Plant RidA proteins protect an enzyme of branched-chain amino acid biosynthesis from inactivation by hydrolyzing reactive pathway intermediates before they can damage the enzyme. RidA proteins are thus crucial for the efficient biosynthesis of branched-chain amino acids in plants and provide an iconic example of the preemption of metabolite damage.
Abstract
RidA (for Reactive Intermediate Deaminase A) proteins are ubiquitous, yet their function in eukaryotes is unclear. It is known that deleting Salmonella enterica ridA causes Ser sensitivity and that S. enterica RidA and its homologs from other organisms hydrolyze the enamine/imine intermediates that Thr dehydratase forms from Ser or Thr. In S. enterica, the Ser-derived enamine/imine inactivates a branched-chain aminotransferase; RidA prevents this damage. Arabidopsis thaliana and maize (Zea mays) have a RidA homolog that is predicted to be plastidial. Expression of either homolog complemented the Ser sensitivity of the S. enterica ridA mutant. The purified proteins hydrolyzed the enamines/imines formed by Thr dehydratase from Ser or Thr and protected the Arabidopsis plastidial branched-chain aminotransferase BCAT3 from inactivation by the Ser-derived enamine/imine. In vitro chloroplast import assays and in vivo localization of green fluorescent protein fusions showed that Arabidopsis RidA and Thr dehydratase are chloroplast targeted. Disrupting Arabidopsis RidA reduced root growth and raised the root and shoot levels of the branched-chain amino acid biosynthesis intermediate 2-oxobutanoate; Ser treatment exacerbated these effects in roots. Supplying Ile reversed the root growth defect. These results indicate that plastidial RidA proteins can preempt damage to BCAT3 and Ile biosynthesis by hydrolyzing the Ser-derived enamine/imine product of Thr dehydratase.
INTRODUCTION
Many metabolites are subject to damage by spontaneous chemical reactions and enzymatic errors that generate useless or toxic side-products (Golubev, 1996; D’Ari and Casadesús, 1998; Tawfik, 2010). It is becoming increasingly clear that such metabolite damage is countered in all organisms by a suite of damage-control systems (Galperin et al., 2006; Linster et al., 2013; Van Schaftingen et al., 2013). These systems fall into two categories: those that repair metabolite damage and those that preempt it (Linster et al., 2013). Repair involves the reconversion of a damaged metabolite to its original form in a one- or multistep process. Damage preemption typically works by converting an aggressively reactive product into a more benign one, thereby forestalling damage; the Nudix family hydrolases that degrade noncanonical, genotoxic NTPs are classic examples (Bessman et al., 1996). Emerging evidence indicates that the RidA family of proteins (formerly called the YjgF/YER057c/UK114 family) is a new class of damage preemption enzymes (Lambrecht et al., 2013).
The members of the RidA family are small, sequence-diverse proteins that occur in all domains of life; prokaryote genomes often encode several of them (Lambrecht et al., 2013). Various phenotypes have been reported for ridA mutants in bacteria, yeast, and other organisms (Christopherson et al., 2012), and several crystal structures have been solved (Manjasetty et al., 2004; Burman et al., 2007; Pu et al., 2011). However, only a few RidA family proteins have been assigned clear biochemical functions. These are (1) the AmnD and NbaF proteins of Pseudomonas spp and Burkholderia cepacia, which mediate deamination of muconate in specialist aromatic degradation pathways (Takenaka et al., 2000; Colabroy and Begley, 2005), and (2) the YjgF protein (renamed RidA) of Salmonella enterica, which has a role in Ile biosynthesis (Enos-Berlage et al., 1998; Lambrecht et al., 2010, 2012).
