Identification and analysis of the maize boron (B) transporter mutant tassel-less1 demonstrated that the primary symptoms of B deficiency are defects in vegetative and reproductive meristems, thus providing an explanation for the reductions in yield observed under B-limited conditions.
Abstract
The element boron (B) is an essential plant micronutrient, and B deficiency results in significant crop losses worldwide. The maize (Zea mays) tassel-less1 (tls1) mutant has defects in vegetative and inflorescence development, comparable to the effects of B deficiency. Positional cloning revealed that tls1 encodes a protein in the aquaporin family co-orthologous to known B channel proteins in other species. Transport assays show that the TLS1 protein facilitates the movement of B and water into Xenopus laevis oocytes. B content is reduced in tls1 mutants, and application of B rescues the mutant phenotype, indicating that the TLS1 protein facilitates the movement of B in planta. B is required to cross-link the pectic polysaccharide rhamnogalacturonan II (RG-II) in the cell wall, and the percentage of RG-II dimers is reduced in tls1 inflorescences, indicating that the defects may result from altered cell wall properties. Plants heterozygous for both tls1 and rotten ear (rte), the proposed B efflux transporter, exhibit a dosage-dependent defect in inflorescence development under B-limited conditions, indicating that both TLS1 and RTE function in the same biological processes. Together, our data provide evidence that TLS1 is a B transport facilitator in maize, highlighting the importance of B homeostasis in meristem function.
INTRODUCTION
Boron (B) is an essential micronutrient that is required for growth and development of vascular plants (Warrington, 1923). B-deficient soils are a widespread and growing problem around the world, resulting in severe defects in plant growth and reduced yield (Shorrocks, 1997; Blevins and Lukaszewski, 1998; Goldbach et al., 2001). Cereals such as maize (Zea mays) and rice (Oryza sativa) are especially sensitive to B deficiency during reproductive development (Struckmeyer et al., 1961; Hu et al., 1996; Shorrocks, 1997; Lordkaew et al., 2011). B is required mainly in developing tissues, as opposed to mature tissues, and the first symptoms of B deficiency are visible in the growing tips of the plant, followed by aborted growth in the root and shoot apices, stunted and narrow leaves, and an increase in axillary bud outgrowth (Sommer and Sorokin, 1928; Lovatt, 1985; Jiao et al., 2005; Krug et al., 2009; Lordkaew et al., 2011). Studies on the cellular effects of B deficiency in the root revealed that the premature cessation of growth of root tips is the result of defects in the root meristem (Dell and Huang, 1997; Goldbach et al., 2001; Yu et al., 2002, 2003; Abreu et al., 2014). Whether similar defects occur in the shoot apical meristem has yet to be determined.
To date, the only established molecular function for B in vascular plants is to cross-link two molecules of the cell wall pectic polysaccharide referred to as rhamnogalacturonan II (RG-II) (Ishii and Matsunaga, 1996; Kobayashi et al., 1996; O’Neill et al., 1996, 2001; Blevins and Lukaszewski, 1998; Bolaños et al., 2004). This cross-linking is believed to be required for the formation of a pectin network composed of RG-II, rhamnogalacturonan I, and homogalacturonan within the wall. Plants or suspension cells grown in B-deficient conditions have reduced amounts of the RG-II dimer together with defects in cell wall organization and properties (Fleischer et al., 1999; Matoh et al., 2000; Ishii et al., 2001; Noguchi et al., 2003; Miwa et al., 2013; Chormova et al., 2014). Similarly, mutants that are unable to synthesize normal RG-II, such as the murus1 mutant of Arabidopsis thaliana, have severe developmental defects similar to B-deficient plants, including short stature, small leaves, or defective pollen (O’Neill et al., 2001; Delmas et al., 2008; Voxeur et al., 2011). Several of these mutants are rescued when grown under high B conditions, indicating that the B-dependent stabilization of RG-II is essential for the structure of the cell wall (Kobayashi et al., 1996; O’Neill et al., 2001, 2004; Reboul et al., 2011; Voxeur et al., 2011).
Additional roles for B have been proposed in other parts of the cell, such as the plasma membrane and the cytoskeleton, although none have been demonstrated unequivocally (Cohen, 1977; Loomis and Durst, 1992; Cakmak and Römheld, 1997; Dell and Huang, 1997; Power and Woods, 1997; Bassil et al., 2004). However, several genes have been found to be regulated by B levels, suggesting that plants possess mechanisms to sense, respond to, and maintain B homeostasis, either directly or indirectly (Kobayashi et al., 2004; Takano et al., 2006; Camacho-Cristóbal et al., 2008; Ozhuner et al., 2013; Abreu et al., 2014). Most notable, however, is the fact that B is proposed to play an important role in organisms lacking cell walls such as zebra fish and mice, suggesting that there are other functions for B that remain unresolved (Lanoue et al., 1998; Bennett et al., 1999; Rowe and Eckhert, 1999).
B is present in the soil, mainly in the form of uncharged boric acid [B(OH)3] (Marschner, 1995). As B is not easily remobilized from mature to newly developing organs, plants need to continually take up B from the soil and deliver it to growing tissues (Raven, 1980; Brown and Shelp, 1997). The identification of influx channels and efflux transporters from Arabidopsis provided the first evidence that directed uptake and transport of B occurs in plants (Noguchi et al., 1997; Takano et al., 2002, 2006; Tanaka et al., 2008). The BOR1 gene was shown to encode an efflux-type transporter, similar to bicarbonate transporters in animal cells (Takano et al., 2002). NIP5;1 and its close relative NIP6;1 encode channel proteins in the aquaporin family that were reported to facilitate the influx of boric acid (Takano et al., 2006; Tanaka et al., 2008). Loss-of-function bor1, nip5;1, and nip6;1 mutants have normal phenotypes under sufficient B conditions, but when B is limited have reduced leaf and root growth, reduced apical dominance, and defects in the inflorescence, including sterility (Noguchi et al., 1997; Takano et al., 2006; Tanaka et al., 2008). These defects have mostly been attributed to impaired cell elongation; however, many of these phenotypes resemble meristem defects, which have not previously been investigated.
Based on the available data and analogy with other nutrient pathways, a model has been developed describing the uptake of B from the soil and subsequent transport to the shoots (Takano et al., 2008; Miwa and Fujiwara, 2010; Miwa et al., 2010; Baxter and Dilkes, 2012). In this model, B diffuses into the root epidermal apoplast from the soil and is then transported into the cytosol by influx proteins localized in the plasma membrane of the epidermis, cortex, and endodermis. B then moves via the symplast through the cells of the stele until it reaches the pericycle, at which point it is transported into the xylem by efflux proteins. Once in the xylem, B is transported to shoot tissue in the transpiration stream (Kohl and Oertli, 1961). Another influx protein may be involved in transfer of B from xylem to phloem and export to sink tissues (Brown and Shelp, 1997; Miwa et al., 2010). By contrast, little is known about the mechanism of B transport within the shoots from the vasculature to more distant cells, such as the shoot apical and inflorescence meristems.
Here, we report the identification and functional characterization of the maize tassel-less1 (tls1) gene. Mutations in tls1 result in plants with reduced or completely absent tassels and small, ball-shaped ears. Additionally, in some environmental conditions, tls1 mutants display vegetative defects, including reduced shoot apical meristem size, reduced stature, small and narrow leaves, and premature arrest of growth. We show that tls1 encodes an aquaporin co-orthologous to B transporters in other species and provide evidence that the TLS1 protein facilitates the influx of boric acid and water. We further show that tls1 mutant tissues have reduced amounts of B and borate cross-linked RG-II and that tls1 growth defects are rescued by exogenous application of B. The results of this study indicate that tls1 mediates the delivery of B to the shoot apex and highlight the importance of directed transport of B for vegetative and reproductive meristem development.
RESULTS
tls1 Mutants Have Defects in Reproductive Development
To identify genes functioning in tassel and ear development, mutant populations generated by ethyl methanesulfonate (EMS) treatment were screened for plants with defects in reproductive development (http://www.maizeinflorescence.org/). Six mutants with either a reduced or completely absent tassel and the same suite of phenotypes as the original tls1-reference (tls1-ref) allele were identified (Albertsen et al., 1993; Leonard et al., 2014) (M. Albertsen, personal communication). The tls1-ref allele had been introgressed into B73 and hence was used for further analyses (unless otherwise stated) and is hereafter referred to as tls1.
In maize, the male inflorescence, or tassel, contains a central rachis, the main spike, with multiple long branches at the base (Figure 1A). The branches and main spike produce short branches consisting of a pair of spikelets, each of which makes two florets enclosed by two glumes. tls1 mutants were characterized by either the complete absence of a tassel, with no structure being present inside the flag leaf (Figure 1B) or a reduced tassel with a short main spike, short branches, and a sparse appearance due to the production of fewer spikelets (Figure 1C, Table 1). In addition to tassel defects, phenotypes in the female inflorescence were observed. tls1 mutants produced ears that were either small and ball shaped (48.6%; Figure 1E), were aborted with no kernels at the tip of the ear shank (45.9%; Figure 1F, arrow), or did not produce an ear (5.5%). In cases where mutants produced spikelets, the plants were fertile and produced viable seed.
Figure 1.
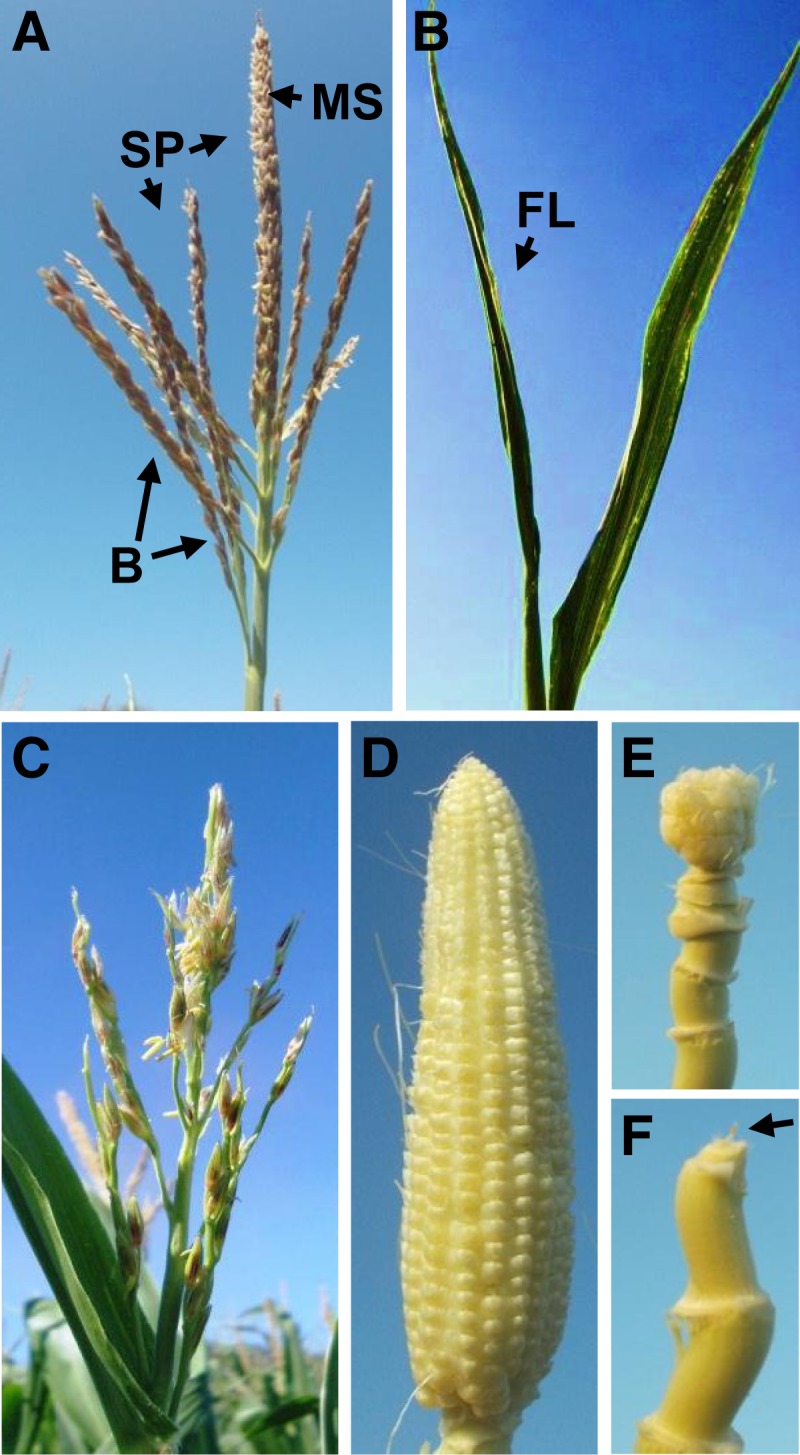
tls1 Tassel and Ear Phenotypes.
(A) Normal tassel with long branches (B) at the base of the main spike (MS). Spikelet pairs (SP) cover the main spike and the branches.
(B) tls1-ref mutant lacking a tassel. When the flag leaf (FL) is unwrapped there is nothing inside.
(C) tls1-ref mutant with a reduced main spike and short branches with fewer spikelets.
(D) Normal ear (with silks removed).
(E) tls1-ref mutant with a small, ball-shaped ear and long shank.
(F) tls1-ref mutant with a completely aborted ear (arrow) at the top of the shank.
[See online article for color version of this figure.]
Table 1. Quantification of Tassel Phenotypes of tls1 Mutants Grown in HI.
| Normal | tls1-ref | |
|---|---|---|
| Length main spike (cm) | 21.13 ± 0.71 | 14.69 ± 1.68* |
| Branch number | 7.46 ± 0.26 | 7.45 ± 0.38 |
| Branch length (cm) | 14.24 ± 0.39 | 10.53 ± 0.55* |
| No. of spikelets/cm | 9.11 ± 0.40 | 5.63 ± 0.76* |
Each measurement is shown as mean ± se. For all measurements, n = 10. *P < 0.01.
