-PET/CT imaging may overcome the limitation of the current Ann Arbor staging and play a role as a prognostic indicator in patients with DLBCL, regardless of nodal or extranodal sites.
Materials and methods
Patients
Patients were included in the current study if they were: (1) aged ≥20 years, (2) pathologically confirmed as DLBCL according to the 2008 World Health Organization (WHO) Criteria [23], (3) subject to a pretreatment torso FDG-PET/CT scan taken within 10 days before the initiation of lymphoma treatment, (4) treated with R-CHOP as the first-line treatment with or without consolidative therapy, such as involved-field radiotherapy or autologous stem cell transplantation, according to the protocol of Gachon University Gil Medical Center (GUGMC) as previously described [24], and (5) provided with a complete set of clinical and radiologic data from electronic medical records sufficient for performance of survival analysis. Patients were excluded if they had undergone PET/CT scan after the initiation of treatment, surgical resection of a lymphomatous lesion, or use of granulocyte colony stimulating factor.
This study was reviewed and approved by the Institutional review board (IRB) of GUGMC and informed consent was waived by the IRB considering the retrospective nature of the current analysis (IRB approval no.: 2013–310).
Acquisition of FDG-PET/CT
FDG-PET/CT scan of each analyzed patient was performed according to the GUGMC protocol as previously described [25]. Briefly, following fasting for at least 6 h, patients were injected with 370–555 MBq of FDG, with imaging 60 min later on a PET/CT instrument (Biograph6; Siemens Healthcare Systems, Erlangen, Germany). Emission PET images were acquired in a three-dimensional mode from skull base to the upper thigh. Helical CT imaging was performed for attenuation correction. All reported blood glucose levels were under 160 mg/dl at the time of injection. Images were reconstructed by iteration using the ordered subset-expectation maximization (OSEM) algorithm.
FDG PET/CT Analysis
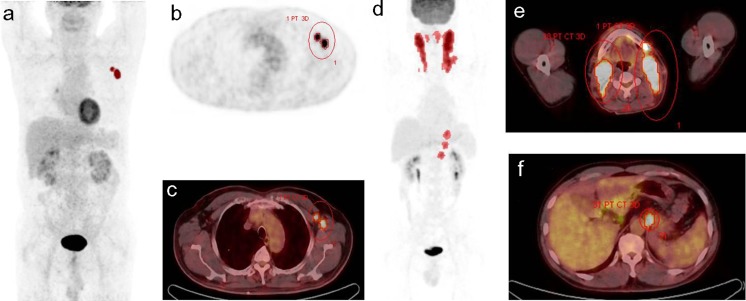
Display of all images and their analyses were performed on a workstation that allowed multiplanar reformatting of images. All FDG-PET/CT scans were interpreted in a dichotomous fashion as positive or negative. A positive lesion was defined as focal or diffuse FDG uptake above the background in a location incompatible with normal anatomy or physiology [8]. With pre-treatment FDG PET/CT scans, the metabolic tumor volume (MTV) was defined as the volume of hypermetabolic tissue with a SUV greater than a threshold value of 2.5 using volume viewer software (MMWP Syngo TrueD; Siemens Healthcare Systems). The threshold value of SUV 2.5 was decided according to previous reports [26–28]. Figure 1 illustrates two examples of the method used for tumor delineation. The contour around the target lesions inside the boundaries was automatically produced, and the voxels presenting SUV intensity greater than 2.5 within the contouring margin were incorporated in order to define the tumor volumes.
Fig. 1.
Measurement of the MTV of tumorous lesions using a standardized uptake value (SUV)-based automated contouring program. a-c A 75-year-old male patient with stage II DLBCL. Volume of interest (VOI) was drawn in the axillar lymph nodes. d-f A 47-year-old male patient with stage III DLBCL. Red-dotted lesions on maximum intensity projection image (d) show segmented MTV
Ann Arbor staging of the analyzed patients was determined according to results of FDG-PET/CT and other diagnostic tests, including physical examination, bone marrow trephine biopsy, and imaging studies, such as enhanced CT scan. First, a nuclear medicine physician and a physician estimated stage of each patient individually. If there was a discrepancy in determination of staging between the two investigators, a conclusion was made after discussion.