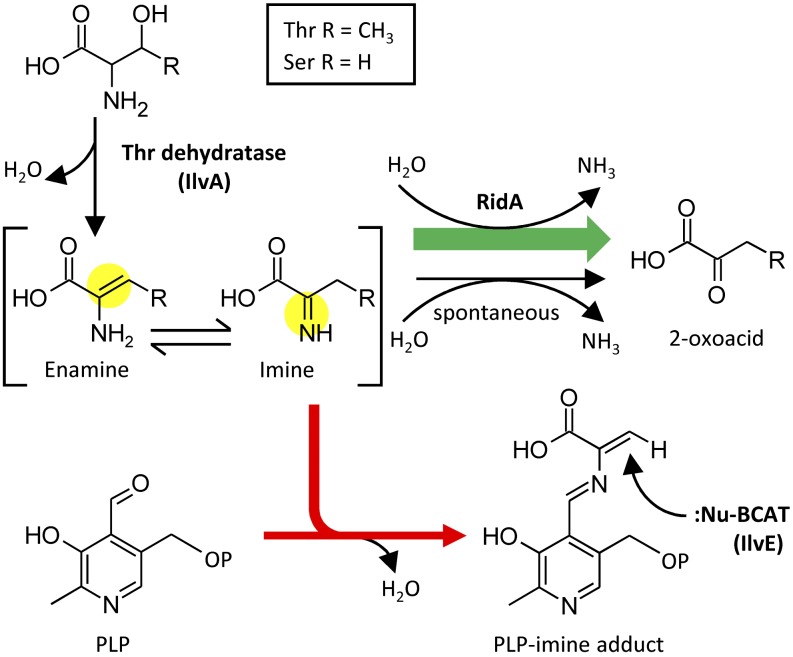
S. enterica RidA facilitates hydrolysis of short-lived reactive enamine/imine intermediates produced by Thr dehydratase (IlvA) (Figure 1). Thr dehydratase acts on Ser or Thr, and in both cases an enamine is produced that tautomerizes to an imine (Chargaff and Sprinson, 1943). The imine is then hydrolyzed to a 2-oxoacid, a reaction formerly thought to be purely spontaneous (Chargaff and Sprinson, 1943). The reactive enamine/imine tautomers (henceforth simply called imines) can attack other compounds before being hydrolyzed (Datta and Bhadra, 1978; Hillebrand et al., 1979), and in S. enterica, the imine formed from Ser attacks the pyridoxal 5′-phosphate (PLP) cofactor of the Ile biosynthesis enzyme IlvE, a branched-chain aminotransferase (BCAT), forming an adduct that inactivates the enzyme (Figure 1) (Lambrecht et al., 2013). By rapidly hydrolyzing the imine, RidA preempts this damage to IlvE.
Figure 1.
Potential Fates in Bacteria of the Reactive Enamine/Imine Produced by Thr Dehydratase.
Thr dehydratase (IlvA) acts on Thr or Ser to produce an enamine that tautomerizes to an imine. The Ser-derived enamine/imine can react with PLP; the resulting adduct can then react with nucleophilic residue(s) of BCAT (IlvE), inactivating this enzyme. The imine also undergoes hydrolysis to the corresponding 2-oxoacid; this occurs spontaneously but is greatly accelerated by RidA. RidA-mediated hydrolysis thus prevents the enamine/imine from reacting with PLP and inactivating BCAT.
[See online article for color version of this figure.]
RidA proteins almost surely have functions beyond aromatic catabolism and Ile biosynthesis. The high sequence diversity of the family and the presence of multiple ridA genes in many genomes point to diverse roles, and experimental data for various RidA proteins favor this possibility. Thus, Escherichia coli RutC is implicated in pyrimidine degradation (Kim et al., 2010), rat liver L-PSP is reported to have ribonuclease activity (Morishita et al., 1999), and the RidA domain of the Xanthomonas campestris XanB2 protein is needed for hydrolysis of chorismate to 3-hydroxybenzoate (Zhou et al., 2013).