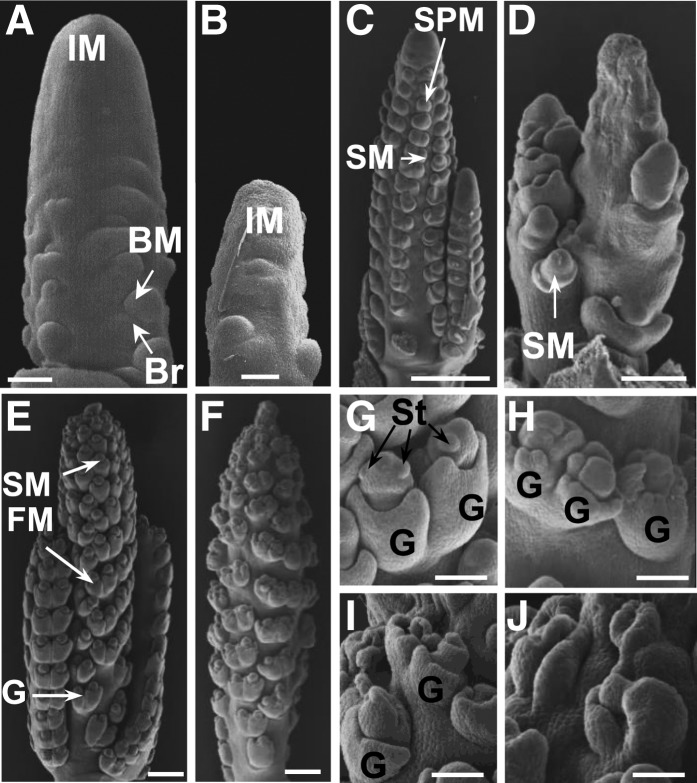
To better understand the development of the tls1 phenotype, immature tassels of plants grown in the greenhouse where the phenotype was less severe than in the field were examined by scanning electron microscopy. In maize, multiple types of axillary meristems are produced from the inflorescence meristem (IM) to give rise to the branches, spikelets, florets, and floral organs of the tassel (McSteen et al., 2000; Tanaka et al., 2013). At stages immediately following the transition to reproductive growth when the tassel was ∼1 to 2 mm long, normal tassels consisted of an apical IM that produced branch meristems in the axils of suppressed bracts (Figure 2A). In tls1 mutants, the IM appeared smaller in size, and fewer branch meristems were present (Figure 2B). At later stages of development (∼4 to 5 mm), normal tassels had several long branches and the IM produced spikelet-pair meristems (SPMs), which produced pairs of spikelet meristems (SMs) (Figure 2C). In tls1 mutants, the IM appeared to have collapsed, and fewer SPMs and SMs were present, causing the formation of barren patches (Figure 2D). In more mature tassels (∼7 to 8 mm) when normal IM had finished producing SPMs and begun to terminate (Figure 2E), tls1 mutants frequently had a sparse appearance due to a reduced number of SMs (Figure 2F). At this stage in normal tassels, the floral meristems produced by the SM gave rise to the floral organs (Figure 2G). In tls1 mutants, few floral meristems were observed, and those that did form appeared defective with few stamen primordia visible (Figures 2H to 2J). Often, only glumes were observed that were either empty or fused into tubular structures (Figures 2I and 2J). These results suggest that the defects observed in tls1 mutant tassels are due to early defects in apical and axillary meristems in the inflorescence.
Figure 2.
Scanning Electron Microscopy Analysis of tls1 Tassels.
(A), (C), (E), and (G) Normal siblings grown in the greenhouse.
(B), (D), (F), and (H) to (J) tls1-ref mutants grown in the greenhouse.
(A) and (B) 1- to 2-mm stage tassels just after the floral transition.
(A) Normal tassel producing branch meristems (BM) in the axils of bract (Br) primordia.
(B) The apical IM of the tls1-ref mutant tassel is shorter than normal.
(C) and (D) 4- to 5-mm stage tassels.
(C) Normal tassel producing SPMs at the tip and pairs of SMs at the middle and base.
(D) Extreme example of tls1-ref mutant. The IM is collapsed and few SMs are produced.
(E) and (F) 7- to 8-mm stage tassels.
(E) Normal tassel has finished producing SPM. SMs are producing glumes (G) and floral meristems (FM).
(F) Weak example of tls1-ref mutant phenotype. The tassel has a sparse appearance due to the production of fewer spikelet pairs.
(G) to (J) Spikelets from 7- to 8-mm stage tassels.
(G) Close-up of normal spikelet pair showing the formation of stamen (St) primordia in the upper floret.
(H) to (J) tls1-ref mutant spikelet pairs showing abnormal glumes and aberrant organ primordia formation in the floral meristems.
Bars = 50 μm in (A) and (B), 500 μm in (C) to (F), and 250 μm in (G) to (J).
tls1 Mutants Have Defects in Vegetative Development
tls1 mutants grown in the greenhouse or grown in the field in Hawaii (HI) did not display any obvious vegetative defects compared with normal siblings (Figure 3A). However, dramatic differences in plant height, tiller number, and leaf blade size were observed in addition to premature termination of growth (Figures 3B and 3C, Table 2), when seed from the same ear was planted in the field in Missouri (MO). In MO field-grown tls1 mutants, germination occurred normally with the first three to four leaves exhibiting no visible phenotype (Supplemental Figures 1A and 1B). However, starting with leaf 4 or 5, the leaves became significantly shorter and narrower (Figure 3C, Table 2) with the phenotypes becoming more severe with each subsequent leaf (Supplemental Figures 1A to 1C). The leaves often had lesions that became progressively larger and more numerous (Supplemental Figure 1C). MO field-grown mutants typically did not produce more than 12 to 13 leaves (Table 2), and growth terminated ∼3 weeks after germination with the plants senescing soon afterwards in hot weather (Supplemental Figure 1D) and later in cooler conditions (Figure 3B). Therefore, MO field-grown tls1 mutants exhibit progressive defects in leaf development appearing around the time of the floral transition.
Figure 3.
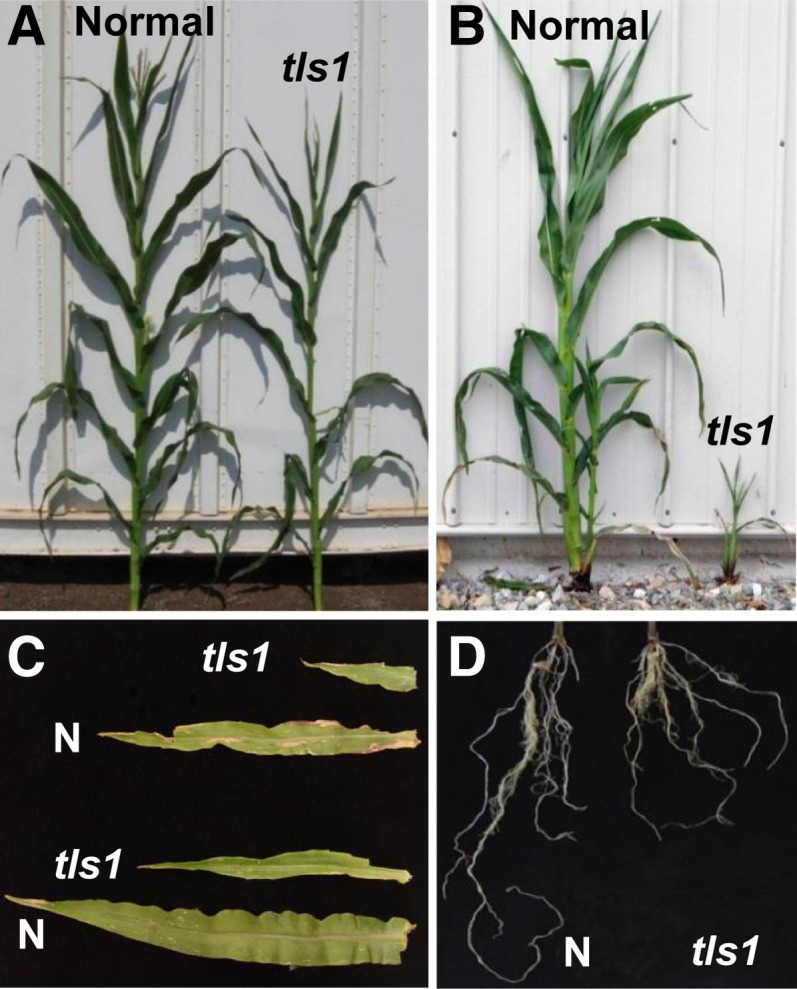
tls1 Vegetative Phenotypes.
(A) Normal sibling and tls1-ref mutant plants grown in the winter nursery in HI.
(B) Normal and tls1-ref mutant plants grown in the summer nursery in MO.
(C) Blades from leaf 5 (bottom) and leaf 6 (top) from normal (N) and tls1-ref mutant plants grown in MO at 3 weeks old.
(D) The roots from 2-week-old normal and tls1-ref mutant plants.
[See online article for color version of this figure.]
Table 2. Quantification of Vegetative Phenotypes in 3-Week-Old tls1 Mutants Grown in MO.
| Normal | tls1-GN5 | tls1-ref | |
|---|---|---|---|
| Plant height (cm) | 25.98 ± 0.93 | 22.99 ± 2.98 | 8.26 ± 0.36* |
| No. of leaves | 19.2 ± 0.46 | 13.9 ± 0.98* | 12.2 ± 0.44* |
| Leaf 5 width (cm) | 2.94 ± 0.54 | 2.21 ± 1.60* | 1.28 ± 1.27* |
| Leaf 5 length (cm) | 27.89 ± 0.67 | 21.54 ± 2.52 | 10.19 ± 0.83* |
| Tiller/axillary bud no. | 0.08 ± 0.07 | 2.53 ± 0.40* | 3.50 ± 0.37* |
Each measurement is shown as mean ± se. For normals, n = 15; tls1-GN5, n = 14; tls1-ref, n = 18. *P < 0.01.
Histological Analysis of tls1 Meristem Defects
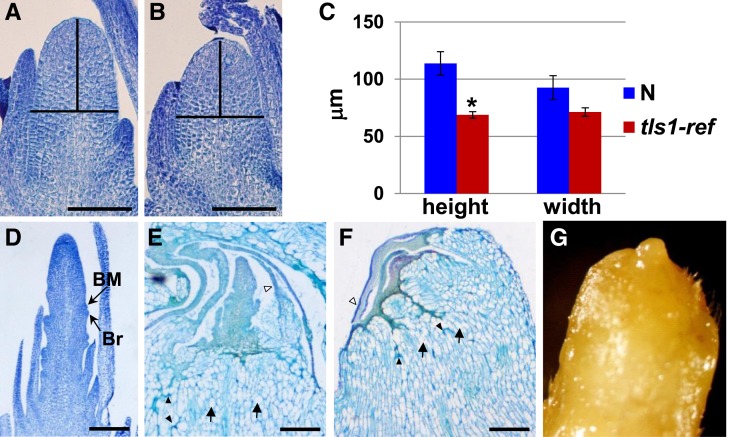
The progressive nature of the leaf defects observed in MO field-grown tls1 mutants, along with the increased tiller outgrowth, were indicative of a defect in the shoot apical meristem (SAM). Therefore, SAMs from MO field-grown tls1 mutants were examined for defects in morphology and size. SAMs were collected from plants 8 to 12 d after germination, prior to the appearance of a visible phenotype. Longitudinal sectioning of the SAMs revealed that the morphology of the SAM appeared normal but smaller (Figures 4A and 4B). Quantification revealed that the height of the apical dome was significantly shorter indicating a reduction in SAM size (Figure 4C). The defect in SAMs was more apparent at 10 d (Figure 4C; P < 0.01) than at 8 d (Supplemental Figure 2; P < 0.05), suggesting that the meristem defect was also progressive.
Figure 4.
Analysis of Vegetative and Transition Stage Meristems in MO-Grown Plants.
(A) and (B) Longitudinal sections of vegetative meristems from 10-d-old plants stained with Toluidine Blue O.
(A) Normal sib.
(B) tls1-ref.
(C) Quantification of vegetative SAM height and width from 10-d-old plants. n = 8. *P < 0.01. Bars represent se.
(D) to (F) Longitudinal sections from immature tassels from 25-d-old plants.
(D) Normal sibling that has produced an immature tassel with branch meristem in the axils of bract primordia.
(E) tls1-ref immature tassel that appears to have arrested shortly after the transition to reproductive growth.
(F) tls1-ref shoot apex that failed to undergo the transition to reproductive growth. Arrows in (E) and (F) indicate examples of highly vacuolated cells, black arrowheads indicate regions of cell collapse, and white arrowheads indicate necrotic leaves.
(G) Stereomicroscope picture of the apex of a dissected tls1-ref plant with a similar phenotype to (F).
Bars = 50 μm in (A) and (B) and 500 μm in (D) to (F).
The transition to reproductive growth occurred 20 to 25 d after germination in the field, with 1- to 2-mm transition stage tassels observed in apices from normal plants (Figure 4D). At this stage, the tls1 mutant phenotype was visible in the leaves, and in some cases, an abnormal immature tassel was visible (Figure 4E). In more extreme cases, structures resembling SAMs that had aborted prior to undergoing the reproductive transition were observed (Figures 4F and 4G). While removing leaves, it was noted that the innermost leaves were often abnormally shaped and fused around the shoot apex. In addition, necrosis of the leaves and stem was commonly seen (Figures 4E and 4F). Necrosis in the leaves appeared to be progressive, as the innermost immature leaves were found to be the most severely affected, followed by necrosis and premature senescence of the entire plant (Supplemental Figures 1C and 1D). In addition, the cells in the stem appeared to be highly vacuolated or collapsed (Figures 4E and 4F). In summary, histological analysis of tls1 mutants grown in the field in MO indicated that the first apparent defects were a reduction in SAM size visible soon after planting, followed by abortion of the SAM, necrosis of the immature leaves, and vacuolation of the cells in the stem, which occurred before or soon after the time that normal plants transitioned to reproductive development.
tls1 Mutants Have Defects in Root Development
Although plants grown in the greenhouse under normal B conditions did not exhibit any visible shoot defects, when roots were examined, it was observed that the root system was shorter in tls1 mutants (Figure 3D; Supplemental Figure 3A). Quantification revealed that nodal roots were shorter in tls1 mutants than in normal siblings (Supplemental Figure 3B). Transverse sectioning of nodal roots revealed that tls1 mutants have thinner cell walls in the xylem and a reduced Casparian strip compared with normal siblings (Supplemental Figures 3C and 3D). Additionally, cells in the tls1 mutants were less uniform in shape, particularly in the pith, and appeared to have reduced wall integrity, as the cells were often damaged or ruptured (Supplemental Figure 3D), similar to the defects seen during shoot development. These results suggest that tls1 plays a role in both root and shoot development. Although the roots of tls1 mutants were shorter, this defect did not appear to cause general nutritional defects in tls1 mutants as the leaves did not exhibit chlorosis, and there was no reduction of the major nutrients nitrogen, phosphorous, or potassium in the leaves (Supplemental Table 1).