Estimation of Serum Lactate Dehydrogenase
Serum lactate dehydrogenase (LDH) levels were measured using an autoanalyzer (ADVIA 2400; Siemens Healthcare Diagnostics, Tarrytown, NY, USA), according to the manufacturer’s instructions. The upper limit of the normal value of serum LDH for the GUGMC was 485 U/l.
Statistical Analysis
Mann–Whitney U test was performed for determination of whether non-parametric variables of two independent groups were significantly different from each other (MTV according to stages and MTV according to LDH status). Correlations between two continuous variables (serum level of LDH and MTV) were estimated using Spearman’s Rho Correlation test. Clinical, laboratory, and radiologic parameters were dichotomized and their relationship to prognosis was analyzed. Event-free survival (EFS) was defined as survival free from progression or relapse of lymphoma, discontinuation of treatment for any reason, such as intolerable toxicity, or death from any cause. Overall survival (OS) was defined as survival free from death from any cause. Survival analysis was performed using the Kaplan-Meier method and compared using the log-rank test. The variables associated with EFS in the univariate analysis with a p < 0.2, were included for multivariate analysis by entering into Cox regression model. Each value was two-sided and acceptable statistical significance was at the level of p < 0.05.
Results
Patient Characteristics and Overall Treatment Outcomes
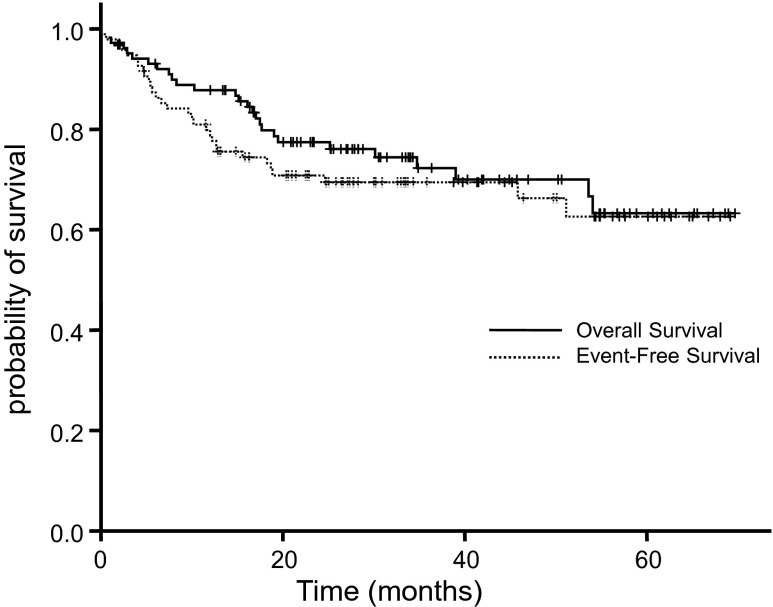
From October 2006 to May 2012, 120 patients were diagnosed as DLBCL and treated in the GUGMC. Among them, 96 patients (median age 57.5 years, range 26–87) satisfied the inclusion criteria; 19 patients were excluded due to complete or partial resection of tumor before PET/CT, 2 patients could not be included due to absence of pre-treatment PET/CT, and 3 patients due to lack of the medical record of major clinical data. A summary of patient characteristics is shown in Table 1. Out of 93 evaluable patients, 83 (89.2 %) achieved a complete response (CR) and four patients had a progressive disease (PD) during the first-line therapy according to the revised criteria for non-Hodgkin’s lymphoma proposed by Cheson et al. [8]. During a median follow-up period of 27.8 months, 3-year EFS and OS were 69.5 % and 72.9 %, respectively (Fig. 2).
Table 1.