The only plant RidA family members investigated so far are the chromoplast protein D proteins of cucumber (Cucumis sativus) and tomato (Solanum lycopersicum), the former being reported to localize to chromoplasts, i.e., plastids (Libal-Weksler et al., 1997), and the latter being reported to influence carotenoid accumulation and photosynthesis (Leitner-Dagan et al., 2006). If plant RidA proteins are indeed in plastids, they would be in the same compartment as Thr dehydratase and the other enzymes of branched-chain amino acid biosynthesis. Accordingly, to investigate whether plant RidA proteins function in the branched-chain pathway, we devised a dynamic assay to test the ability of RidA proteins from Arabidopsis thaliana and maize (Zea mays) to hydrolyze imines generated by Thr dehydratase. We then used an improved version of an existing assay to show that these RidA proteins can preempt imine damage to branched-chain aminotransferase in vitro and localized RidA and Thr dehydratase to plastids. Finally, we confirmed the in vivo involvement of RidA in branched-chain amino acid biosynthesis by physiological and metabolomic analyses of Arabidopsis RidA knockouts.
RESULTS
Arabidopsis and Maize Have a Single RidA Gene That Is Coexpressed with Thr Dehydratase and BCAT3
BLASTP searches of Arabidopsis and maize protein databases using S. enterica RidA and 10 other diverse RidA family proteins (Supplemental Table 1) identified a single stand-alone RidA homolog in each plant (At3g20390 and GRMZM2G117642). These proteins are 44 and 39% identical to S. enterica RidA, respectively, and 66% identical to each other. Both proteins have N-terminal extensions relative to S. enterica RidA that are predicted to encode transit peptides (Figure 2A).
Figure 2.
Plants Have Homologs of S. enterica RidA That Can Relieve the Ser Sensitivity of RidA-Deficient S. enterica.
(A) Alignment of S. enterica (Se) RidA with its Arabidopsis (At) and maize (Zm) homologs. Identical residues are shaded black; similar residues are in gray; dashes are introduced to maximize alignment. The Leu residues that were changed to Met when targeting peptides were removed are highlighted in yellow. Conserved Arg residues that were mutated to Ala are in red.
(B) RidA-deficient (ΔridA) S. enterica was transformed with pBAD24 alone or encoding wild-type RidA from S. enterica (Se), Arabidopsis (At), or maize (Zm), or mutant RidA from Arabidopsis (R165A) or maize (R157A). Strains were grown at 37°C in minimal medium containing ampicillin, arabinose, and 5 mM Ser. Data are means and se for three independent strains. All strains grew equally well in medium without Ser.
The CSB.DB Arabidopsis (Steinhauser et al., 2004) and qTeller maize expression databases show that the RidA genes in both plants are coexpressed during development with the Ile biosynthesis genes encoding Thr dehydratase (l-O-methylthreonine resistance 1 [OMR1] in Arabidopsis) and plastidial branched-chain aminotransferase-3 (BCAT3 in Arabidopsis) (Supplemental Figures 1A and 1B). Furthermore, CSB.DB ranks Arabidopsis BCAT3 as the gene most highly correlated with RidA during development. Similarly, the ATTED-II database (Obayashi et al., 2009) places Arabidopsis RidA in a coexpressed gene network with BCAT3, Thr dehydratase (OMR1), and two other Ile synthesis genes, dihydroxy-acid dehydratase (At3g23940) and ketol-acid reductoisomerase (At3g58610) (Supplemental Figure 1C). Neither CSB.DB nor ATTED-II associated RidA with genes of aromatic catabolism, pyrimidine degradation, or chorismate metabolism. Transcriptomic evidence thus predicts that plant RidA proteins, like S. enterica RidA, play a damage preemption role in Ile biosynthesis and does not predict any of the other metabolic functions established or inferred thus far for other RidA family proteins.
Expression of Plant RidA Proteins Relieves Ser Sensitivity in RidA- S. enterica
S. enterica ridA deletants do not grow in glucose minimal medium when Ser is added (Christopherson et al., 2008). This Ser sensitivity, which is likely due to Ile deficiency caused by inactivation of the branched-chain aminotransferase IlvE, enables a complementation test for RidA activity of candidate proteins. We therefore cloned Arabidopsis and maize RidA sequences (minus their predicted targeting peptides; Figure 2A) into the pBAD24 expression vector and introduced the constructs into a S. enterica ΔridA strain, together with a construct encoding S. enterica ridA as a positive control, and the empty vector. When inoculated in medium containing 5 mM Ser, growth of the strain harboring the empty vector was inhibited, as expected (Figure 2B). Expressing Arabidopsis or maize RidA restored growth as effectively, or nearly so, as the plasmid-borne S. enterica ridA gene (Figure 2B). Furthermore, mutating a conserved Arg residue (Figure 2A) that is catalytically essential in S. enterica RidA (Lambrecht et al., 2012) to Ala abolished the complementing activity of the plant proteins (Figure 2B). These functional complementation results show that plant RidA homologs have RidA activity in vivo, at least in S. enterica.