Positional Cloning of tls1
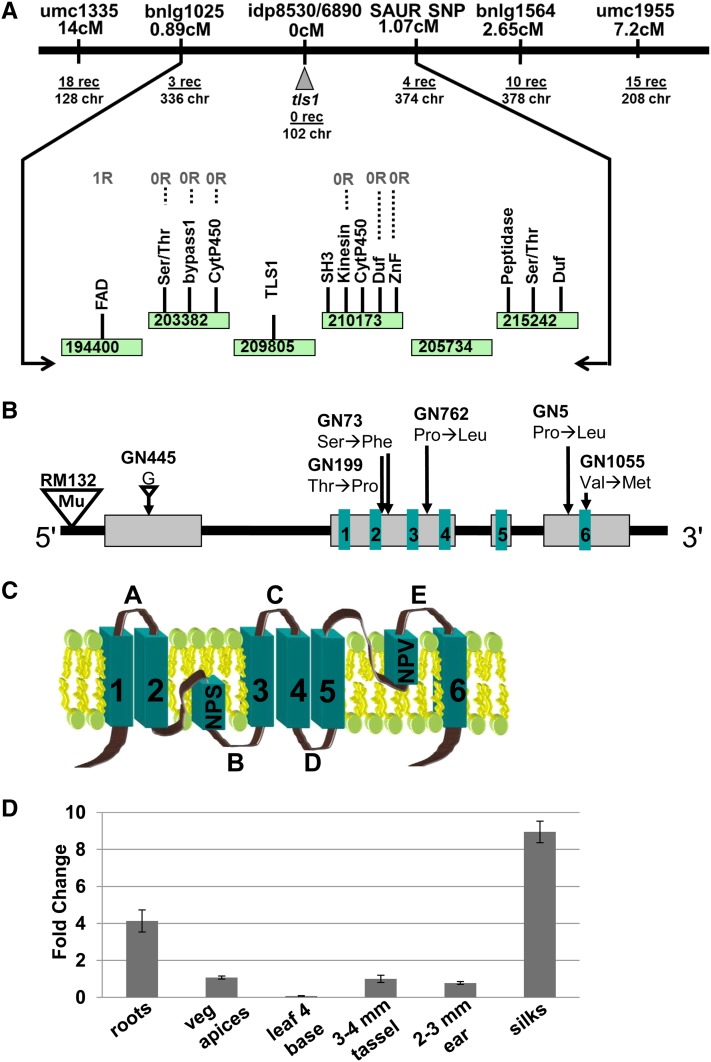
The six EMS-induced tls1 alleles were mapped to bin 7 on the long arm of chromosome 1 using bulked segregant analysis, indicating a more proximal location than what had previously been reported using BA translocation mapping and restriction fragment length polymorphism analysis (Albertsen et al., 1993). Fine mapping of these six populations using publicly available simple sequence repeat (SSR) markers indicated linkage to a region between markers bnlg1025 and bnlg1564. Further fine mapping with single nucleotide polymorphism (SNP) markers derived from genes in the region narrowed the tls1-containing region to five BACs encompassing 13 genes (Figure 5A). All 13 genes were sequenced in multiple EMS-induced tls1 mutant alleles and compared with sequences from parental backgrounds. The six EMS-induced tls1 alleles were found to have nonsynonymous mutations in the coding region of a gene encoding the major intrinsic protein NIP3;1 (Figure 5B). An additional allele identified from the RescueMu population had a Mu insertion in the 5′ untranslated region (Figure 5B). Attempts to amplify any part of the tls1 coding region (AGPv2 Chromosome 1: 223,842,177-223,846,120) from the tls1-ref allele failed but sequences immediately upstream (1: 223,840,370-223,841,707) and downstream (1: 223,846,013-223,847,483) could be amplified in both B73 and tls1-ref mutants. Thus, the tls1-ref allele is likely caused by an ∼4-kb deletion that includes the entire tls1 coding region but does not affect any other genes, as demonstrated in a recent paper (Leonard et al., 2014). In summary, the identification of eight tls1 alleles with mutations in the maize NIP3;1 confirms that tls1 encodes the major intrinsic protein NIP3;1.
Figure 5.
Positional Cloning of tls1.
(A) A genetic map of the tls1 region after fine mapping with publicly available markers and SNPs identified in genes in the region. BACs are represented as rectangles (green in online version).
(B) Model of the tls1 gene structures and tls1 alleles. Gray boxes represent exons and black lines indicate introns. Boxes 1 to 6 (green in online version) represent the location of the transmembrane domains. Small triangle represents a deletion, while large triangle indicates a Mutator (Mu) insertion.
(C) Model of the TLS1 protein structure. A to E indicate loop domains and 1 to 6 indicate transmembrane domains. NPS and NPV are conserved motifs in the core predicted to function in specificity.
(D) Quantitative RT-PCR analysis of tls1 expression in different tissues. The y axis represents the fold change in expression relative to tassels. Bars represent se.
[See online article for color version of this figure.]
Quantitative real-time RT-PCR performed in roots, leaves, vegetative apices, immature tassels, and ears showed that the tls1 transcript was present in all tissues with the highest expression detected in silks (Figure 5D). Comparison to publicly available RNA-seq expression data (eFB browser and qTeller) (Li et al., 2010; Sekhon et al., 2011) was consistent with this broad expression pattern (Supplemental Figures 4A to 4C).
tls1 Encodes an Aquaporin and Is Co-Orthologous to the NIP5;1 Boron Transporter in Arabidopsis
The tls1 gene encodes a major intrinsic protein, a member of the aquaporin channel protein family (Maurel, 2007; Hove and Bhave, 2011). Sequence analysis revealed that tls1 falls in the plant-specific NOD26-like intrinsic proteins (NIP) subfamily of aquaporins, which function in the transport of water and other small uncharged molecules, such as glycerol and urea (Chaumont et al., 2001). NIPs, like other aquaporins, contain six transmembrane domains connected by two intracellular and three extracellular loops, and cytoplasmic N- and C-terminal extensions (Figure 5C). Aquaporins also contain the dual NPA motif and the aromatic/arginine (ar/R) motif, which have amino acid residues involved in regulating which molecules can pass through the channel (Figure 5C; Supplemental Figure 5) (Wallace and Roberts, 2004, 2005; Kosinska Eriksson et al., 2013).
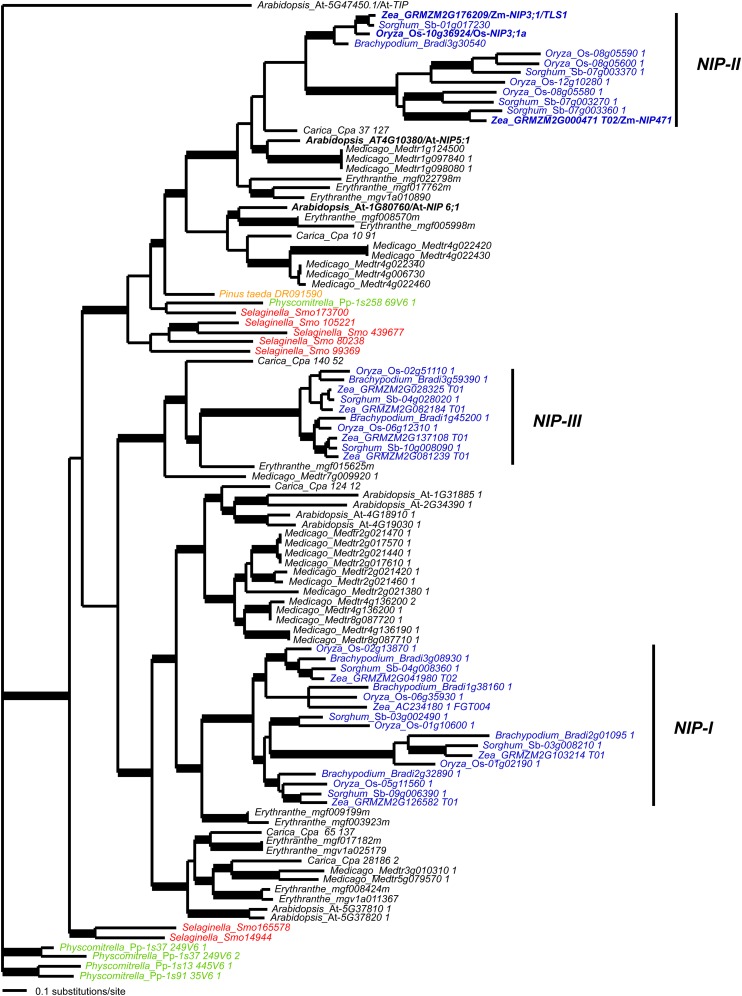
Bayesian phylogenetic analysis of maize tls1 and similar NIPs from other plant species revealed that tls1 falls in a well-supported (>0.95 posterior probability) monocot-specific clade along with rice NIP3;1a (Figure 6) (Hanaoka et al., 2014; Leonard et al., 2014). This grass tls1 clade is sister to another well-supported grass clade containing four rice sequences, three sorghum (Sorghum bicolor) sequences, and the maize NIP471 sequence. Although sampling is limited, this phylogeny estimates that the grass tls1 and maize NIP471 clades are likely the product of a gene duplication event within monocots and potentially at the base of the grass family. The two grass clades are sister to a clade of eudicot sequences including Arabidopsis NIP5;1. The tls1/At-NIP5;1 clade is sister to a eudicot clade containing Arabidopsis NIP6;1, and the combined tls1/At-NIP5;1 and At-NIP6;1 clade is sister to the gymnosperm Pinus taeda DR091590 sequence. This pattern of relationships suggests that the Arabidopsis NIP5;1 and NIP6;1 clades were produced from a duplication event near the base of flowering plants and that co-orthologs of the At-NIP6;1 duplication have been lost in monocots. Hence, of the Arabidopsis genes, tls1 is most closely related phylogenetically to NIP5;1.
Figure 6.
Bayesian Consensus Phylogram of the NIP Subfamily of Aquaporins.
The position of the monocot NIP-I, NIP-II, and NIP-III clades are indicated. Bold branches, ≥0.95 posterior probability; black, eudicots; blue, monocots; green, mosses; orange, gymnosperms; red, lycophytes.
Alignment of the TLS1 protein sequence to orthologs from other plant species showed that TLS1 shares 74 and 63% identity with Arabidopsis NIP5;1 and NIP6;1, respectively, and 90% identity with rice NIP3;1a (Supplemental Figure 5) (Hanaoka et al., 2014; Leonard et al., 2014). TLS1, At-NIP5;1 and Os-NIP3;1a are conserved at the dual NPA and ar/R motifs (Figure 5C; Supplemental Figure 5; NPS/NPV and AIGR, respectively), which regulate substrate specificity, suggesting that all three proteins may function in the transport of similar molecules. Maize NIP471 shares only 43% identity with other NIP II class proteins and does not share identity within the ar/R region residues or NPA signature as is found with the other B transporters, suggesting that maize NIP471 is likely not involved in the transport of B. We propose that this and the other monocot members in this clade represent a subclass of NIPs that originated by gene duplication either before or at the base of the grasses.
The TLS1 Protein Transports Water and Boric Acid in Xenopus laevis Oocytes
Based on the conservation of functional residues among TLS1 and other known B transporters (Takano et al., 2006; Hanaoka et al., 2014), we hypothesized that tls1 may also function in B and water transport in maize. To test this hypothesis, X. laevis oocytes injected with tls1 cRNA were used to assay the transport capabilities of the TLS1 protein. Water channel function is indicated by the capacity of the oocyte to rapidly take up water from a hypotonic bath solution (Maurel et al., 1993; Dordas et al., 2000; Takano et al., 2006; Tanaka et al., 2008). Mock-injected control oocytes (stabbed with the needle, but not injected with cRNA) showed a linear increase in diameter for the first ∼6 min, after which the rate slowed down considerably (Figure 7A). The minimal swelling in the stabbed control oocytes is likely due to passive diffusion of water across the plasma membrane. TLS1-expressing oocytes, on the other hand, showed an exponential initial rate of swelling, which then became linear after the first ∼6 min (Figure 7A). Therefore, it was concluded from these results that the TLS1 protein can facilitate the transport of water.
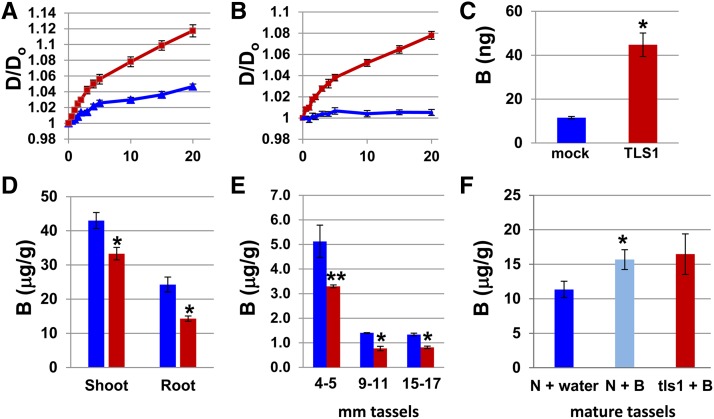
Figure 7.
Oocyte Swelling Assays and Analysis of Boron Concentration.
(A) Rate of oocyte swelling in water in mock injected controls (blue line) versus TLS1-expressing oocytes (red line) (n = 7).
(B) Rate of oocyte swelling in boric acid in mock injected versus TLS1-expressing oocytes (n = 7).
(C) B content in 10 TLS1-expressing oocytes versus 10 mock-injected controls measured by ICP-MS (n = 6).
(D) to (F) B content shown as μg of B/g of sample in various tissues and stages of development. Blue bars, normal; red bars, tls1 mutant.
(D) B content in 2.5-week-old shoots (n = 10) and roots (n = 7) from normal and tls1-ref plants grown in the greenhouse determined by ICP-OES.
(E) B content in developing normal and tls1-ref tassels grown in the greenhouse determined by ICP-MS. For 4- to 5-mm stage tassels, eight tassels were pooled for each replicate (n = 2), while for 9- to 11-mm tassels, three tassels were pooled for each replicate (n = 2). For 15- to 17-mm tassels, each tassel was measured individually for each replicate (n = 3).
