Abstract
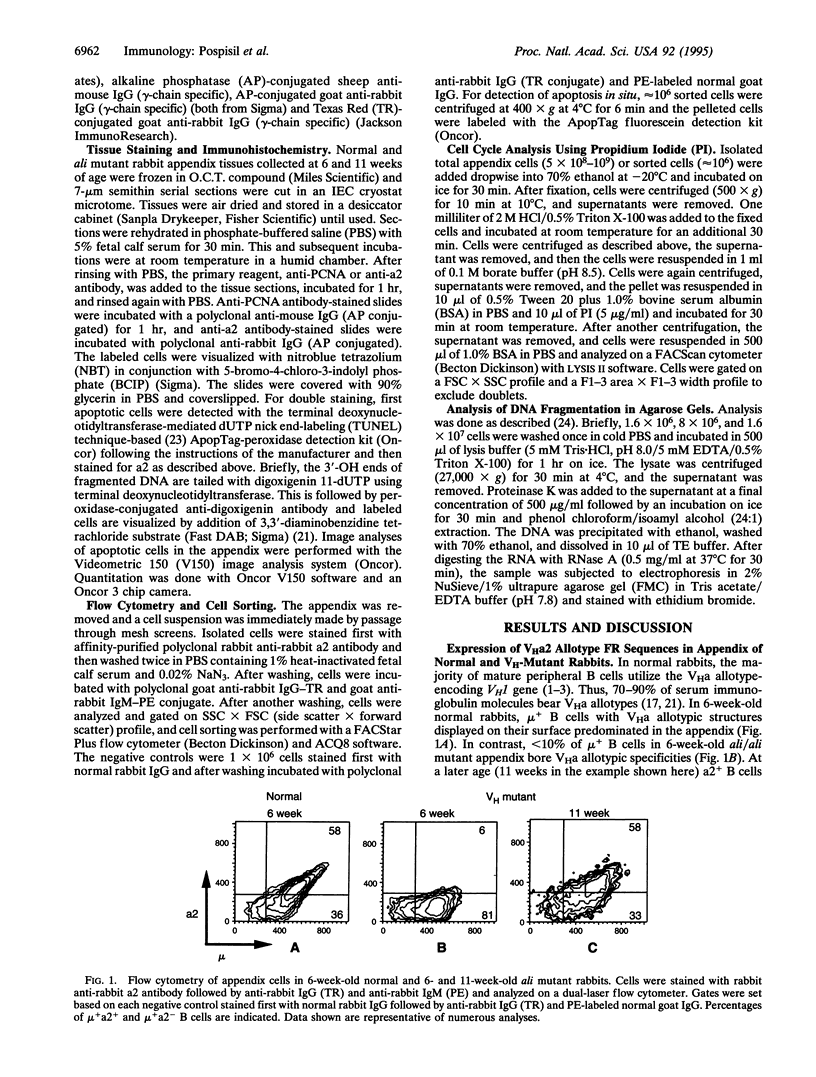
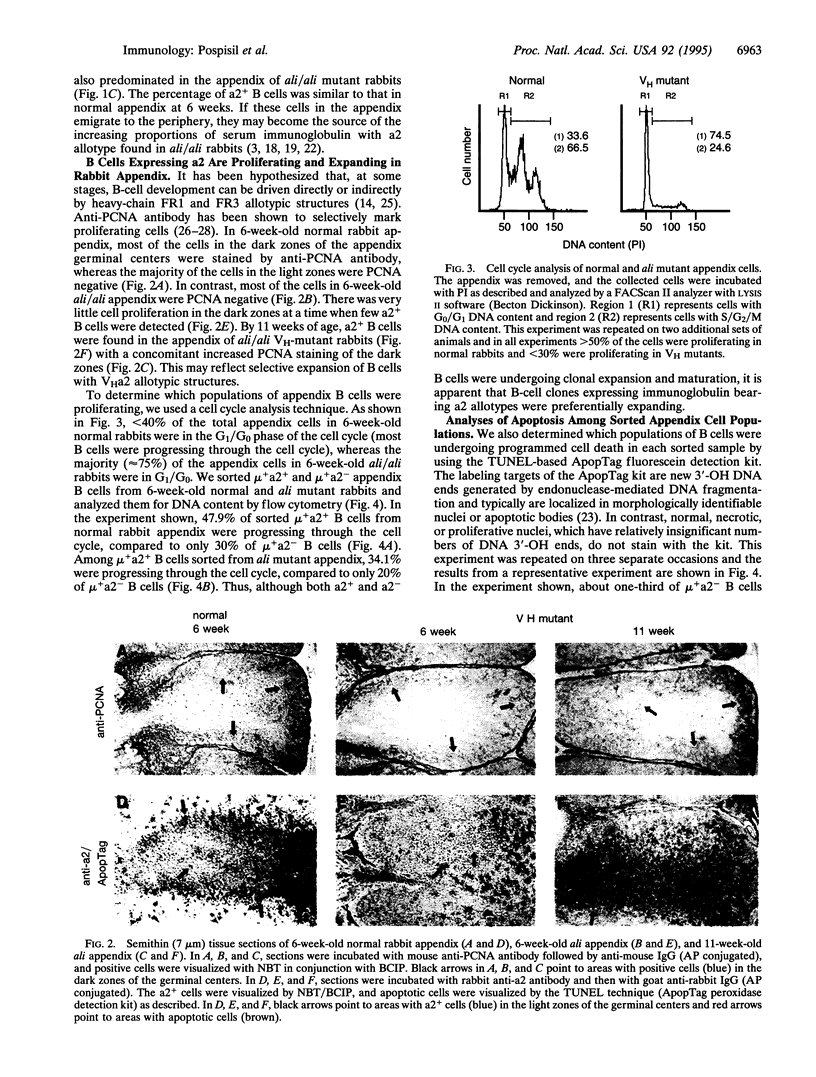
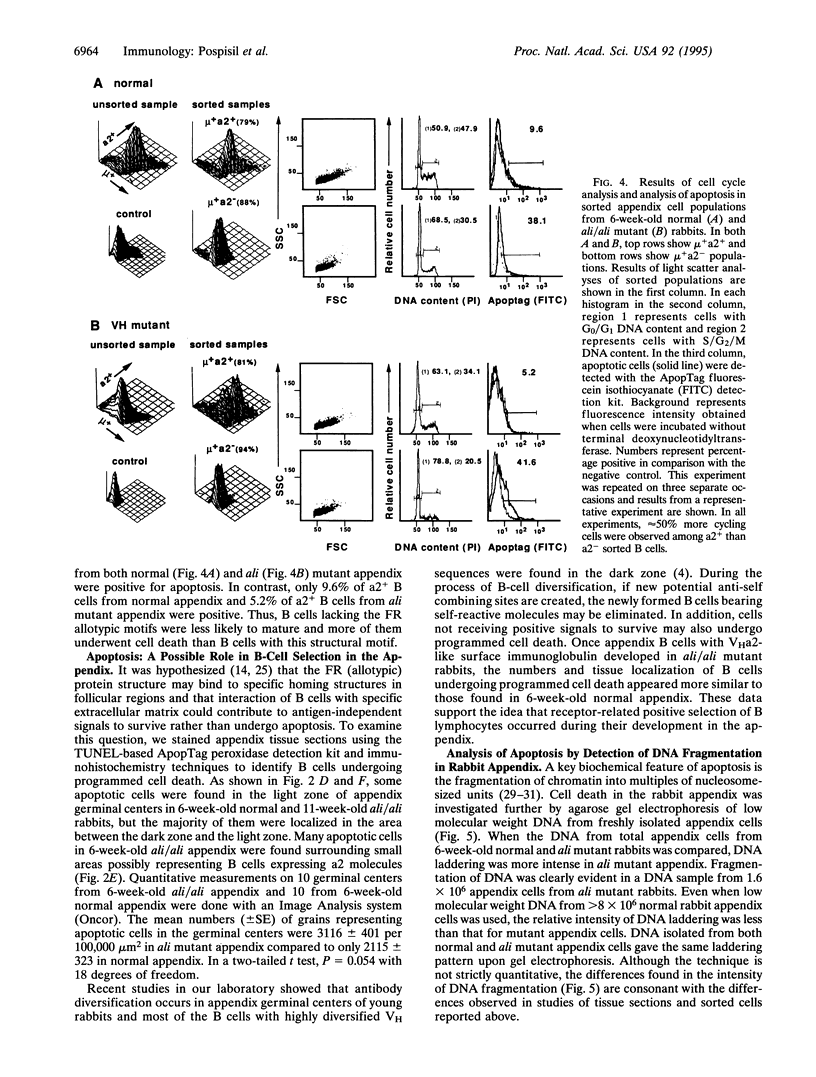
B cells with a rearranged heavy-chain variable region VHa allotype-encoding VH1 gene segment predominate throughout the life of normal rabbits and appear to be the source of the majority of serum immunoglobulins, which thus bear VHa allotypes. The functional role(s) of these VH framework region (FR) allotypic structures has not been defined. We show here that B cells expressing surface immunoglobulin with VHa2 allotypic specificities are preferentially expanded and positively selected in the appendix of young rabbits. By flow cytometry, a higher proportion of a2+ B cells were progressing through the cell cycle (S/G2/M) compared to a2- B cells, most of which were in the G1/G0 phase of the cell cycle. The majority of appendix B cells in dark zones of germinal centers of normal 6-week-old rabbits were proliferating and very little apoptosis were observed. In contrast, in 6-week-old VH-mutant ali/ali rabbits, little cell proliferation and extensive apoptosis were observed. Nonetheless even in the absence of VH1, B cells with a2-like surface immunoglobulin had developed and expanded in the appendix of 11-week-old mutants. The numbers and tissue localization of B cells undergoing apoptosis then appeared similar to those found in 6-week-old normal appendix. Thus, B cells with immunoglobulin receptors lacking the VHa2 allotypic structures were less likely to undergo clonal expansion and maturation. These data suggest that "positive" selection of B lymphocytes through FR1 and FR3 VHa allotypic structures occurs during their development in the appendix.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allegrucci M., Newman B. A., Young-Cooper G. O., Alexander C. B., Meier D., Kelus A. S., Mage R. G. Altered phenotypic expression of immunoglobulin heavy-chain variable-region (VH) genes in Alicia rabbits probably reflects a small deletion in the VH genes closest to the joining region. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5444–5448. doi: 10.1073/pnas.87.14.