Patient characteristics
| Parameters | Entire patients (n = 96) | Analyzed patients (Stage II or III; n = 53) | |
|---|---|---|---|
| Gender | male | 42 (43.8 %) | 22 (41.5 %) |
| Age | >60 years | 43 (44.8 %) | 22 (41.5 %) |
| Ann Arbor stage | I | 15 (15.6 %) | 0 |
| II | 34 (35.4 %) | 34 (64.2 %) | |
| III | 19 (19.8 %) | 19 (35.8 %) | |
| IV | 28 (29.2 %) | 0 | |
| Extranodal sites | 0 or 1 site | 71 (74.0 %) | 53 (100 %) |
| Lactate dehydrogenase | ≥2 sites | 25 (26.0 %) | 0 |
| ECOG performance status | elevated | 48 (50 %) | 20 (37.7 %) |
| ≥2 | 27 (28.1 %) | 8 (15.1 %) | |
| Risk by standard IPI | low (score 0 or 1) | 49 (51.0 %) | 34 (64.2 %) |
| low-intermediate (score 2) | 14 (14.6 %) | 11 (20.8 %) | |
| high-intermediate (score 3) | 10 (10.4 %) | 5 (9.4 %) | |
| high (score 4 or 5) | 23 (24.0 %) | 3 (5.7 %) | |
| B symptom | present | 24 (25.0 %) | 10 (18.9 %) |
| Bone marrow involvement | yes | 17 (17.7 %) | 0 |
| Bulky disease | yes | 22 (22.9 %) | 10 (18.9 %) |
| Consolidative therapy | Radiotherapy | 25 (26.0 %) | 2 (3.8 %) |
| Autologous SCT | 9 (9.4 %) | 16 (30.2 %) | |
ECOG Eastern Cooperative Oncology Group, IPI international prognostic index, SCT stem cell transplantation
Fig. 2.
A Kaplan-Meier curve for EFS and OS of entire patients
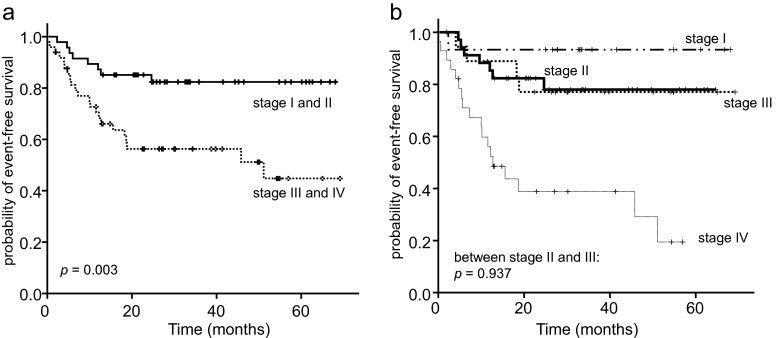
Among 96 patients who satisfied the inclusion criteria, the IPI factors showed an excellent prognostic value: age >60 years [hazard ratio (HR) = 2.6, 95 % confidence interval (CI) 1.2-5.4, p = 0.014], elevated serum LDH (HR = 4.24, 95 % CI 1.8-9.9, p = 0.001), ≥2 ECOG PS (HR = 13.4, 95 % CI 5.9-30.5, p < 0.001), ≥2 extranodal sites HR = 5.8, 95 % CI 2.8-12.1, p < 0.001), and Ann Arbor stage III or IV (HR = 3.3, 95 % CI 1.5-7.3, p = 0.004; Fig. 3a). However, no difference of EFS was observed between patients with Ann Arbor stage II and those with stage III (Fig. 3b).
Fig. 3.
Kaplan-Meier curves for EFS of patients stratified according to Ann Arbor staging. a Patients with a limited stage (Ann Arbor I or II) vs an advanced stage (III or IV). b EFS according to each Ann Arbor stage
Metabolic Tumor Volume and Ann Arbor Staging
We measured MTV of patients with Ann Arbor stage I, II, and III disease. MTV of stage IV was not estimated because: (1) the nature of stage IV defined by the Ann Arbor staging system, such as bone marrow involvement, cannot be reflected by MTV, (2) a poor prognosis of patients with stage IV was already well demonstrated (Fig. 3b), and (3) acquisition of MTV in patients with stage IV disease was too laborious for measurement due to the fact that most of them had very high tumor burden. Median values and range of MTV according to Ann Arbor staging are shown in Table 2. In evaluation of difference of MTV of patients according to Ann Arbor stage, no significant difference of MTV was observed between patients with stage I and stage II (p = 0.398). Patients with stage III disease showed significantly higher MTV compared with those with Ann Arbor stage I (p < 0.001) and II DLBCL (p < 0.001), respectively.
Table 2.