(F) B content in mature tassels from plants treated with either water or B (n = 8). *P < 0.01; **P < 0.05. For (A) to (F), bars represent se.
To test the ability of the TLS1 protein to transport B in the form of boric acid, oocytes were observed while the full-strength standard ND96 incubation medium was modified by replacing the 96 mM NaCl with 200 mM boric acid. As the osmolarity of the two solutions is the same, any oocyte swelling is presumed to be due to accumulation of boric acid in the oocyte leading to the concomitant osmotic uptake of water. The diameter of the stabbed control oocytes was found to fluctuate slightly over a 20-min period (Figure 7B), likely due to initial osmotic fluxes incurred when the new solution was encountered. However, the diameter of the control oocytes was never significantly greater after 20 min than at time 0. In contrast, the TLS1-expressing oocytes showed significant swelling, similar to that observed for oocytes placed in a hypotonic solution (Figure 7B). These results indicate that TLS1 is able to transport boric acid under these conditions.
To confirm that B is being transported from the extracellular buffer solution to the inside of the oocytes, the B content of TLS1-expressing and stabbed control oocytes was determined after incubation in standard ND96 medium containing 1 mM boric acid. The TLS1-expressing oocytes contained 4 times as much B as the mock-injected oocytes (Figure 7C). These results provide additional support for the hypothesis that the TLS1 protein facilitates the transport of boric acid into Xenopus oocytes.
tls1 Mutants Have Reduced B and Reduced Dimerization of RG-II in Inflorescences
If the physiological in planta role of tls1 is to facilitate the transport of B, as indicated by the X. laevis oocyte assays, then B concentration should be altered in tls1 mutants. Therefore, we measured the amount of B in the roots and shoots of 2.5-week-old greenhouse-grown plants using inductively coupled plasma optical emission spectroscopy (ICP-OES). A significant reduction in the amount of B was observed in both the roots (∼42%) and shoots (∼24%) of tls1 mutants compared with normal siblings (Figure 7D). The amount of B in immature tassels from tls1 mutants and normal siblings at different developmental stages was also measured using inductively coupled plasma mass spectrometry (ICP-MS). The plants were grown in the greenhouse where the phenotype was not severe and tassels were produced (see Figures 2D and 2F). In 4- to 5-mm, 9- to 11-mm, and 15- to 17-mm stage tassels, tls1 mutant tassels were found to have a 36 to 45% reduction in B levels compared with normal siblings (Figure 7E). The reduced B concentration in the shoots, roots, and tassels of tls1 mutants provides further evidence that tls1 plays a role in B transport in maize.
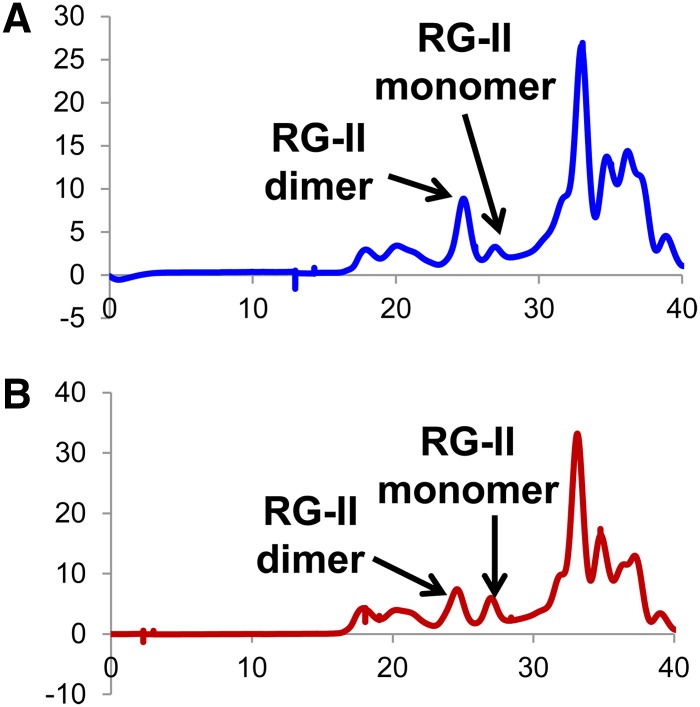
In vascular plants, B is known to cross-link the cell wall pectic polysaccharide RG-II. Thus, the cell walls of immature inflorescences and mature leaves from tls1 mutants and normal siblings were treated with endopolygalacturonase and the solubilized material analyzed by size-exclusion chromatography to determine the ratio of the borate cross-linked RG-II dimer (d-RG-II-B) and RG-II monomer (mRG-II) (O’Neill et al., 1996, 2004). Walls from pooled immature (2.2 to 5.5 cm) tls1 mutant tassels or ears grown in the greenhouse showed on average a 30% reduction in the percentage of d-RG-II-B (and concomitant increase in mRG-II) compared with normal siblings (Figures 8A and 8B). This reduction is comparable to the reduced amounts of B found in immature tassels (Figure 7E). The reduction in d-RG-II-B in tls1 mutant tassels and ears suggests that the defects observed in the mutants are, at least in part, due to defects in the cell wall. By contrast, mature flag leaves from tls1 mutants grown in HI had levels of d-RG-II-B (>90% dimer) similar to normal siblings (Supplemental Figure 6). As this degree of RG-II dimerization occurs when sufficient B is present (Brown and Hu, 1997; Ishii et al., 2001; Noguchi et al., 2003), it is possible that even though B was reduced in tls1 leaves, there was still enough present to allow for cross-linking of >90% of the RG-II in leaves (Figure 7D). However, the overall lower amounts of B seen in normal tassels, combined with the greater B deficit in tls1 tassels (Figure 7E), prevented complete RG-II cross-linking, indicating a greater sensitivity to low B levels in the tassel.
Figure 8.
Size-Exclusion Chromatography Profiles of the Material Solubilized by EPG Treatment of the Destarched AIR from Normal and tls1 Mutant Tassels.
The elution positions of RG-II dimer and monomer are shown. The spectra are from normal immature tassels (A) and tls1-ref mutant immature tassels (B) grown in the greenhouse. These spectra are representative of the results obtained for two biological replicates of tassels and one replicate of ears, with each replicate containing a pool of 6 to 18 inflorescences.
[See online article for color version of this figure.]
Addition of B Rescues the tls1 Mutant Phenotype
As HI soils, particularly in Molokai, have high B levels (Hue et al., 1988; Chatterjee et al., 2014) and MO soils may be B deficient (Shorrocks, 1997), we tested the hypothesis that tls1 mutants had a more severe phenotype in MO than HI due to low soil B content in MO. We determined amounts of B at several field sites in MO that were all found to be B deficient (0.41 ppm ± 0.05 se, n = 6), as it is recommended to apply B to field maize when soil levels are <0.75 ppm (Heckman, 2009). Thus, tls1 plants grown in the MO field were watered every other day with a 4.8% borax solution. On off days, the plants were given water only in order to ensure that enough water was available to allow B uptake from the soil. Another set of tls1 plants were planted in a nearby field and treated with water only daily. Normal siblings from both the water-treated and B-treated populations showed no differences in plant height or number of leaves after 4 weeks of growth (Supplemental Table 2). tls1 mutants receiving the water-only treatment began to show a leaf phenotype at ∼2 weeks and had completely arrested growth by 4 weeks (Figure 9A), consistent with what had been observed previously in tls1 mutants grown in MO. However, tls1 mutants watered with B were mostly indistinguishable from normal siblings after 2 weeks. After 4 weeks, the B-treated tls1 mutants were taller and had more leaves than water-treated tls1 mutants (Figure 9B). Subsequent quantification of height and leaf number revealed that B-treated tls1 plants were indistinguishable from normal siblings (Supplemental Table 2). Approximately 10% of B-treated tls1 mutants displayed weak leaf phenotypes, including narrow leaves or lesions, on the 4th or 5th leaf (Figure 9B, arrows). However, by week 4, all subsequent leaves appeared normal (Figure 9B). After 9 weeks, all water treated tls1 mutants were completely senesced, while the B-treated tls1 mutants were healthy, although the mutants were slightly shorter than normal siblings (136 cm ± 4.34 versus 153 cm ± 1.99, P < 0.03). After flowering, B-treated tls1 mutants produced tassels with no significant difference in tassel length or spikelet density compared with either B-treated or water-treated normal siblings, although both B-treated tls1 mutants and B-treated normal siblings had fewer branches than water-treated normal siblings (Figure 9C; Supplemental Table 2). tls1 mutants treated with B also produced normal looking ears with the same number of ear rows as normal siblings, although ear length was slightly reduced (Figure 9D; Supplemental Table 2). To confirm that B was taken up by the plants, B content of mature tassels was determined and was found to be similar in B-treated tls1 mutants to B-treated normal siblings, which were both slightly increased on average compared with water-treated normal plants (Figure 7F). These results indicate that the vegetative phenotype observed in tls1 mutants grown in MO is due to B deficiency and not water deficiency, as the application of water could not rescue the vegetative phenotype. They further indicate that the reproductive phenotype is also due to B deficiency and that HI soils have sufficient B to sustain vegetative growth but not to sustain reproductive growth.
Figure 9.
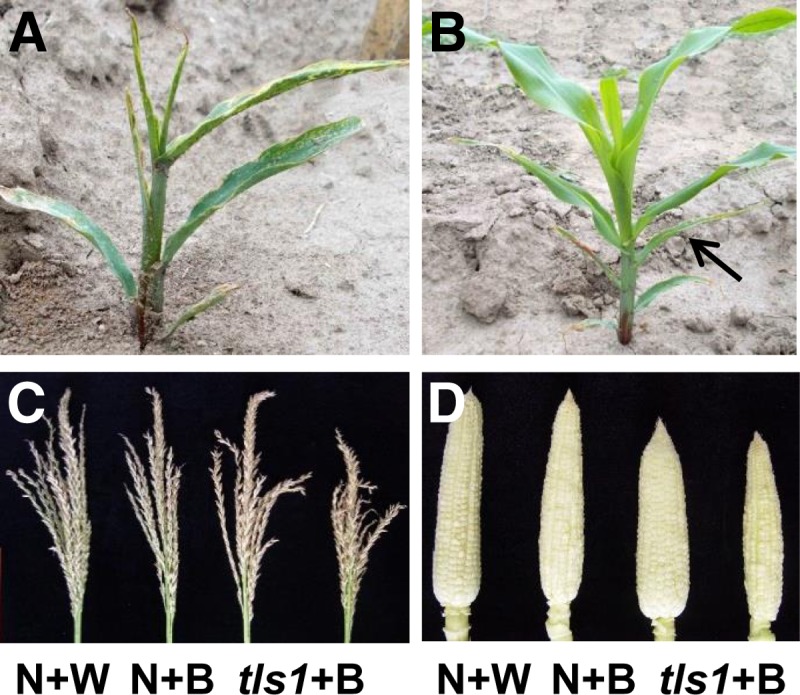
Rescue of the tls1 Phenotype by Application of Exogenous B.
(A) and (B) Twenty-eight days after germination.
(A) tls1-ref mutant in MO treated with water only.
(B) tls1-ref mutant in MO treated with water + B.
(C) and (D) Nine weeks after germination.
(C) Mature tassels from normal siblings treated with water only (N+W) or boron (N+B) and tls1-ref mutants rescued by application of boron.
(D) Ears from 9-week-old plants treated with water or boron.
[See online article for color version of this figure.]
Mutations in B Influx and B Efflux Genes Result in Nonallelic Noncomplementation
The movement of B from the soil to the shoots likely requires the coordination of passive B influx into the cell, which facilitates B uptake into the plant, and active B efflux out of the cell, which is essential for xylem loading and long-range movement of B (Takano et al., 2002, 2006, 2010). To dissect the effects of impaired B influx and efflux in maize, double mutants were generated between tls1 and rotten ear (rte), the putative B efflux transporter in maize (Chatterjee et al., 2014). rte has developmental phenotypes similar though less severe than tls1, with the exception that rte is sterile. Both mutants are completely recessive in all growing conditions (Supplemental Table 3; Chatterjee et al., 2014). As the tls1-ref phenotype is so severe in MO, the weaker tls1-GN5 allele was used in which some plants have phenotypes similar to those observed in tls1-ref grown in HI (Table 2; Supplemental Figure 7). By using a weaker tls1 allele, it was hypothesized that any enhanced effects that may occur when both B influx and efflux were altered would be discernible. Plants heterozygous for tls1-GN5 were crossed with plants heterozygous for rte-1, the F1 population was self-crossed in HI and the tls1;rte F2 segregating population was planted in MO in two different field seasons. The tls1 rte double mutants were similar to the most severe phenotype of tls1-GN5 single mutants in MO (n = 16; Figure 10A). As the tls1 phenotype is more severe than the rte phenotype, this result is not unexpected, but the fact that the phenotype was not significantly enhanced in the double mutant, even though a weak allele was used, could be interpreted as evidence that the two genes act in the same pathway.
Figure 10.

Genetic Interaction between tls1 and rte.
(A) Normal and mutant plants grown in MO showing typical single and double mutant phenotypes.
(B) Normal, tls1/+;rte/+, and rte mutant tassels. tls1 and double mutant plants do not make tassels in MO.
(C) Normal, tls1/+;rte/+, rte, and tls1 mutant ears grown in MO.
[See online article for color version of this figure.]
More significant evidence that tls1 and rte act in the same pathway was obtained from the nonallelic noncomplementation exhibited in MO. Although the F1 generation planted in HI did not exhibit a mutant phenotype, when the F1 plants were grown in MO, approximately one-quarter of the plants displayed reproductive defects, including small, sparse tassels, and short ears (Figures 10B and 10C). Attempts to self-cross these plants failed, as the few spikelets produced were empty and silks did not grow well. It was suspected that these plants were tls1/+;rte/+ and that a dosage effect was being exhibited in MO but not in HI. The F2 population planted in MO was therefore genotyped, and plants belonging to the tls1/+;rte/+ class were identified and determined to be segregating in the expected ratio (Supplemental Table 4) and to have the same phenotype observed in the F1 population in MO. Quantification of the tassel and ear phenotypes revealed that the tls1/+;rte/+ plants grown in MO had a significantly shorter main spike and reduced spikelet number in the tassel, as well as shorter ears and reduced kernel row number (n = 10; Figures 10B and 10C, Table 3). As the double heterozygous plants still contain one functional copy of both tls1 and rte, the tassel and ear phenotypes observed are likely due to gene dosage effects resulting in reduced B transport capacity. These data provide further evidence that tls1 and rte function together to transport B and maintain B homeostasis in maize.