Plant RidA Proteins Facilitate Hydrolysis of Imines Formed by Thr Dehydratase
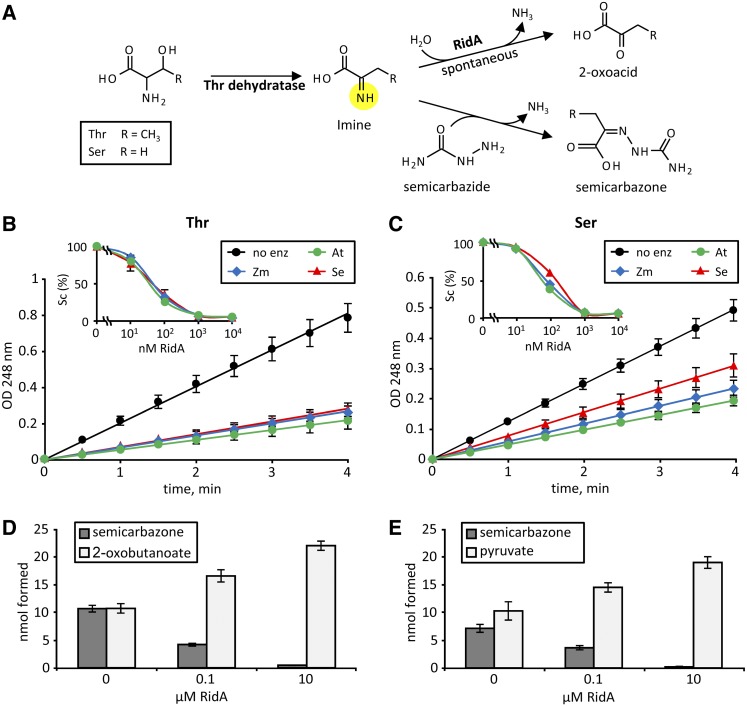
To assess the ability of plant RidA proteins to hydrolyze the reactive imines produced by Thr dehydratase, we developed a new RidA activity assay based on the rapid reaction of semicarbazide with imines to produce UV-absorbing semicarbazones (Hafner and Wellner, 1979). In this assay, the RidA-mediated hydrolysis of imines produced by Thr dehydratase from Thr or Ser is monitored spectrophotometrically via the ability of hydrolysis to compete with, and thereby reduce, semicarbazone formation (Figure 3A).
Figure 3.
Plant RidA Proteins Accelerate the Rate of Hydrolysis Thr- and Ser-Derived Reactive Imines Produced by Arabidopsis Thr Dehydratase.
(A) The imines produced by Thr dehydratase can be hydrolyzed to the corresponding 2-oxoacids spontaneously or via RidA action. Alternatively, the imines can react with semicarbazide to produce semicarbazones that absorb strongly at 248 nm.
(B) and (C) Plant RidA proteins accelerate hydrolysis of Thr- and Ser-derived imine intermediates, respectively. Assays contained 100 nM Arabidopsis (At), maize (Zm), or S. enterica (Se) RidA, 0.5 μM (Thr) or 2.5 μM (Ser) Arabidopsis Thr dehydratase, 10 mM semicarbazide, and were started by addition of Thr or Ser (final concentration 2 mM). Insets show the rate of semicarbazone (Sc) formation in assays containing various concentrations of RidA, expressed as a percentage of that in reactions without RidA. Data are means and se for three independent assays. Where no error bars are shown they were smaller than the symbols.
(D) and (E) The RidA-mediated reduction in semicarbazone formation is accompanied by an increase in 2-oxoacid formation from Thr or Ser, respectively. Arabidopsis RidA was used; reaction time was 5 min. Data are means and se for three independent assays.