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegrucci M., Young-Cooper G. O., Alexander C. B., Newman B. A., Mage R. G. Preferrential rearrangement in normal rabbits of the 3' VHa allotype gene that is deleted in Alicia mutants; somatic hypermutation/conversion may play a major role in generating the heterogeneity of rabbit heavy chain variable region sequences. Eur J Immunol. 1991 Feb;21(2):411–417. doi: 10.1002/eji.1830210224. [DOI] [PubMed] [Google Scholar]
- Becker R. S., Suter M., Knight K. L. Restricted utilization of VH and DH genes in leukemic rabbit B cells. Eur J Immunol. 1990 Feb;20(2):397–402. doi: 10.1002/eji.1830200224. [DOI] [PubMed] [Google Scholar]
- Boehme S. A., Lenardo M. J. Propriocidal apoptosis of mature T lymphocytes occurs at S phase of the cell cycle. Eur J Immunol. 1993 Jul;23(7):1552–1560. doi: 10.1002/eji.1830230724. [DOI] [PubMed] [Google Scholar]
- Chen H. T., Alexander C. B., Mage R. G. Characterization of a rabbit germ-line VH gene that is a candidate donor for VH gene conversion in mutant Alicia rabbits. J Immunol. 1995 Jun 15;154(12):6365–6371. [PubMed] [Google Scholar]
- Chen H. T., Alexander C. B., Young-Cooper G. O., Mage R. G. VH gene expression and regulation in the mutant Alicia rabbit. Rescue of VHa2 allotype expression. J Immunol. 1993 Apr 1;150(7):2783–2793. [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C., Fadok V. A., Sellins K. S. Apoptosis and programmed cell death in immunity. Annu Rev Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- DiPietro L. A., Sethupathi P., Kingzette M., Zhai S. K., Suter M., Knight K. L. Limited repertoire of utilized VH gene segments in a VHa3-allotype-suppressed rabbit. Int Immunol. 1992 May;4(5):555–561. doi: 10.1093/intimm/4.5.555. [DOI] [PubMed] [Google Scholar]