Table 3. Quantification of tls1/+;rte/+ Tassel and Ear Phenotypes in MO.
| Normal | tls1/+;rte/+ | |
|---|---|---|
| Length main spike (cm) | 22.35 ± 0.95 | 10.70 ± 1.08* |
| No. of spikelets/cm | 10.20 ± 3.89 | 5.10 ± 9.96* |
| Ear length (cm) | 14.60 ± 0.51 | 6.05 ± 0.64* |
| Ear row no. | 12.60 ± 0.31 | 8.40 ± 0.88* |
Each measurement is shown as mean ± se. n = 10. *P < 0.01.
DISCUSSION
We provide evidence that tls1 plays a critical role in vegetative and reproductive development in maize by facilitating the transport of B to the shoot apex. tls1 mutants consistently display defects early in tassel and ear development, but under low B conditions, they also display defects in vegetative development, including a smaller SAM, progressively narrow leaves, and premature termination of growth. The tls1 gene encodes an aquaporin that can transport B and water, and tls1 mutants have reduced B levels and are rescued by application of sufficient B. Additionally, tls1 immature inflorescences were found to have reduced RG-II cross-linking in the cell walls, indicating that the phenotypes observed are in part due to reduced cell wall integrity. Our data reveal that tls1 functions as a B transporter in maize and reveal a critical role for B in the function of meristems during vegetative and reproductive development.
The tls1 Mutant Phenotype Is Dependent on B Availability
Our ability to rescue the vegetative and reproductive phenotypes of tls1 mutants with the application of excess B, but not water, indicates that the defects observed in tls1 mutants are due to B deficiency. The near-complete rescue of a B transporter mutant by application of excess B may be due to increased diffusion of B under high B concentrations or is indicative of the presence of other B transporters in the plant that are B induced or have low affinity for B. The extreme phenotypic variation seen in the tls1 mutant phenotype when grown in different conditions is likely due to differences in B availability in the soil. tls1 mutants grown in HI, where soil B levels are relatively high, show only reproductive phenotypes, whereas mutants grown in the B-deficient soils in MO also display vegetative phenotypes. Our results show that early stages of tassel and ear development are particularly sensitive to B deficiency and are in agreement with the observation that more B is required during reproductive development in cereals (Shorrocks, 1997; Blevins and Lukaszewski, 1998).
The distribution of B throughout the plant is achieved through the coordination of B influx and B efflux through cells (Miwa and Fujiwara, 2010; Miwa et al., 2010). Mutations in either tls1 or rte, the maize ortholog of BOR1 (Chatterjee et al., 2014), result in phenotypes similar to those observed in wild-type maize grown under B-deficient conditions (Eltinge, 1936; Struckmeyer et al., 1961; Lordkaew et al., 2011), supporting the hypothesis that tls1 and rte function in B homeostasis. The nonallelic noncomplementation exhibited by tls1/+;rte/+ plants can be interpreted as evidence that the two genes interact in the same pathway (Hawley and Gilliland, 2006; Baker and Braun, 2008). This nonallelic noncomplementation was only observed in B-limited conditions in MO, indicating that it may be dependent on B availability. The fact that changes in B levels correlate with severity of the tls1 and rte phenotypes provides further evidence that the primary function of tls1 and rte are in the movement of B in maize.
The Role of B in Maize Shoot Development
Historically, the cellular effects of B deficiency on growth have been studied mainly in the root (Sommer and Sorokin, 1928; Eltinge, 1936; Lovatt, 1985; Shorrocks, 1997; Lordkaew et al., 2011). Research on several species, including maize, has shown that B deficiency results in impaired cell elongation and division in the meristematic tips of primary and lateral roots (Loomis and Durst, 1992; Dell and Huang, 1997; Goldbach et al., 2001; Baluška et al., 2002). Low B has also been well documented to cause many defects in shoots, including reduced stature, aberrant leaf formation, impaired inflorescence development, and reduced yield (Eltinge, 1936; Struckmeyer et al., 1961; Lovatt, 1985; Shorrocks, 1997; Lordkaew et al., 2011). However, a close examination of the causal defects responsible for low B-induced shoot inhibition has not received much attention. We show that low B conditions cause early defects in development, particularly in the SAM and the IM.
Our developmental studies of the maize tls1 mutant identified a progressive reduction in the height of the SAM, prior to an obvious whole plant growth phenotype, indicating that the SAM is particularly sensitive to reduced B transport. We propose that during seedling development when the B requirement is low in grasses, a minimal B level is sufficient to maintain normal cellular functions in the SAM (Shorrocks, 1997). As the plant continues to grow, B becomes depleted from the SAM in tls1 mutants, resulting in a progressively smaller SAM, which leads to the production of fewer and increasingly narrower leaves until the plant ceases growth prematurely. In cases where B levels are sufficient for vegetative growth, but not for reproductive growth when the requirement for B in the grasses increases dramatically (Blevins and Lukaszewski, 1998), tls1 mutants succeed in transitioning but display reproductive defects, including short main spike and branches in the tassel and ball-shaped or aborted ears. Analysis of early reproductive development in tls1 mutants by scanning electron microscopy and histology reveals smaller IMs and fewer and aberrant axillary meristem production, which indicates IM defects similar to those observed in the SAM. Therefore, we propose that meristems are particularly sensitive to B deficiency and that many of the phenotypes observed in mature tissues are, at least in part, due to defects in meristem function.
Cell Wall Integrity Is Critical for Meristem Function
Meristems function to maintain a stem cell population and regulate organogenesis (Barton, 2010); therefore, coordination of new cell wall formation is critical for meristem function (Baskin, 2005; Cosgrove, 2005). There has been a recent resurgence of interest in the role of mechanical forces in the regulation of meristem function (Hamant et al., 2008; Mirabet et al., 2011; Robinson et al., 2013). Atomic force microscopy and other methods indicate that the central zone of the meristem has a rigid cell wall, while organ primordia in the peripheral zone have more elastic walls (Milani et al., 2011; Peaucelle et al., 2011; Kierzkowski et al., 2012). Modification of another pectic polysaccharide, homogalacturon, contributes to this difference in rigidity, as demethylesterification of homogalacturon is correlated with loosening of the cell wall during organ formation (Peaucelle et al., 2008, 2011). Reduction of RG-II cross-linking alters the tensile strength of cell walls in stems and hypocotyls (Ryden et al., 2003) and hence may have similar effects in meristems.
Cell wall structure, particularly the amount, distribution, and type of pectins present, varies greatly among plant families (Carpita and Gibeaut, 1993), and it has been shown that a strong correlation exists between pectin content and B requirement (Hu et al., 1996; Matoh et al., 1996). The low B requirement during vegetative development in maize is likely due to the low pectin content in the primary cell wall of grasses, which increases during reproductive development (Carpita and Gibeaut, 1993; Hu et al., 1996; Matoh et al., 1996). We hypothesize that this high requirement for B during reproductive development may be due to the increased primary cell wall materials required for the large number of axillary meristems produced shortly after the transition to reproductive growth (McSteen et al., 2000; Tanaka et al., 2013). This is supported by the observation that B concentration is higher in 4- to 5-mm tassels, when much of the tissue is meristematic, compared with later stages when the tassel is in the process of differentiating (9 to 11 mm) or has completely differentiated (15 to 17 mm) and no additional meristems are being produced.
The majority of B in the plant is associated with the cell wall (Hu and Brown, 1994; Matoh et al., 1996), where over 90% of the RG-II in the plant cell wall is cross-linked by B (Brown and Hu, 1997; O’Neill et al., 2001, 2004; Matsunaga et al., 2004). In dicots, plants grown in B-deficient conditions exhibit reduced RG-II cross-linking, but the effect of B deficiency on RG-II formation in cereals has not previously been examined (Fleischer et al., 1999; Matoh et al., 2000; Ishii et al., 2001; Noguchi et al., 2003; Miwa et al., 2013; Chormova et al., 2014). We found than in normal mature leaves >90% of the RG-II is cross-linked with borate, as had previously been shown for dicots (Ishii et al., 2001; Noguchi et al., 2003), but in normal immature inflorescences grown in the greenhouse only 65 to 87% of the RG-II was cross-linked. That reproductive structures have <90% RG-II dimers may explain why reproductive development is more sensitive to B deficiency in cereals (Hu et al., 1996; Shorrocks, 1997; Lordkaew et al., 2011). Moreover, there was no discernible decrease in RG-II cross-linking in mature tls1 leaves, while immature tassels and ears showed an ∼30% reduction in the percentage of d-RG-II-B compared with normal siblings. Although <50% cross-linking of RG-II is typically needed to manifest significant growth defects in dicots (O’Neill et al., 2001; Noguchi et al., 2003), we propose that the reduction in RG-II dimer formation observed in tassels and ears may be sufficient to elicit growth defects in maize. As B cross-linking occurs during synthesis of RG-II, it has been proposed that only cells that are synthesizing cell walls at the time of deficiency will be affected (Chormova et al., 2014). This may explain why meristem-rich tissues that contain many cells undergoing division and expansion, such as immature tassels and ears, are more strongly affected than mature leaves. The defects in tls1 mutants seen later in development, such as altered cell shape and reduced cell integrity, as visible by histology, are also likely due to defects in the cell wall integrity (Fleischer et al., 1998; Ryden et al., 2003; Ahn et al., 2006; Koshiba et al., 2009; Reboul and Tenhaken, 2012; Oiwa et al., 2013). However, the fact that the leaves have defects despite having normal cross-linking of RG-II suggests that B is also involved in other processes occurring after organogenesis has occurred (Bassil et al., 2004).
Although many of the phenotypes in the tls1 mutants can be explained by defects in the cell wall, other explanations are possible. The loss of apical dominance seen in tls1 mutants could be due to the premature arrest of the SAM; alternatively, this phenotype could be due to interactions between B and other factors including altered homoeostasis of other minerals (Baxter, 2009) and/or hormones, such as auxin. Historically, there are many reports of interactions between B and auxin dating back to the 1960s (Dyar and Webb, 1961), but the exact nature of the relationship remains unclear. The fact that the tls1 mutant displays many phenotypes similar to known auxin mutants, such as the auxin biosynthetic mutant vanishing tassel2, suggests that B and auxin could intersect at certain points of development (Phillips et al., 2011). Indeed, a correlation between low B and increased auxin and reduced cytokinin has been reported in the root (Martín-Rejano et al., 2011; Abreu et al., 2014). The identification of the tls1 mutant in maize will be an invaluable tool for understanding the nature of the relationship between B and hormones in the future. The tls1 tassel and ear phenotypes (seen in HI) are very similar to the tls3 mutant, which is caused by thiamine deficiency (Woodward et al., 2010). Thiamine and B are essential for plant growth, and notably both are essential in tissue culture media, along with the plant growth hormones auxin and cytokinin (Murashige and Skoog, 1962). Therefore, the identification of the tls class of mutants highlights the importance of micronutrients in plant development, particularly in the meristem.
METHODS
Plant Materials
The tls1-ref allele was obtained from Marc Albertson (DuPont Pioneer, Johnston, IA) (Albertsen et al., 1993). tls1-GN5, GN73, GN199, GN445, GN762, GN1055, and rte-GN128 were generated by EMS mutagenesis in defined genetic backgrounds by the Maize Inflorescence Project (http://www.maizeinflorescence.org/) and the seed provided by the Maize Genetics Cooperation Stock Center. The tls1-RM132 insertion allele was identified in the Rescue Mu population (http://dev.maizegdb.org/rescuemu-phenotype.php) and the seed obtained from the Maize Coop Stock Center.
Phenotypic Analysis of Mature Phenotypes
All tassel and ear analyses were performed using the tls1-ref allele, which was backcrossed to B73 at least four times. Plants were grown either in the greenhouse in Columbia, MO or State College, PA to analyze early stages of tassel development (4 to 6 weeks) or in the field in Molokai, HI for mature tassel and ear analysis. Plants in the greenhouse were grown under long-day conditions with an average temperature of 25°C. Plants were germinated in Promix soil with 0.1% iron sulfate and slow release fertilizer (DynaGreen) and were subsequently treated with 0.2% Peter’s 20-20-20 Solution (Scott) and 0.2% iron sulfate every week. Plants were genotyped by PCR to identify mutants, either with a linked marker (idp8530) or by the presence or absence of a band using primers within the deleted region of the tls1-ref allele (MIP-5A/5B2; Supplemental Table 5). For field-grown plants, the data are representative of one field season, although similar phenotypes were quantified over multiple field seasons. Main spike length was measured as the distance from the top-most long branch to the tip of the main spike. Spikelets/centimeter was measured as the number of total spikelets on the main spike divided by the length of the main spike, except for tassels from B rescue assay, which were measured as the number of spikelets in the middle 10 cm of the main spike. Ear phenotypes were determined by opening the first ear of each mature plant. A ball ear was scored as any ear containing at least one spikelet, while an aborted ear was scored as an ear that had no spikelets at the end of the shank.
Analyses of vegetative phenotypes were performed on 3-week-old MO-grown plants using the tls1-ref allele backcrossed to B73 at least four times or the tls1-GN5 allele backcrossed to B73 at least three times. Plant height was measured as the distance from the ground to the top of the whorl, and leaf number was quantified by marking every 4th leaf after germination, followed by dissection of the plant. Leaf width was determined by measuring three points along the approximate middle of the blade of leaf 5 and recording the largest value. Leaf length was determined by measuring the blade from the auricle to the tip. Tiller/axillary bud number was determined by removing all the leaves and counting any bud that was greater than 1 cm.
For double mutant analysis, tls1 rte segregating F2 families were planted in two different fields in MO in 2012, 2 weeks apart. For this analysis, the tls1-GN5 allele was crossed with rte-1 both in hybrid genetic backgrounds as the two mutants had not been introgressed into the same genetic background. Plants were genotyped using cleaved amplified polymorphic sequence markers for both tls1-GN5 and rte-1 (MIP-5A/5B and RE-StyI-F2/RE-T25-R10; Supplemental Table 5) and cut with FseI and StyI, respectively. For quantification of significant differences, Student’s two-tailed t tests were performed in Microsoft Excel.