Metabolic tumor volume of the analyzed patients
| Ann Arbor stage | MTV (cm3), both nodal and extranodal mean/median (range) | MTV (cm3), nodal mean/median (range) | MTV (cm3), extranodal mean/median (range) |
|---|---|---|---|
| I (n = 15) | 119.8/65.1 (0.1- 782.3) | 16.1/21.0 (0–65.0) | 187.1/141.8 (11.3- 782.3) |
| II (n = 34) | 176.7/72.4 (0.9- 1,131.4) | 106.1/21.3 (0–1,121.0) | 141.2/42.9 (0.9- 788.9) |
| III (n = 19) | 697.5/346.3 (22.3- 3,117.2) | 613.6/219.9 (23.0- 3,117.0) | 199.5/21.3 (0.37- 1,090.8) |
Treatment Outcomes According to MTV—Among Patients with Stage II and III Disease
Because patients with stage I disease showed significantly favorable survival compared with those with stage II or III DLBCL (Fig. 3b), we evaluated the prognostic role of MTV in patients limited to Ann Arbor stage II and III. For comparison of survival according to MTV volume among patients with stage II and III disease (n = 53), we dichotomized the patients according to the median value of MTV of 53 patients, 130.7 cm3. Patients with stage III disease correlated to MTV ≥130.7 cm3 (p = 0.002 by chi-square test; Table 3). The high MTV group showed significantly inferior EFS compared with the low MTV group (Fig. 4a). In estimation of the prognostic value of MTV for each stage, patients with high MTV stage II disease showed poorer EFS compared with those with low MTV stage II disease (Fig. 4b), whereas there was no difference of EFS between patients with high MTV stage III disease and those with low MTV stage III disease. Univariate and multivariate analysis of MTV along with IPI factors have showed that higher MTV is an independent prognostic factor for EFS of the patients with Ann Arbor stage II or III DLBCL (Table 4).
Table 3.
MTV according to Ann Arbor stage in patients with stage II and III disease
| Ann Arbor stage | MTV <130.7 | MTV ≥130.7 | Total |
|---|---|---|---|
| Stage II | 12 | 22 | 34 |
| Stage III | 15 | 4 | 19 |
| Total | 27 | 26 | 53 |
Fig. 4.
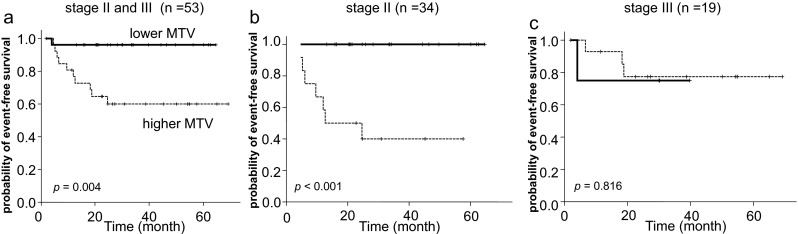
Kaplan-Meier plots in patients with stage II and III disease. The cutoff value of MTVs is 130.7 cm3. a EFS according to MTV in patients with stage II or III disease (n = 53). b EFS according to MTV in patients with Ann Arbor stage II (n = 34). c EFS according to MTV in patients with stage III disease (n = 19)
Table 4.
Univariate and multivariate analysis for EFS in patients with Ann Arbor stage II or III disease (n = 53)
| Parameters | Hazard ratio (95 % confidence interval) | p valuea |
|---|---|---|
| Univariate analysis | ||
| International Prognostic Index (IPI) score ≥3 | 2.6 (0.7-9.7) | 0.166 |
| Age >60 years | 1.8 (0.6-6.1) | 0.300 |
| Elevated lactose dehydrogenase | 2.2 (0.7-7.2) | 0.198 |
| Ann Arbor stage III | 1.1 (0.3-3.6) | 0.937 |
| ECOG PS ≥2 | 6.3 (1.9-20.6) | 0.003 |
| Metabolic tumor volume ≥ 130.7 cm3 | 11.2 (1.4-88.1) | 0.021 |
| B symptom | 0.9 (0.2-4.0) | 0.871 |
| Presence of bulky lesion | 2.7 (0.3-21.0) | 0.347 |
| Multivariate analysis: IPI as single parameter | ||
| IPI score ≥3 | 1.5 (0.4-5.6) | 0.573 |
| Metabolic tumor volume ≥ 130.7 cm3 | 10.4 (1.3-83.4) | 0.027 |
| With individual IPI parameters | ||
| Age >60 years | 1.3 (0.3-5.2) | 0.756 |
| Elevated lactose dehydrogenase | 1.1 (0.3-5.2) | 0.891 |
| ≥2 | 2.6 (0.6-12.2) | 0.219 |
| Metabolic tumor volume ≥ 130.7 cm3 | 7.7 (0.9-67.5) | 0.065 |
ECOG PS Eastern Cooperative Oncology Group Performance Status
aBy Cox proportional regression method
Serum LDH and MTV
Among patients with Ann Arbor stage II and III DLBCL, we evaluated the relationship of MTV to serum LDH. MTV showed significant correlation with blood level of LDH (correlation coefficient 0.473, p < 0.001, by Spearman’s Rho Correlation test). Serum LDH level of patients with MTV ≥130.7 cm3 was higher compared with those with MTV <130.7 cm3 (p = 0.009 by Mann–Whitney U test). Patients with stage III DLBCL also had a higher serum LDH level compared with those with stage II disease (p = 0.026 by Mann–Whitney U test).