Recombinant Arabidopsis and maize RidA proteins were compared with S. enterica RidA as a benchmark. These proteins and Arabidopsis Thr dehydratase were purified to near-homogeneity by Ni2+-affinity chromatography (Supplemental Figure 2). Rapid semicarbazone formation was observed when Thr dehydratase was incubated with Thr and semicarbazide, and without RidA (Figure 3B). Addition of 100 nM Arabidopsis, maize, or S. enterica RidA caused 74, 68, or 65% decreases in semicarbazone formation, respectively (Figure 3B). Very similar results were obtained when Ser replaced Thr. All three RidA proteins cut semicarbazone formation by >90% when their concentration was raised to 1 or 10 μM, with Ser or Thr as substrate (Figures 3B and 3C, insets).
To confirm that the decrease in semicarbazone formation was due to RidA-mediated hydrolysis of imines and not to some other effect, we measured both semicarbazone and 2-oxoacid formation. Reactions were run as above and stopped after 5 min by placement on ice and addition of Ile to a final concentration 10 mM; Ile almost completely inhibits Arabidopsis Thr dehydratase at concentrations above 8 mM (Wessel et al., 2000). The amount of 2-oxobutanoate or pyruvate formed was determined enzymatically using lactate dehydrogenase (Meister, 1950). With either Thr or Ser as the substrate, the decrease in semicarbazone formation in the presence of RidA was matched by an increase in 2-oxobutanoate or pyruvate formation (Figures 3D and 3E).
Collectively, these data show that plant RidA proteins greatly increase the rate of hydrolysis of Ser- or Thr-derived imine intermediates (2-iminopropanoate or 2-iminobutanoate, respectively) produced by Thr dehydratase. Plant RidA proteins, like their bacterial homologs, can thus potentially intercept these short-lived imines before they damage branched-chain aminotransferases or other cell constituents.
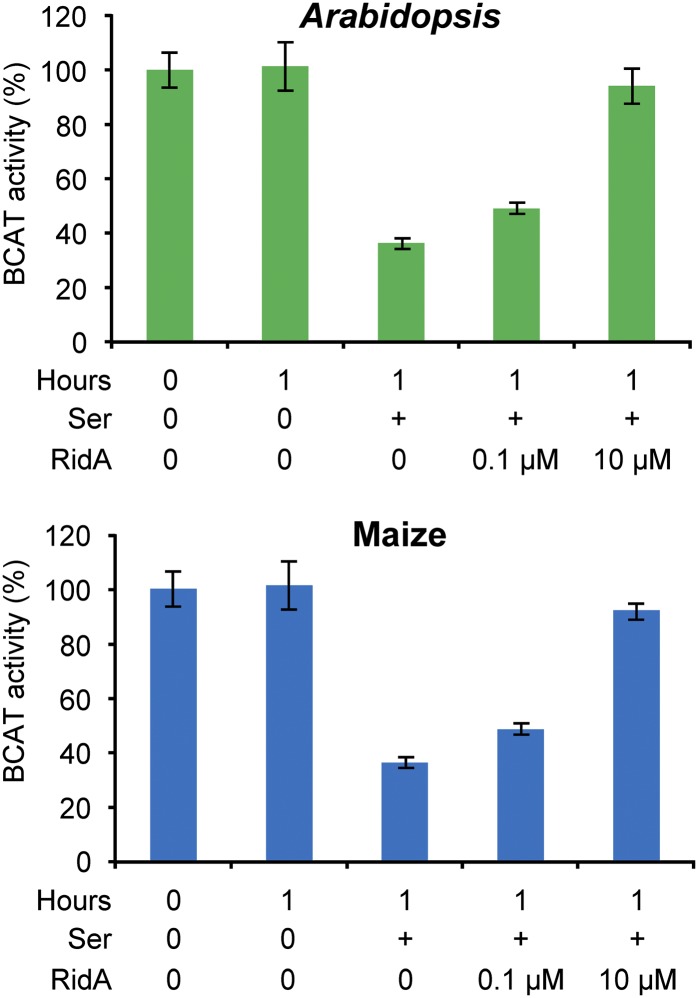
Plant RidA Proteins Protect BCAT3 from Inactivation by the Ser-Derived Imine
To test directly whether plant RidAs can intercept reactive imines before they damage branched-chain aminotransferase, we first exposed Arabidopsis BCAT3 to the Ser- or Thr-derived imine (produced in situ by Thr dehydratase) in the absence or presence of plant RidA and then measured BCAT activity in a continuous coupled spectrophotometric assay. This assay is simpler and more suited to kinetic analysis than the end-point radioassay used previously for S. enterica proteins (Lambrecht et al., 2013). BCAT3 was chosen as the inactivation target based on its