Histology and Scanning Electron Microscopy
Plants used for quantification of vegetative shoot apical meristem size were grown in the field in MO and were genotyped, as plants look normal at this stage, and collected 8 to 12 d and 3 weeks after germination. Fixation, preparation for histology, and staining with Toluidine Blue O were performed as previously described (Wu and McSteen, 2007). Longitudinal sections were analyzed on a Nikon 80i microscope and photographed with a Nikon DM1200F camera. Vegetative meristems were measured using ImageJ software (http://imagej.nih.gov/ij/). For each sample, several sections were measured, with the largest being reported, in order to ensure that the midpoint of the meristem was measured. For root histology, prop roots from 5-week-old plants grown in the greenhouse were fixed, prepared, stained, and imaged as described above.
For scanning electron microscopy analysis of inflorescence meristems, plants were grown in the greenhouse and harvested 4 to 6 weeks after planting in order to capture different stages of development from transition to flowering to differentiation of the IM. Samples were fixed and prepared for scanning electron microscopy as previously described (Wu and McSteen, 2007). Samples were critical point dried, sputter coated with platinum or gold, and analyzed using a Hitachi S-4700 cold field emission scanning electron microscope at the MU Electron Microscopy Core or on a JEOL JSM5400 scanning electron microscope at the Penn State Huck Institutes Electron Microscopy Facility.
Mapping of tls1 EMS-Induced Alleles
For mapping the EMS-induced tls1 alleles, six different mapping populations were used. tls1-GN445, tls1-GN762, and tls1-GN1055 originated in the Mo17 background and were backcrossed to B73 once or twice and selfed. tls1-GN199 and tls1-GN5 originated in A632 and were crossed to Oh43 and selfed twice. tls1-GN73 originated in B73 and was crossed to A619 and selfed twice. From each family, DNA from a pool of 24 to 41 mutants and a separate pool of 24 to 41 normal siblings was extracted as previously described (Chen and Dellaporta, 1994) and sent to Iowa State University for bulked segregant analysis using Sequenom detection of 1000 SNPs across the genome (Liu et al., 2010), which indicated that all of the mutants mapped to bin 1.07-1.08 on the long arm of chromosome 1. To confirm this location, public SSR and insertion deletion polymorphism markers in the region (IBM2 2008 Neighbors map at http://www.maizegdb.org/) were used on individuals from each mapping population to confirm that tls1 mapped to bin 1.07. Fine mapping with public markers using 189 mutant individuals from these six F3 mapping populations indicated that tls1 mapped between bnlg1025 and bnlg1564. After the public mapping resources were exhausted, genes in the region were sequenced to identify SNPs, which enabled tls1 to be further fine mapped to a five BAC region containing 13 genes between FAD-SNP and SAUR-SNP (Supplemental Table 5). Amplification of Zm-NIP3;1 with primers (MIP2A/2B, MIP3A/3B, MIP4A/4B, and MIP5A/5B; Supplemental Table 5) and sequencing identified mutations in all six EMS-induced alleles. The tls1-RM123 allele was not mapped due to an absence of polymorphism but allelism with the tls1-ref allele was confirmed by complementation testing. Amplification with a Mu terminal inverted repeat primer (Mu9242; Supplemental Table 5) together with gene-specific primers and sequencing indicated the insertion of a Mu element in the 5′ untranslated region.
Phylogenetic Analysis
tls1-like genes were identified using the gene family search feature on the Phytozome website (http://www.phytozome.org). Full-length sequences were assembled and translated into conceptual amino acids using Mesquite 2.75 (Maddison and Maddison, 2003) and then aligned using MUSCLE (Edgar, 2004) before being manually adjusted using Mesquite. Nucleotide positions 1 to 435, 1039 to 1254, 1309 to 1509, and 2047 to 2247 (in Supplemental Data Set 1) were considered unalignable and excluded from subsequent analysis. Bayesian phylogenetic analyses of 101 genes of the tls1-like data set were performed according to Christensen and Malcomber (2012) using MrBayes 3.2 (Ronquist and Huelsenbeck, 2003) except that the split frequencies between the two runs was 0.052889. After convergence had been assured, the first 25% of trees were removed and burn-in and clade credibility values estimated using MrBayes. The distantly related Arabidopsis At5g47450/TIP was chosen as an outgroup.
Expression Analysis
RNA was extracted from various tissues from B73 plants using the Machnery-Nagel plant RNA kit. Samples used in the analysis included seedling roots, 1 to 2 cm of leaf tissue from the base of the blade of leaf 4 from 3-week-old plants, vegetative apices (the vegetative meristem including a few leaf primordia and a small amount of stem tissue of the topmost node), 3- to 4-mm immature tassels, 2- to 3-mm immature ears, and silks from 8- to 10-cm ears. cDNA was generated from 1 μg of total RNA for each replicate using the Superscript III first-strand synthesis system (Invitrogen). RT-PCR was performed using the SYBR-green method using the TLS1-RT-F3/TLS1-RT-R2 primers to detect tls1 (Supplemental Table 5). The data were analyzed using the ΔΔCT method with CT threshold values normalized against gap1 (Supplemental Table 5). There were four biological replicates for each tissue and three technical replicates for each sample.
Expression of TLS1 in Xenopus laevis Oocytes
The full-length coding region of tls1 was amplified from cDNA generated from RNA extracted from 4- to 6-mm ears as described above. The full-length tls1 coding region was cloned into the pGEM-T Easy vector (Promega) and the sequence confirmed by DNA sequencing. Next, the full-length coding region was amplified using primers against tls1 containing NcoI and XhoI recognition sites (TLS1-5′-NcoI-F/TLS1-3′-XhoI-R; Supplemental Table 5). Amplicons were digested and cloned into the X. laevis-specific p002 vector (Ludewig et al., 2002), and insertion and orientation was confirmed by DNA sequencing. cRNA was transcribed from linearized p002 vector containing the tls1 coding region using the mMessage mMachine kit (Ambion). X. laevis oocytes were harvested, maintained, and injected as described (Osawa et al., 2006; Pike et al., 2009) with modifications; oocytes were defoliculated for 30 to 90 min and injected with 46 ng cRNA on the day after isolation. The ND96 Ringer incubation solution contained 96 mM NaCl, 2 mM KCl, 1 mM MgCL2, 1.8 mM CaCl2, 5 mM HEPES, 2.5 mM sodium pyruvate, 10 μL/mL streptomycin sulfate, and 50 μL/mL gentamicin sulfate, pH 7.4.
Swelling Assays and Boron Uptake in Oocytes
For swelling assays, oocytes were incubated in 48-well or six-well plates, one oocyte per well, and analyzed 1 to 3 d after injection. To minimize injury to the oocytes, they were not transferred from their incubation wells for swelling assays. Instead, solutions were changed within each well while under the microscope. To measure swelling in the presence of water, full-strength incubation ND96 was diluted 70% with milliQ water (Rivers et al., 1997; Wallace and Roberts, 2005). To measure swelling in the presence of B, the incubation ND96 was removed and replaced with boron-ND96. Boron-ND96 consisted of standard ND96 without sodium pyruvate and antibiotics and with the NaCl replaced by 200 mM boric acid (Wallace and Roberts, 2005; Takano et al., 2006; Tanaka et al., 2008). To measure the rate of swelling, oocytes were visualized using an Olympus SZ61 dissecting microscope outfitted with a Nikon Coolpix 5000 camera in one of the oculars. Images of the oocytes were taken every 30 s for the first 2 min, every minute from 2 to 5 min and every 5 min until 20 min had elapsed. The diameters of the oocytes were measured using ImageJ software, and the rate of swelling was calculated as the diameter divided by the initial diameter at time 0. The swelling assays were performed a total of seven times with oocytes from two different frogs.
For uptake assays, oocytes were incubated in six-well plates, 10 per well in 5 mL ND96. One to two days after injection, the ND96 was removed and replaced with boron-ND96 containing 1 mM boric acid. After 30 min incubation at room temperature, each 10-oocyte sample was rinsed five times with ice-cold ND96, transferred to a specially cleaned 15-mL tube, weighed, and frozen at −20°C until sampled for elemental analysis using ICP-MS by the MU Research Reactor facility (http://www.murr.missouri.edu/ps_analytical_ICP.php). The oocytes were digested in Teflon digestion vessels with nitric acid, heated to 190°C in a microwave system, and analyzed on an Axiom high-resolution ICP-MS run at a nominal resolution of 6000. The uptake assay was repeated twice, using three replicates of mock and TLS1 oocytes with similar results using a newly extracted batch of oocytes from different frogs each time.
Measurement of Plant B Levels
For B content of shoots and roots, normal sib and tls1-ref mutants were grown in the greenhouse, genotyped by PCR as described above, and harvested after 2.5 weeks at the V4-V5 stage. The plants were cut at the base of the stem and the entire aboveground portion was used for analysis. The tips of the leaves were removed prior to analysis, as excess B and other salts accumulate at the tips due to the movement of B in the transpiration stream and guttation (Curtis, 1943; Kohl and Oertli, 1961; Ivanoff, 1963). For roots, the entire root system was used in the analysis. Each replicate contained the shoots or roots of two plants. B content was measured by ICP-OES by the MU Plant and Soil Analysis Facility (http://soilplantlab.missouri.edu/soil/). For B content in B-treated tassels, entire mature tassels were collected ∼2 weeks post pollen shed and were dried and ground (all branch and stem tissue from the bottom branch to the tip plus all spikelets), and B content was measured by ICP-OES by the MU Plant and Soil Analysis Facility. For B content of immature tassels, normal sib and tls1-ref mutants were grown in the greenhouse, genotyped by PCR as described above, and harvested after 6 to 8 weeks to obtain tassels at 4- to 17-mm stages. Meristems were harvested and weighed individually. For analysis of 4- to 5-mm tassels, two replicates of eight tassels were pooled, and for 9- to 11-mm tassels, two replicates of three tassels were pooled for both normal and mutants in order to obtain the minimum mass required for ICP-MS. For 15- to 17-mm tassels, three tassels were analyzed individually. The B content of fresh tassels was measured by ICP-MS by the MU Research Reactor Facility as described above.
Preparation of the Alcohol-Insoluble Residue from Maize Leaves and Inflorescences
Flag leaf tissue was obtained from tls1-ref mutants and normal siblings grown in HI in the winter. In this season, a leaf phenotype was exhibited with mutant flag leaves being narrower and shorter than normal siblings. The freeze-dried flag leaves (1.0 to 2.3 g dry weight, pooled from one to five plants) were ground to a fine powder using a coffee grinder and the powder then suspended in aqueous 80% ethanol (110 mL). The suspensions were transferred to ceramic grinding mill jars (0.3 liters; US Stoneware) containing a mixture of 1/4” × 1/4” and 1/2” × 1/2” Burundum grinding media (US Stoneware). The suspensions were milled for 16 h at 96 rpm and 4°C. The suspensions were filtered through a kitchen strainer to remove the grinding media and then centrifuged for 15 min at 3000g. The alcohol-insoluble resides (AIRs) were then washed with aqueous 80% ethanol (3 × 40 mL), absolute ethanol (2 × 40 mL), and with acetone (1 × 40 mL) and then vacuum dried at room temperature.
The frozen immature tassels and ears from normal and mutant plants (1.0 to 2.0 g fresh weight, pooled from 6 to 18 plants) grown in the greenhouse were cut into small pieces and then suspended in aqueous 80% ethanol (30 mL). The tissues were homogenized using a Polytron blender (Kinematica). The suspensions were then centrifuged for 15 min at 3000g. The AIRs were washed with aqueous 80% ethanol (3 × 40 mL), absolute ethanol (2 × 40 mL), and with acetone (1 × 40 mL) and then vacuum dried at room temperature.
Release of RG-II Using Endopolygalacturonase
Suspensions of the AIR from the leaves, tassels, and ears of normal and mutant plants in 50 mM NaOAC, pH 5.2 (30 mL/g), were destarched for 24 h at 45°C using Spirizyme Excel (3 μL/mg AIR; Novozymes) and Liquozyme SC DS (15 μL/mg AIR; Novozymes). The suspensions were centrifuged and the insoluble residue washed with deionized water (2 × 40 mL). Suspensions of the destarched AIR in 50 mM NaOAc, pH 5.0, were then treated for 24 h at 23°C with homogeneous Aspergillus niger endopolygalacturonase (EPG; 1 unit/500 mg AIR, a gift of Carl Bergman of the Complex Carbohydrate Research Center). The suspensions were centrifuged and the insoluble residues again treated with EPG. The combined EPG-soluble materials were dialyzed (3.5-kD molecular mass cutoff) against several changes of deionized water and freeze dried.
Size-Exclusion Chromatography
Solutions of the EPG-soluble materials in 50 mM ammonium formate, pH 5 (200 μL), were filtered using centrifugal spin-filters (0.45μm Costar) and fractionated on a Superdex-75 HR10/30 column (GE Healthcare) using a Dionex Ultimate 3000 HPLC equipped with a Shodex RI-101 refractive index detector. The column was eluted with 50 mM ammonium formate, pH 5, and calibrated with a mixture (∼1 mg) of the borate cross-linked RG-II dimer and the RG-II monomer obtained from red wine RG-II (Pellerin et al., 1996). In preliminary experiments, the peak eluting in the region for the maize (Zea mays) RG-II dimer was collected and its glycosyl residue composition determined to confirm the presence of RG-II (Pellerin et al., 1996).
B Supplementation
Lines segregating for tls1-ref were planted in 20-foot rows with 30 plants per row in the field in MO and genotyped as described above. For B treatment, the plants in each row were watered with 5 liters of a solution containing 2.4 g/L of soluble B powder containing 62% B2O2 and 20.5% elemental B (Solubor) every other day after two leaves were visible. On days between B treatments, each row was watered with 5 liters of water only. In a nearby but separate field, tls1-ref segregating lines were watered daily with 5 liters of water only.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL databases under accession number GRMZM2g176209 (tls1).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. tls1 Mutant Phenotypes in MO.
Supplemental Figure 2. Analysis of 8-d-Old Vegetative Meristems in MO-Grown Plants.
Supplemental Figure 3. Root Phenotypes from Normal and tls1 Plants.
Supplemental Figure 4. Expression Analysis of tls1 in Various Tissues.
Supplemental Figure 5. Alignment of TLS1 with Closely Related NIPs.