Discussion
In the current study, stratification of patients with DLBCL of Ann Arbor stage II or III according to their MTV value was prognostic, whereas no significant difference of EFS was observed between patients with Ann Arbor stage II and those with stage III. These results are compatible with the report of Song et al. [17] in general, who evaluated the prognostic role of MTV in patients with nodal DLBCL of Ann Arbor stage II or III.
There are several differences between the study reported by Song et al. [17] and the current study: first, they excluded patients with extranodal involvement in order to improve homogeneity of the analyzed patients, because several types of extranodal DLBCL have different clinical characteristics and overall outcomes compared with nodal DLBCL. By contrast, we included patients of DLBCL regardless of the existence of an extranodal lesion, because (1) we thought that the sum of overall lymphomatous MTV is still a reliable indicator of disease extent, only if there is single extranodal lesion (i.e., not stage IV disease), and (2) as a matter of fact, some of the patients with DLBCL have both nodal and one extranodal lesion (i.e., stage IIE or IIIE). Second, they used a cutoff point of MTV estimated using the receiver operative characteristic (ROC) curve, although we used just a median MTV of the analyzed patients (stage II and III) reflecting the result of statistic consultation; application of an ROC curve may not be optimal because survival outcomes vary for each patient, and using a median value is convenient and not unreasonable in this exploratory analysis with a limited number of patients. We believe that both studies adequately support the potential role of MTV in overcoming limitation of the Ann Arbor staging system, especially according to our study, irrespective of nodal or extranodal lesion.
For the abovementioned reasons, we did not estimate MTV of patients with Ann Arbor stage I or IV disease. Indeed measurement of MTV of multiple, disseminated, and extensive lymphomatous lesions is too laborious, not only for stage IV disease but also for some stage III disease. For those patients, measurement of MTV for prediction of treatment outcome or survival seems not to be realistic in daily practice. More advanced, delicate, and automatic programs may improve this limitation. However, delineation of tumor lesions is not easy because discrimination between tumorous hypermetabolism and benign or normally observed hypermetabolism, such as bowel or ureteric activity, basically necessitates significant manual labor. Although we did not evaluate MTV of patients with stage IV DLBCL, it is not conclusive that there is no role of MTV for those patients in prediction of survival. Kim et al. [22], who evaluated the prognostic value of total lesion glycolysis, calculated by SUVmean × MTV, for patients with DLBCL, and concluded that the quantitative metabolic parameter (i.e., TLG) is predictive of survival in DLBCL patients treated with R-CHOP [22]. They consolidated the potential prognostic role of TLG in view of volume-based PET parameters, which was reported by Esfahani et al. [21] (n = 20), in line with our study. In their study, they included patients with stage IV disease, although no discussion on how they consider patients with disease not estimated by FDG-PET/CT, such as bone marrow involvement, was provided [22]. Conduct of future studies especially focusing on impact of MTV on stage IV patients with low tumor burden is warranted.
We also did not include patients with stage I disease because we thought that it would not be necessary to incorporate them because they showed excellent EFS and OS according to the Ann Arbor staging system. As they have relatively lower MTV, including them in survival analysis would probably not have an impact on the results.