coexpression with RidA (see above), its similarity to S. enterica branched-chain aminotransferase IlvE, and its known role in branched-chain amino acid synthesis (Knill et al., 2008). After 1 h exposure to the Ser-derived imine alone, BCAT3 activity had fallen by 64%, but when Arabidopsis or maize RidA was added at concentrations of 0.1 or 10 μM, the activity loss was proportionately less (Figure 4). No activity was lost in controls incubated without imine for 1 h (Figure 4) nor was there significant activity loss when Thr replaced Ser as the source of the imine, as observed in analogous experiments with S. enterica IlvE (Lambrecht et al., 2013). These data show that, as with bacterial RidA, IlvA, and IlvE (Figure 1), so with their plant counterparts: plant RidA proteins can hydrolyze the imine formed from Ser by plant Thr dehydratase before it attacks plant branched-chain aminotransferase.
Figure 4.
Arabidopsis and Maize RidA Proteins Prevent Arabidopsis BCAT3 Inactivation by the Ser-Derived Enamine/Imine Produced by Arabidopsis Thr Dehydratase.
Arabidopsis BCAT3 was preincubated at 22°C for 1 h with Arabidopsis Thr dehydratase and 160 mM Ser, minus or plus 0.1 or 10 μM Arabidopsis or maize RidA. BCAT activity was then assayed. Controls that were not preincubated, or preincubated without Ser, were included. Data are expressed as percentages of the activity before preincubation (4.3 μmol min−1 mg−1 protein) and are means and se for three independent assays. Preincubation with Thr instead of Ser caused no significant BCAT3 inactivation.
[See online article for color version of this figure.]
RidA and Thr Dehydratase Are Plastid-Targeted
Arabidopsis BCAT3 has been shown to localize to plastids (Diebold et al., 2002), and multiple proteomics analyses, summarized at the Plant Proteome Database (Sun et al., 2009), show that Arabidopsis RidA, Thr dehydratase, and BCAT3 are found in chloroplasts. Furthermore, the consensus of targeting prediction programs is that Arabidopsis and maize RidA, Thr dehydratase, and BCAT proteins all have N-terminal plastid targeting sequences (Supplemental Table 2).
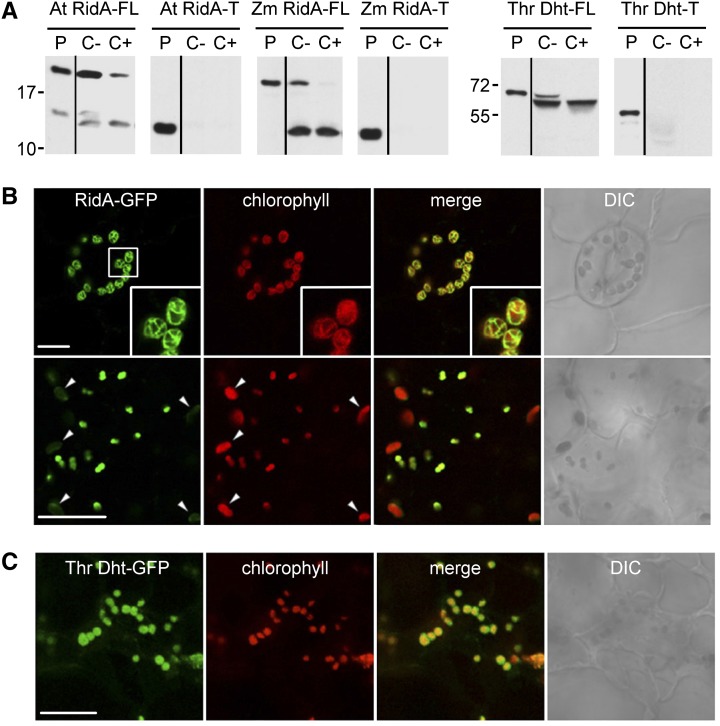
To confirm their plastid targeting sequences, in vitro chloroplast import assays were performed with Arabidopsis and maize RidA proteins and Arabidopsis Thr dehydratase. As shown in Figure 5A, the full-length forms of all three proteins entered, and were proteolytically processed in, isolated pea (Pisum sativum) chloroplasts, but versions of each protein lacking its predicted N-terminal targeting peptide did not.