Supplemental Figure 6. Size Exclusion Chromatography Profiles of the Material Solubilized by EPG Treatment of the Destarched AIR from Normal and tls1 Flag Leaves.
Supplemental Figure 7. Image of Normal and tls1-GN5 Plants Grown in MO Showing the Variation Observed in the tls1-GN5 Mutant Phenotype.
Supplemental Table 1. Nutrient Analysis of tls1 Mutant Leaves Measured by ICP-OES.
Supplemental Table 2. Quantification of Phenotypes of B Rescued Plants Grown in MO.
Supplemental Table 3. Chi Square Analysis of tls1.
Supplemental Table 4. Chi Square Analysis of a tls1 × rte F2 Segregating Population.
Supplemental Table 5. Primers Used in This Study.
Supplemental Data Set 1. Alignment Used for Phylogenetic Analysis.
Supplementary Material
Acknowledgments
We thank Dale Blevins (University of Missouri) for his advice on boron physiology and David Mendoza for critical reading of the article. We thank Marc Albertson, Gerry Neuffer, the Maize Inflorescence Project, the RescueMu project, and the Maize Coop Stock Center for generating the tls1 and rte alleles and Lu Gao and Pat Schnable (Iowa State University) for bulked segregant analysis. We thank Jessica Levy, Tom Slewinski, Katherine Suman, and Shelbie Wooten for assistance with phenotypic analysis and mapping. We thank Nathanial Oswald (Hawaiian Research), Chris Browne, Michelle Brooks (MU), W. Scott Harkom, Tony Omeis, and Tom Slewinski (Penn State) for plant care in the greenhouse and the field. We thank Jim Guthrie and David Robertson (MU Research Reactor Analytical Chemistry facility) and Xingyao Wang and Susan Leaver (MU Department of Chemistry) for assistance with ICP-MS analysis and the MU Plant and Soil Analysis Facility for performing the ICP-OES analysis. We thank April Leonard and Bailin Li for advice on B supplementation. We thank Missy Hazen, Ruth Haldeman (Penn State), and Cheryl Jensen (MU) for training with scanning electron microscopy. We thank the Huck Institutes Genomics core facility and MU DNA core facility for DNA sequencing and the University of Missouri–St. Louis for access and computer time on the Grethor parallel processing cluster. This research was supported by a National Science Foundation grant (IOS-0820729/1114484) to P.M., S.T.M., and A.G. Some of this material is based upon work by S.T.M. while serving at the National Science Foundation. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. The authors acknowledge the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy through Grant DE-FG02-12ER16326 (to M.A.O.) for funding the structural studies of maize RG-II.
AUTHOR CONTRIBUTIONS
A.R.D., K.A.P., S.P., M.A.O., J.M., S.T.M., W.G., and P.M. designed the research. A.R.D., K.A.P., S.P., M.A.O., and J.M. performed the research. A.G. provided materials. A.R.D., K.A.P., S.P., M.A.O., J.M., S.T.M., W.G., and P.M. analyzed the data. A.R.D. and P.M. wrote the article with input from all authors.
Glossary
- RG-II
rhamnogalacturonan II
- EMS
ethyl methanesulfonate
- IM
inflorescence meristem
- SPM
spikelet-pair meristem
- SM
spikelet meristem
- SAM
shoot apical meristem
- SSR
simple sequence repeat
- ICP-OES
inductively coupled plasma optical emission spectroscopy
- ICP-MS
inductively coupled plasma mass spectrometry
- SNP
single nucleotide polymorphism
- AIR
alcohol-insoluble reside
- EPG
endopolygalacturonase
Footnotes
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Paula McSteen (mcsteenp@missouri.edu).
Some figures in this article are displayed in color online but in black and white in the print edition.
Online version contains Web-only data.
Articles can be viewed online without a subscription.
References
- Abreu I., Poza L., Bonilla I., Bolaños L. (2014). Boron deficiency results in early repression of a cytokinin receptor gene and abnormal cell differentiation in the apical root meristem of Arabidopsis thaliana. Plant Physiol. Biochem. 77: 117–121. [DOI] [PubMed] [Google Scholar]
- Ahn J.W., Verma R., Kim M., Lee J.Y., Kim Y.K., Bang J.W., Reiter W.D., Pai H.S. (2006). Depletion of UDP-D-apiose/UDP-D-xylose synthases results in rhamnogalacturonan-II deficiency, cell wall thickening, and cell death in higher plants. J. Biol. Chem. 281: 13708–13716. [DOI] [PubMed] [Google Scholar]
- Albertsen M.C., Trimnell M.R., Fox T.W. (1993). Description and mapping of the tassel-less (tls1) mutation. Maize Newsletter 67: 51–52. [Google Scholar]
- Baker R.F., Braun D.M. (2008). Tie-dyed2 functions with tie-dyed1 to promote carbohydrate export from maize leaves. Plant Physiol. 146: 1085–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluška F., Hlavacka A., Šamaj J., Palme K., Robinson D.G., Matoh T., McCurdy D.W., Menzel D., Volkmann D. (2002). F-actin-dependent endocytosis of cell wall pectins in meristematic root cells. Insights from brefeldin A-induced compartments. Plant Physiol. 130: 422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton M.K. (2010). Twenty years on: the inner workings of the shoot apical meristem, a developmental dynamo. Dev. Biol. 341: 95–113. [DOI] [PubMed] [Google Scholar]
- Baskin T.I. (2005). Anisotropic expansion of the plant cell wall. Annu. Rev. Cell Dev. Biol. 21: 203–222. [DOI] [PubMed] [Google Scholar]
- Bassil E., Hu H., Brown P.H. (2004). Use of phenylboronic acids to investigate boron function in plants. Possible role of boron in transvacuolar cytoplasmic strands and cell-to-wall adhesion. Plant Physiol. 136: 3383–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter I. (2009). Ionomics: studying the social network of mineral nutrients. Curr. Opin. Plant Biol. 12: 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter I., Dilkes B.P. (2012). Elemental profiles reflect plant adaptations to the environment. Science 336: 1661–1663. [DOI] [PubMed] [Google Scholar]
- Bennett A., Rowe R.I., Soch N., Eckhert C.D. (1999). Boron stimulates yeast (Saccharomyces cerevisiae) growth. J. Nutr. 129: 2236–2238. [DOI] [PubMed] [Google Scholar]
- Blevins D.G., Lukaszewski K.M. (1998). Boron in plant structure and function. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49: 481–500. [DOI] [PubMed] [Google Scholar]
- Bolaños L., Lukaszewski K., Bonilla I., Blevins D. (2004). Why boron? Plant Physiol. Biochem. 42: 907–912. [DOI] [PubMed] [Google Scholar]
- Brown P.H., Shelp B.J. (1997). Boron mobility in plants. Plant Soil 193: 85–101. [Google Scholar]
- Brown P.H., Hu H. (1997). Does boron play only a structural role in the growing tissues of higher plants? Plant Soil 196: 211–215. [Google Scholar]
- Cakmak I., Römheld V. (1997). Boron deficiency-induced impairments of cellular functions in plants. Plant Soil 193: 71–83. [Google Scholar]
- Camacho-Cristóbal J.J., Herrera-Rodríguez M.B., Beato V.M., Rexach J., Navarro-Gochicoa M.T., Maldonado J.M., González-Fontes A. (2008). The expression of several cell wall-related genes in Arabidopsis roots is down-regulated under boron deficiency. Environ. Exp. Bot. 63: 351–358. [Google Scholar]
- Carpita N.C., Gibeaut D.M. (1993). Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 3: 1–30. [DOI] [PubMed] [Google Scholar]
- Chatterjee M., Tabi Z., Galli M., Malcomber S., Buck A., Muszynski M., Gallavotti A. (2014). The boron efflux transporter ROTTEN EAR is required for maize inflorescence development and fertility. Plant Cell 26: 2962–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont F., Barrieu F., Wojcik E., Chrispeels M.J., Jung R. (2001). Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol. 125: 1206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J., and Dellaporta, S. (1994). Urea-based plant DNA miniprep. In The Maize Handbook, M. Freeling and V. Walbot, eds (New York: Springer-Verlag), pp. 526–538. [Google Scholar]
- Chormova D., Messenger D.J., Fry S.C. (2014). Boron bridging of rhamnogalacturonan-II, monitored by gel electrophoresis, occurs during polysaccharide synthesis and secretion but not post-secretion. Plant J. 77: 534–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A.R., Malcomber S.T. (2012). Duplication and diversification of the LEAFY HULL STERILE1 and Oryza sativa MADS5 SEPALLATA lineages in graminoid Poales. Evodevo 3: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M.S. (1977). Effect of boron on cell elongation and division in squash roots. Plant Physiol. 59: 884–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D.J. (2005). Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 6: 850–861. [DOI] [PubMed] [Google Scholar]
- Curtis L.C. (1943). Deleterious effects of guttated fluids on foliage. Am. J. Bot. 30: 778–782. [Google Scholar]
- Dell B., Huang L. (1997). Physiological response of plants to low boron. Plant Soil 193: 103–120. [Google Scholar]
- Delmas F., Séveno M., Northey J.G.B., Hernould M., Lerouge P., McCourt P., Chevalier C. (2008). The synthesis of the rhamnogalacturonan II component 3-deoxy-D-manno-2-octulosonic acid (Kdo) is required for pollen tube growth and elongation. J. Exp. Bot. 59: 2639–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dordas C., Chrispeels M.J., Brown P.H. (2000). Permeability and channel-mediated transport of boric acid across membrane vesicles isolated from squash roots. Plant Physiol. 124: 1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyar J.J., Webb K.L. (1961). A relationship between boron & auxin in C translocation in bean plants. Plant Physiol. 36: 672–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. (2004). MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltinge E.T. (1936). Effect of boron deficiency upon the structure of Zea mays. Plant Physiol. 11: 765–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer A., O’Neill M.A., Ehwald R. (1999). The pore size of non-graminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiol. 121: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer A., Titel C., Ehwald R. (1998). The boron requirement and cell wall properties of growing and stationary suspension-cultured Chenopodium album L. cells. Plant Physiol. 117: 1401–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach H.E., Yu Q., Wingender R., Schulz M., Wimmer M., Findeklee P., Baluška F. (2001). Rapid response reactions of roots to boron deprivation. J. Plant Nutr. Soil Sci. 164: 173–181. [Google Scholar]
- Hamant O., Heisler M.G., Jönsson H., Krupinski P., Uyttewaal M., Bokov P., Corson F., Sahlin P., Boudaoud A., Meyerowitz E.M., Couder Y., Traas J. (2008). Developmental patterning by mechanical signals in Arabidopsis. Science 322: 1650–1655. [DOI] [PubMed] [Google Scholar]
- Hanaoka H., Uraguchi S., Takano J., Tanaka M., Fujiwara T. (2014). OsNIP3;1, a rice boric acid channel, regulates boron distribution and is essential for growth under boron-deficient conditions. Plant J. 78: 890–902. [DOI] [PubMed] [Google Scholar]
- Hawley R.S., Gilliland W.D. (2006). Sometimes the result is not the answer: the truths and the lies that come from using the complementation test. Genetics 174: 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman, J.R. (2009). Boron: Needs of Soils and Crops in New Jersey. (Rutgers NJAES Cooperative Extension).