Some may argue that measurement of volume-based PET parameters would be more time-consuming than conventional PET parameters, such as SUV. Moreover, a recent study conducted by Gallicchio et al. [29] (n = 52) even showed that SUVmax rather than MTV or TLG is a more reliable prognostic indicator in patients with DLBCL. Park et al. [30] (n = 108) also reported that higher SUVsum (sum of SUVmax) from pre-treatment PET/CT, as well as higher SUVmax and SUVsum from interim PET/CT, were significantly correlated with poorer outcome in DLBCL patients with an IPI score of 1, 2, or 3, whereas TLG was not. However, several limitations do not support the superiority of SUV compared with MTV or TLG; first, those two studies had a limited number of patients to be conclusive. Second, the prognostic value of SUV was discordant in two studies; Gallicchio et al.[29] reported that a higher SUVmax associated with longer EFS, while Park et al.[30] asserted that patients with higher pre-treatment SUVsum showed the inferior survival outcomes. Third, studies may not include the entire tumor volume and metabolism. For example, Park et al. [30] defined target lesions up to a maximum of five measurable lesions per organ and ten measurable lesions in total showing high FDG uptake for response analysis, and measured SUVmax, SUVmean, and TLG only from these target lesions. In addition, it might also be time consuming to measure the number of SUVmax from disseminated and multiple lesions in order to acquire SUVsum. Although it is almost impossible to obtain the daily measurement of MTV in all DLBCL, it is more feasible to measure MTV in stage II or III, also suggested by the current study.
In the current study, serum LDH level was significantly related to MTV of the analyzed patients with stage II or III disease, and relationship of MTV to serum LDH was stronger (p = 0.009) compared with that of Ann Arbor staging (II vs III; p = 0.026). Considering that serum LDH level is a biochemical parameter reflecting burden of tumors [31] and a component of the IPI [32], we can cautiously suggest that the prognostic power of the IPI would be more improved according to a new staging system that reflects a more precise tumor burden by incorporating MTV estimated by PET/CT, rather than Ann Arbor staging, proposed and validated in future studies.
This study is limited by the relatively small patient cohort and the retrospective nature. The number of patients with stage III was small and there were too few patients with lower MTV in stage III. If we could have analyzed more patients with III disease, MTV might have been a prognostic value among them. Thus, prospective validation in a larger cohort and longer follow-up would lend more statistical power in order to better define the relationship between MTV and outcomes in DLBC found in our study. However, despite these limitations, the main ideas of the results of our exploratory analysis provide us with insight regarding the importance of measuring baseline tumor burden of DLBCL and still warrant further investigation. Recent studies suggested that metabolic tumor response might be a more powerful prognostic factor compared with baseline PET/CT [30]. However, the application of interim PET/CT has limitations in terms of interpretation and reproducibility [33], and several studies even have failed to demonstrate the prognostic role of interim PET/CT in patients with DLBCL in the rituximab era [34, 35]. Therefore, baseline PET/CT still remains a major field of research to predict the treatment outcomes in patients with lymphomas. We anticipate that this method will be useful for stratification of patients with DLBCL into high- and low-risk subgroups within Ann Arbor stage groups, and ultimately for selection of patients for risk-adapted therapies.
Conclusion
Our study shows that pre-treatment MTV of FDG-PET/CT is correlated with survival in patients with DLBCL treated with R-CHOP immunochemotherapy, at least Ann Arbor stage II and III disease. Further evaluation of MTV may improve the prognostication of DLBCL and enable development of a risk-adapted therapy.
Acknowledgments
Conflict of Interest
Jihyun Kim, Junshik Hong, SeogGyun Kim, Kyung Hoon Hwang, Minsu Kim, Hee Kyung Ahn, Sun Jin Sym, Jinny Park, Eun Kyung Cho, Dong Bok Shin, and Jae Hoon Lee declare that they have no conflicts of interest.
Ethics Statement
The current study was reviewed and approved by our Institutional Review Board (Approval No.:2013–310) and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki, and its later amendments. Waiver of informed consent from patients was applied by the Institutional Review Board considering the retrospective nature of the current analysis with minimal risk.
Footnotes
J. Hong and S.G. Kim made equal contributions and should be regarded as co-corresponding authors.
Contributor Information
Junshik Hong, Phone: +82-32-460-3229, Email: alertjun@hanmail.net.
Seog Gyun Kim, Email: d970012@hanmail.net.