- DiPietro L. A., Short J. A., Zhai S. K., Kelus A. S., Meier D., Knight K. L. Limited number of immunoglobulin VH regions expressed in the mutant rabbit "Alicia". Eur J Immunol. 1990 Jun;20(6):1401–1404. doi: 10.1002/eji.1830200629. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay D., Saunders T., Camper S., Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993 Apr 1;177(4):999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow C. C. Transgenic mice and analysis of B-cell tolerance. Annu Rev Immunol. 1992;10:489–518. doi: 10.1146/annurev.iy.10.040192.002421. [DOI] [PubMed] [Google Scholar]
- Kelus A. S., Weiss S. Mutation affecting the expression of immunoglobulin variable regions in the rabbit. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4883–4886. doi: 10.1073/pnas.83.13.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham P. M., Mortari F., Newton J. A., Schroeder H. W., Jr Immunoglobulin VH clan and family identity predicts variable domain structure and may influence antigen binding. EMBO J. 1992 Feb;11(2):603–609. doi: 10.1002/j.1460-2075.1992.tb05092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight K. L., Becker R. S. Molecular basis of the allelic inheritance of rabbit immunoglobulin VH allotypes: implications for the generation of antibody diversity. Cell. 1990 Mar 23;60(6):963–970. doi: 10.1016/0092-8674(90)90344-e. [DOI] [PubMed] [Google Scholar]
- Kurki P., Lotz M., Ogata K., Tan E. M. Proliferating cell nuclear antigen (PCNA)/cyclin in activated human T lymphocytes. J Immunol. 1987 Jun 15;138(12):4114–4120. [PubMed] [Google Scholar]
- Kurki P., Ogata K., Tan E. M. Monoclonal antibodies to proliferating cell nuclear antigen (PCNA)/cyclin as probes for proliferating cells by immunofluorescence microscopy and flow cytometry. J Immunol Methods. 1988 Apr 22;109(1):49–59. doi: 10.1016/0022-1759(88)90441-3. [DOI] [PubMed] [Google Scholar]
- Mage R. G., Bernstein K. E., McCartney-Francis N., Alexander C. B., Young-Cooper G. O., Padlan E. A., Cohen G. H. The structural and genetic basis for expression of normal and latent VHa allotypes of the rabbit. Mol Immunol. 1984 Nov;21(11):1067–1081. doi: 10.1016/0161-5890(84)90117-2. [DOI] [PubMed] [Google Scholar]