- Hove R.M., Bhave M. (2011). Plant aquaporins with non-aqua functions: deciphering the signature sequences. Plant Mol. Biol. 75: 413–430. [DOI] [PubMed] [Google Scholar]
- Hu H., Brown P.H. (1994). Localization of boron in cell walls of squash and tobacco and its association with pectin. Evidence for a structural role of boron in the cell wall. Plant Physiol. 105: 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Brown P.H., Labavitch J.M. (1996). Species variability in boron requirement is correlated with cell wall pectin. J. Exp. Bot. 47: 227–232. [Google Scholar]
- Hue N.V., Hirunburana N., Fox R.L. (1988). Boron status of Hawaiian soils as measured by B sorption and plant uptake. Commun. Soil Sci. Plant Anal. 19: 517–528. [Google Scholar]
- Ishii T., Matsunaga T. (1996). Isolation and characterization of a boron-rhamnogalacturonan-II complex from cell walls of sugar beet pulp. Carbohydr. Res. 284: 1–9. [Google Scholar]
- Ishii T., Matsunaga T., Hayashi N. (2001). Formation of rhamnogalacturonan II-borate dimer in pectin determines cell wall thickness of pumpkin tissue. Plant Physiol. 126: 1698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanoff S.S. (1963). Guttation injuries of plants. Bot. Rev. 29: 202–229. [Google Scholar]
- Jiao X.Y., Zhu Y.G., Jarvis B.C., Quick W.P., Christie P. (2005). Effects of boron on leaf expansion and intercellular airspaces in mung bean in solution culture. J. Plant Nutr. 28: 351–361. [Google Scholar]
- Kierzkowski D., Nakayama N., Routier-Kierzkowska A.L., Weber A., Bayer E., Schorderet M., Reinhardt D., Kuhlemeier C., Smith R.S. (2012). Elastic domains regulate growth and organogenesis in the plant shoot apical meristem. Science 335: 1096–1099. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Matoh T., Azuma J. (1996). Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol. 110: 1017–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Mutoh T., Matoh T. (2004). Boron nutrition of cultured tobacco BY-2 cells. IV. Genes induced under low boron supply. J. Exp. Bot. 55: 1441–1443. [DOI] [PubMed] [Google Scholar]
- Kohl H.C., Oertli J.J. (1961). Distribution of boron in leaves. Plant Physiol. 36: 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba T., Kobayashi M., Matoh T. (2009). Boron nutrition of tobacco BY-2 cells. V. oxidative damage is the major cause of cell death induced by boron deprivation. Plant Cell Physiol. 50: 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosinska Eriksson U., Fischer G., Friemann R., Enkavi G., Tajkhorshid E., Neutze R. (2013). Subangstrom resolution X-ray structure details aquaporin-water interactions. Science 340: 1346–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug B.A., Whipker B.E., Frantz J., McCall I. (2009). Characterization of calcium and boron deficiency and the effects of temporal disruption of calcium and boron supply on pansy, petunia, and gerbera plugs. HortScience 44: 1566–1572. [Google Scholar]
- Lanoue L., Taubeneck M.W., Muniz J., Hanna L.A., Strong P.L., Murray F.J., Nielsen F.H., Hunt C.D., Keen C.L. (1998). Assessing the effects of low boron diets on embryonic and fetal development in rodents using in vitro and in vivo model systems. Biol. Trace Elem. Res. 66: 271–298. [DOI] [PubMed] [Google Scholar]
- Leonard A., et al. (2014). tassel-less1 encodes a boron channel protein required for inflorescence development in maize. Plant Cell Physiol. 55: 1044–1054. [DOI] [PubMed] [Google Scholar]
- Li P., et al. (2010). The developmental dynamics of the maize leaf transcriptome. Nat. Genet. 42: 1060–1067. [DOI] [PubMed] [Google Scholar]
- Liu S., Chen H.D., Makarevitch I., Shirmer R., Emrich S.J., Dietrich C.R., Barbazuk W.B., Springer N.M., Schnable P.S. (2010). High-throughput genetic mapping of mutants via quantitative single nucleotide polymorphism typing. Genetics 184: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis W.D., Durst R.W. (1992). Chemistry and biology of boron. Biofactors 3: 229–239. [PubMed] [Google Scholar]
- Lordkaew S., Dell B., Jamjod S., Rerkasem B. (2011). Boron deficiency in maize. Plant Soil 342: 207–220. [Google Scholar]
- Lovatt C. (1985). Evolution of xylem resulted in a requirement for boron in the apical meristems of vascular plants. New Phytol. 99: 509–522. [Google Scholar]
- Ludewig U., von Wirén N., Frommer W.B. (2002). Uniport of NH4+ by the root hair plasma membrane ammonium transporter LeAMT1;1. J. Biol. Chem. 277: 13548–13555. [DOI] [PubMed] [Google Scholar]
- Maddison, D.R., and Maddison, W.P. (2003). MacClade: Analysis of Phylogeny and Character Evolution. (Sunderland, MA: Sinauer Associates). [Google Scholar]
- Marschner, M. (1995). Mineral Nutrition of Higher Plants. (London, New York: Academic Press). [Google Scholar]
- Martín-Rejano E.M., Camacho-Cristóbal J.J., Herrera-Rodríguez M.B., Rexach J., Navarro-Gochicoa M.T., González-Fontes A. (2011). Auxin and ethylene are involved in the responses of root system architecture to low boron supply in Arabidopsis seedlings. Physiol. Plant. 142: 170–178. [DOI] [PubMed] [Google Scholar]
- Matoh T., Kawaguchi S., Kobayashi M. (1996). Ubiquity of a borate-rhamnogalacturonan II complex in the cell walls of higher plants. Plant Cell Physiol. 37: 636–640. [Google Scholar]
- Matoh T., Takasaki M., Kobayashi M., Takabe K. (2000). Boron nutrition of cultured tobacco BY-2 cells. III. Characterization of the boron-rhamnogalacturonan II complex in cells acclimated to low levels of boron. Plant Cell Physiol. 41: 363–366. [DOI] [PubMed] [Google Scholar]
- Matsunaga T., Ishii T., Matsumoto S., Higuchi M., Darvill A., Albersheim P., O’Neill M.A. (2004). Occurrence of the primary cell wall polysaccharide rhamnogalacturonan II in pteridophytes, lycophytes, and bryophytes. Implications for the evolution of vascular plants. Plant Physiol. 134: 339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C. (2007). Plant aquaporins: novel functions and regulation properties. FEBS Lett. 581: 2227–2236. [DOI] [PubMed] [Google Scholar]
- Maurel C., Reizer J., Schroeder J.I., Chrispeels M.J. (1993). The vacuolar membrane protein gamma-TIP creates water specific channels in Xenopus oocytes. EMBO J. 12: 2241–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSteen P., Laudencia-Chingcuanco D., Colasanti J. (2000). A floret by any other name: control of meristem identity in maize. Trends Plant Sci. 5: 61–66. [DOI] [PubMed] [Google Scholar]
- Milani P., Gholamirad M., Traas J., Arnéodo A., Boudaoud A., Argoul F., Hamant O. (2011). In vivo analysis of local wall stiffness at the shoot apical meristem in Arabidopsis using atomic force microscopy. Plant J. 67: 1116–1123. [DOI] [PubMed] [Google Scholar]
- Mirabet V., Das P., Boudaoud A., Hamant O. (2011). The role of mechanical forces in plant morphogenesis. Annu. Rev. Plant Biol. 62: 365–385. [DOI] [PubMed] [Google Scholar]
- Miwa K., Fujiwara T. (2010). Boron transport in plants: co-ordinated regulation of transporters. Ann. Bot. (Lond.) 105: 1103–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa K., Tanaka M., Kamiya T., Fujiwara T. (2010). Molecular mechanisms of boron transport in plants: involvement of Arabidopsis NIP5;1 and NIP6;1. Adv. Exp. Med. Biol. 679: 83–96. [DOI] [PubMed] [Google Scholar]
- Miwa K., Wakuta S., Takada S., Ide K., Takano J., Naito S., Omori H., Matsunaga T., Fujiwara T. (2013). Roles of BOR2, a boron exporter, in cross linking of rhamnogalacturonan II and root elongation under boron limitation in Arabidopsis. Plant Physiol. 163: 1699–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T., Skoog F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497. [Google Scholar]
- Noguchi K., Ishii T., Matsunaga T., Kakegawa K., Hayashi H., Fujiwara T. (2003). Biochemical properties of the cell wall in the Arabidopsis mutant bor1-1 in relation to boron nutrition. J. Plant Nutr. Soil Sci. 166: 175–178. [Google Scholar]
- Noguchi K., Yasumori M., Imai T., Naito S., Matsunaga T., Oda H., Hayashi H., Chino M., Fujiwara T. (1997). bor1-1, an Arabidopsis thaliana mutant that requires a high level of boron. Plant Physiol. 115: 901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill M.A., Eberhard S., Albersheim P., Darvill A.G. (2001). Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science 294: 846–849. [DOI] [PubMed] [Google Scholar]
- O’Neill M.A., Ishii T., Albersheim P., Darvill A.G. (2004). Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide. Annu. Rev. Plant Biol. 55: 109–139. [DOI] [PubMed] [Google Scholar]
- O’Neill M.A., Warrenfeltz D., Kates K., Pellerin P., Doco T., Darvill A.G., Albersheim P. (1996). Rhamnogalacturonan-II, a pectic polysaccharide in the walls of growing plant cell, forms a dimer that is covalently cross-linked by a borate ester. In vitro conditions for the formation and hydrolysis of the dimer. J. Biol. Chem. 271: 22923–22930. [DOI] [PubMed] [Google Scholar]
- Oiwa Y., Kitayama K., Kobayashi M., Matoh T. (2013). Boron deprivation immediately causes cell death in growing roots of Arabidopsis thaliana (L.) Heynh. Soil Sci. Plant Nutr. 59: 621–627. [Google Scholar]
- Osawa H., Stacey G., Gassmann W. (2006). ScOPT1 and AtOPT4 function as proton-coupled oligopeptide transporters with broad but distinct substrate specificities. Biochem. J. 393: 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozhuner E., Eldem V., Ipek A., Okay S., Sakcali S., Zhang B., Boke H., Unver T. (2013). Boron stress responsive microRNAs and their targets in barley. PLoS ONE 8: e59543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaucelle A., Braybrook S.A., Le Guillou L., Bron E., Kuhlemeier C., Höfte H. (2011). Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr. Biol. 21: 1720–1726. [DOI] [PubMed] [Google Scholar]
- Peaucelle A., Louvet R., Johansen J.N., Höfte H., Laufs P., Pelloux J., Mouille G. (2008). Arabidopsis phyllotaxis is controlled by the methyl-esterification status of cell-wall pectins. Curr. Biol. 18: 1943–1948. [DOI] [PubMed] [Google Scholar]
- Pellerin P., Doco T., Vidal S., Williams P., Brillouet J.M., O’Neill M.A. (1996). Structural characterization of red wine rhamnogalacturonan II. Carbohydr. Res. 290: 183–197. [DOI] [PubMed] [Google Scholar]
- Phillips K.A., Skirpan A.L., Liu X., Christensen A., Slewinski T.L., Hudson C., Barazesh S., Cohen J.D., Malcomber S., McSteen P. (2011). vanishing tassel2 encodes a grass-specific tryptophan aminotransferase required for vegetative and reproductive development in maize. Plant Cell 23: 550–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike S., Patel A., Stacey G., Gassmann W. (2009). Arabidopsis OPT6 is an oligopeptide transporter with exceptionally broad substrate specificity. Plant Cell Physiol. 50: 1923–1932. [DOI] [PubMed] [Google Scholar]
- Power P.P., Woods W.G. (1997). The chemistry of boron and its speciation in plants. Plant Soil 193: 1–13. [Google Scholar]
- Raven J.A. (1980). Short-and long-distance transport of boric acid in plants. New Phytol. 84: 231–249. [Google Scholar]
- Reboul R., Tenhaken R. (2012). An emerging role of pectic rhamnogalacturonanII for cell wall integrity. Plant Signal. Behav. 7: 298–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboul R., Geserick C., Pabst M., Frey B., Wittmann D., Lütz-Meindl U., Léonard R., Tenhaken R. (2011). Down-regulation of UDP-glucuronic acid biosynthesis leads to swollen plant cell walls and severe developmental defects associated with changes in pectic polysaccharides. J. Biol. Chem. 286: 39982–39992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers R.L., Dean R.M., Chandy G., Hall J.E., Roberts D.M., Zeidel M.L. (1997). Functional analysis of nodulin 26, an aquaporin in soybean root nodule symbiosomes. J. Biol. Chem. 272: 16256–16261. [DOI] [PubMed] [Google Scholar]
- Robinson S., Burian A., Couturier E., Landrein B., Louveaux M., Neumann E.D., Peaucelle A., Weber A., Nakayama N. (2013). Mechanical control of morphogenesis at the shoot apex. J. Exp. Bot. 64: 4729–4744. [DOI] [PubMed] [Google Scholar]
- Ronquist F., Huelsenbeck J.P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Rowe R.I., Eckhert C.D. (1999). Boron is required for zebrafish embryogenesis. J. Exp. Biol. 202: 1649–1654. [DOI] [PubMed] [Google Scholar]
- Ryden P., Sugimoto-Shirasu K., Smith A.C., Findlay K., Reiter W.D., McCann M.C. (2003). Tensile properties of Arabidopsis cell walls depend on both a xyloglucan cross-linked microfibrillar network and rhamnogalacturonan II-borate complexes. Plant Physiol. 132: 1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhon R.S., Lin H., Childs K.L., Hansey C.N., Buell C.R., de Leon N., Kaeppler S.M. (2011). Genome-wide atlas of transcription during maize development. Plant J. 66: 553–563. [DOI] [PubMed] [Google Scholar]
- Shorrocks V.M. (1997). The occurrence and correction of boron deficiency. Plant Soil 193: 121–148. [Google Scholar]
- Sommer A.L., Sorokin H. (1928). Effects of the absence of boron and of some other essential elements on the cell and tissue structure of the root tips of Pisum sativum. Plant Physiol. 3: 237–260.1, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struckmeyer B.E., Heikkinen T., Berger K.C. (1961). Developmental anatomy of tassel and ear shoots of corn grown with different levels of boron. Bot. Gaz. 123: 111–116. [Google Scholar]
- Takano J., Miwa K., Fujiwara T. (2008). Boron transport mechanisms: collaboration of channels and transporters. Trends Plant Sci. 13: 451–457. [DOI] [PubMed] [Google Scholar]
- Takano J., Noguchi K., Yasumori M., Kobayashi M., Gajdos Z., Miwa K., Hayashi H., Yoneyama T., Fujiwara T. (2002). Arabidopsis boron transporter for xylem loading. Nature 420: 337–340. [DOI] [PubMed] [Google Scholar]
- Takano J., Tanaka M., Toyoda A., Miwa K., Kasai K., Fuji K., Onouchi H., Naito S., Fujiwara T. (2010). Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proc. Natl. Acad. Sci. USA 107: 5220–5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J., Wada M., Ludewig U., Schaaf G., von Wirén N., Fujiwara T. (2006). The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 18: 1498–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka W., Pautler M., Jackson D., Hirano H.Y. (2013). Grass meristems II: inflorescence architecture, flower development and meristem fate. Plant Cell Physiol. 54: 313–324. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Wallace I.S., Takano J., Roberts D.M., Fujiwara T. (2008). NIP6;1 is a boric acid channel for preferential transport of boron to growing shoot tissues in Arabidopsis. Plant Cell 20: 2860–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voxeur A., Gilbert L., Rihouey C., Driouich A., Rothan C., Baldet P., Lerouge P. (2011). Silencing of the GDP-D-mannose 3,5-epimerase affects the structure and cross-linking of the pectic polysaccharide rhamnogalacturonan II and plant growth in tomato. J. Biol. Chem. 286: 8014–8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace I.S., Roberts D.M. (2004). Homology modeling of representative subfamilies of Arabidopsis major intrinsic proteins. Classification based on the aromatic/arginine selectivity filter. Plant Physiol. 135: 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace I.S., Roberts D.M. (2005). Distinct transport selectivity of two structural subclasses of the nodulin-like intrinsic protein family of plant aquaglyceroporin channels. Biochemistry 44: 16826–16834. [DOI] [PubMed] [Google Scholar]
- Warrington K. (1923). The effect of boric acid and borax on the broad bean and certain other plants. Ann. Bot. (Lond.) 37: 457–466. [Google Scholar]
- Woodward J.B., Abeydeera N.D., Paul D., Phillips K., Rapala-Kozik M., Freeling M., Begley T.P., Ealick S.E., McSteen P., Scanlon M.J. (2010). A maize thiamine auxotroph is defective in shoot meristem maintenance. Plant Cell 22: 3305–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., McSteen P. (2007). The role of auxin transport during inflorescence development in maize (Zea mays, Poaceae). Am. J. Bot. 94: 1745–1755. [DOI] [PubMed] [Google Scholar]
- Yu Q., Baluška F., Jasper F., Menzel D., Goldbach H.E. (2003). Short-term boron deprivation enhances levels of cytoskeletal proteins in maize, but not zucchini, root apices. Physiol. Plant. 117: 270–278. [Google Scholar]
- Yu Q., Hlavacka A., Matoh T., Volkmann D., Menzel D., Goldbach H.E., Baluška F. (2002). Short-term boron deprivation inhibits endocytosis of cell wall pectins in meristematic cells of maize and wheat root apices. Plant Physiol. 130: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.