References
- 1.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89:3909–18. [PubMed]
- 2.Yoon SO, Suh C, Lee DH, Chi HS, Park CJ, Jang SS, et al. Distribution of lymphoid neoplasms in the Republic of Korea: analysis of 5318 cases according to the World Health Organization classification. Am J Hematol. 2010;85:760–4. doi: 10.1002/ajh.21824. [DOI] [PubMed] [Google Scholar]
- 3.Coiffier B, Lepage E, Brière J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–42. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 4.Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–91. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 5.Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res. 1971;31:1860–1. [PubMed] [Google Scholar]
- 6.Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630–6. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 7.Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–61. doi: 10.1182/blood-2006-08-038257. [DOI] [PubMed] [Google Scholar]
- 8.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 9.Lapela M, Leskinen S, Minn HR, Lindholm P, Klemi PJ, Soderstrom KO, et al. Increased glucose metabolism in untreated non-Hodgkin’s lymphoma: a study with positron emission tomography and fluorine-18-fluorodeoxyglucose. Blood. 1995;86:3522–7. [PubMed] [Google Scholar]
- 10.Bos R, van Der Hoeven JJ, van Der Wall E, van Der Groep P, van Diest PJ, Comans EF, et al. Biologic correlates of (18) fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol. 2002;20:379–87. doi: 10.1200/JCO.20.2.379. [DOI] [PubMed] [Google Scholar]
- 11.Vesselle H, Schmidt RA, Pugsley JM, Li M, Kohlmyer SG, Vallires E, et al. Lung cancer proliferation correlates with [F-18] fluorodeoxyglucose uptake by positron emission tomography. Clin Cancer Res. 2000;6:3837–44. [PubMed] [Google Scholar]
- 12.Haberkorn U, Strauss LG, Reisser C, Haag D, Dimitrakopoulou A, Ziegler S, et al. Glucose uptake, perfusion, and cell proliferation in head and neck tumors: relation of positron emission tomography to flow cytometry. J Nucl Med. 1991;32:1548–55. [PubMed] [Google Scholar]
- 13.Abelson JA, Murphy JD, Trakul N, Bazan JG, Maxim PG, Graves EE, et al. Metabolic imaging metrics correlate with survival in early stage lung cancer treated with stereotactic ablative radiotherapy. Lung Cancer. 2012;78:219–24. doi: 10.1016/j.lungcan.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Chung HH, Kim JW, Han KH, Eo JS, Kang KW, Park NH, et al. Prognostic value of metabolic tumor volume measured by FDG-PET/CT in patients with cervical cancer. Gynecol Oncol. 2011;120:270–4. doi: 10.1016/j.ygyno.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Ryu IS, Kim JS, Roh JL, Lee JH, Cho KJ, Choi SH, et al. Prognostic value of preoperative metabolic tumor volume and total lesion glycolysis measured by 18 F-FDG PET/CT in salivary gland carcinomas. J Nucl Med. 2013;54:1032–8. doi: 10.2967/jnumed.112.116053. [DOI] [PubMed] [Google Scholar]
- 16.Song MK, Chung JS, Lee JJ, Jeong SY, Lee SM, Hong JS, et al. Metabolic tumor volume by positron emission tomography/computed tomography as a clinical parameter to determine therapeutic modality for early stage Hodgkin’s lymphoma. Cancer Sci. 2013;104:1656–61. doi: 10.1111/cas.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song MK, Chung JS, Shin HJ, Lee SM, Lee SE, Lee HS, et al. Clinical significance of metabolic tumor volume by PET/CT in stages II and III of diffuse large B cell lymphoma without extranodal site involvement. Ann Hematol. 2012;91:697–703. doi: 10.1007/s00277-011-1357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song MK, Chung JS, Shin HJ, Moon JH, Ahn JS, Lee HS, et al. Clinical value of metabolic tumor volume by PET/CT in extranodal natural killer/T cell lymphoma. Leuk Res. 2013;37:58–63. doi: 10.1016/j.leukres.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Song MK, Chung JS, Shin HJ, Moon JH, Lee JO, Lee HS, et al. Prognostic value of metabolic tumor volume on PET/CT in primary gastrointestinal diffuse large B cell lymphoma. Cancer Sci. 2012;103:477–82. doi: 10.1111/j.1349-7006.2011.02164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma P, Gupta A, Patel C, Bakhshi S, Malhotra A, Kumar R. Pediatric lymphoma: metabolic tumor burden as a quantitative index for treatment response evaluation. Ann Nucl Med. 2012;26:58–66. doi: 10.1007/s12149-011-0539-2. [DOI] [PubMed] [Google Scholar]