- Mage R. G. Rabbit facts and diversification of VH sequences by gene conversion: comments on "A theory of the ontogeny of the chicken humoral immune system: the consequences of diversification by gene hyperconversion and its extension to rabbit". Res Immunol. 1993 Jul-Sep;144(6-7):476–486. doi: 10.1016/0923-2494(93)80143-m. [DOI] [PubMed] [Google Scholar]
- Nemazee D. A., Bürki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989 Feb 9;337(6207):562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- Nemazee D., Buerki K. Clonal deletion of autoreactive B lymphocytes in bone marrow chimeras. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8039–8043. doi: 10.1073/pnas.86.20.8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J. Cellular mechanisms of immunologic tolerance. Annu Rev Immunol. 1983;1:33–62. doi: 10.1146/annurev.iy.01.040183.000341. [DOI] [PubMed] [Google Scholar]
- Sarli G., Benazzi C., Preziosi R., Marcato P. S. Proliferative activity assessed by anti-PCNA and Ki67 monoclonal antibodies in canine testicular tumours. J Comp Pathol. 1994 May;110(4):357–368. doi: 10.1016/s0021-9975(08)80313-1. [DOI] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Hillson J. L., Perlmutter R. M. Structure and evolution of mammalian VH families. Int Immunol. 1990;2(1):41–50. doi: 10.1093/intimm/2.1.41. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. Acquisition of immunologic self-tolerance. Cell. 1989 Jun 30;57(7):1073–1081. doi: 10.1016/0092-8674(89)90044-5. [DOI] [PubMed] [Google Scholar]
- Schwartz R. S., Stollar B. D. Heavy-chain directed B-cell maturation: continuous clonal selection beginning at the pre-B cell stage. Immunol Today. 1994 Jan;15(1):27–32. doi: 10.1016/0167-5699(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Tiegs S. L., Russell D. M., Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993 Apr 1;177(4):1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
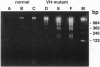
- Weinstein P. D., Anderson A. O., Mage R. G. Rabbit IgH sequences in appendix germinal centers: VH diversification by gene conversion-like and hypermutation mechanisms. Immunity. 1994 Nov;1(8):647–659. doi: 10.1016/1074-7613(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]