- 21.Esfahani SA, Heidari P, Halpern EF, Hochberg EP, Palmer EL, Mahmood U. Baseline total lesion glycolysis measured with (18) F-FDG PET/CT as a predictor of progression-free survival in diffuse large B-cell lymphoma: a pilot study. Am J Nucl Med Mol Imaging. 2013;3:272–81. [PMC free article] [PubMed] [Google Scholar]
- 22.Kim TM, Paeng JC, Chun IK, Keam B, Jeon YK, Lee SH, et al. Total lesion glycolysis in positron emission tomography is a better predictor of outcome than the International Prognostic Index for patients with diffuse large B cell lymphoma. Cancer. 2013;119:1195–202. doi: 10.1002/cncr.27855. [DOI] [PubMed] [Google Scholar]
- 23.Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Journal. 2009; doi:10.1182/asheducation-2009;1.523. [DOI] [PMC free article] [PubMed]
- 24.Hong J, Park S, Park J, Kim HS, Kim KH, Ahn JY, et al. Evaluation of prognostic values of clinical and histopathologic characteristics in diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone therapy. Leuk Lymphoma. 2011;52:1904–12. doi: 10.3109/10428194.2011.588761. [DOI] [PubMed] [Google Scholar]
- 25.Lee Y, Hwang K, Hong J, Park J, Lee J, Ahn J, et al. Usefulness of 18F-FDG PET/CT for the evaluation of bone marrow involvement in patients with high-grade non-Hodgkin’s lymphoma. Nucl Med Mol Imaging. 2012;46:269–77. doi: 10.1007/s13139-012-0153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung MK, Jeong HS, Park SG, Jang JY, Son YI, Choi JY, et al. Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res. 2009;15:5861–8. doi: 10.1158/1078-0432.CCR-08-3290. [DOI] [PubMed] [Google Scholar]
- 27.Roedl JB, Colen RR, Holalkere NS, Fischman AJ, Choi NC, Blake MA. Adenocarcinomas of the esophagus: response to chemoradiotherapy is associated with decrease of metabolic tumor volume as measured on PET–CT: comparison to histopathologic and clinical response evaluation. Radiother Oncol. 2008;89:278–86. doi: 10.1016/j.radonc.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Hong R, Halama J, Bova D, Sethi A, Emami B. Correlation of PET standard uptake value and CT window-level thresholds for target delineation in CT-based radiation treatment planning. Int J Radiat Oncol Biol Phys. 2007;67:720–6. doi: 10.1016/j.ijrobp.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 29.Gallicchio R, Mansueto G, Simeon V, Nardelli A, Guariglia R, Capacchione D, et al. F-18 FDG PET/CT quantisation parameters as predictors of outcome in patients with diffuse large B-cell lymphoma. Eur J Haematol. 2014;92:382–9. doi: 10.1111/ejh.12268. [DOI] [PubMed] [Google Scholar]
- 30.Park S, Moon SH, Park LC, Hwang DW, Ji JH, Maeng CH, et al. The impact of baseline and interim PET/CT parameters on clinical outcome in patients with diffuse large B cell lymphoma. Am J Hematol. 2012;87:937–40. doi: 10.1002/ajh.23267. [DOI] [PubMed] [Google Scholar]
- 31.Coletta DF, Siegel PD. Lactic dehydrogenase. Med Sci. 1964;15:98–101. [PubMed] [Google Scholar]
- 32.Wilder RB, Rodriguez MA, Medeiros LJ, Tucker SL, Ha CS, Romaguera JE, et al. International prognostic index-based outcomes for diffuse large B-cell lymphomas. Cancer. 2002;94:3083–8. doi: 10.1002/cncr.10583. [DOI] [PubMed] [Google Scholar]
- 33.Moskowitz CH. Interim PET-CT in the management of diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2012;2012:397–401. doi: 10.1182/asheducation-2012.1.397. [DOI] [PubMed] [Google Scholar]
- 34.Pregno P, Chiappella A, Bello M, Botto B, Ferrero S, Franceschetti S, et al. Interim 18-FDG-PET/CT failed to predict the outcome in diffuse large B-cell lymphoma patients treated at the diagnosis with rituximab-CHOP. Blood. 2012;119:2066–73. doi: 10.1182/blood-2011-06-359943. [DOI] [PubMed] [Google Scholar]
- 35.Yoo C, Lee DH, Kim JE, Jo J, Yoon DH, Sohn BS, et al. Limited role of interim PET/CT in patients with diffuse large B-cell lymphoma treated with R-CHOP. Ann Hematol. 2011;90:797–802. doi: 10.1007/s00277-010-1135-6. [DOI] [PubMed] [Google Scholar]