Figure 5.
Evidence That Arabidopsis and Maize RidA Proteins, and Arabidopsis Thr Dehydratase, Are Targeted to Chloroplasts.
(A) Protein import into isolated pea chloroplasts. cDNAs that were full length (FL) or truncated to remove the predicted transit peptide (T) were transcribed and translated in vitro using a wheat germ system containing [3H]Leu. The translation products were incubated for 20 min in the light with chloroplasts, which were then reisolated using a Percoll gradient, without (C-) or with (C+) prior thermolysin treatment. Proteins were separated by SDS-PAGE and visualized by fluorography. Samples were loaded next to an aliquot of the translation product (P). The positions of molecular mass markers (kD) are shown. Thr Dht, Thr dehydratase.
(B) and (C) Representative micrographs of Arabidopsis seedling leaf cells stably expressing GFP fused to the C terminus of Arabidopsis RidA or Thr dehydratase. Fluorescence attributable (as indicated by panel labels) to the expressed GFP fusion protein and chlorophyll was observed by confocal microscopy. Merged and differential interference contrast (DIC) micrographs are shown for each set of images. The insets in (B) (top row) are high-magnification images of a portion of the micrographs as indicated. The arrowheads in (B) (bottom row) indicate examples of larger chloroplasts that, compared with smaller chloroplasts, appear to contain relatively less RidA-GFP fusion protein. Bars = 10 μm.
We next employed confocal microscopy to assess the intracellular localization of Arabidopsis RidA or Thr dehydratase fused at their C termini to green fluorescent protein (GFP) in stably transformed Arabidopsis seedlings. As shown in Figure 5B, the RidA-GFP fusion protein was found to localize exclusively to chlorophyll-containing chloroplasts in both guard and mesophyll cells. Notably, in mesophyll cells, the RidA-GFP fusion seemed more abundant in smaller chloroplasts than larger ones (Figure 5B, lower panel), suggesting that, like certain other chloroplast proteins, the targeting of RidA may be influenced by chloroplast age, import capacity, and/or metabolic activity (Kessler and Schnell, 2009; Teng et al., 2012). The Thr dehydratase-GFP fusion protein also localized exclusively to chloroplasts in Arabidopsis mesophyll cells (Figure 5C). Corroborating these results, Arabidopsis RidA-GFP and Thr dehydratase-GFP fusions again localized only to chloroplasts when transiently expressed in Nicotiana benthamiana leaf mesophyll cells (Supplemental Figures 3A and 3C). Furthermore, Arabidopsis RidA and Thr dehydratase-GFP fusions colocalized with the Arabidopsis BCAT3-mCherry fusion in plastids when transiently coexpressed in Nicotiana tabacum (Bright Yellow-2 [BY-2]) suspension-cultured cells (Supplemental Figures 3B and 3D). Taken together, the results of the in vitro import and in vivo localization studies indicate that plant RidA and Thr dehydratase are plastid-targeted and colocalize with plastidial BCATs.
Ablating Arabidopsis RidA Confers a Ser-Sensitive, Ile-Deficient Phenotype in Roots
In order to confirm in vivo the role of RidA in branched-chain amino acid biosynthesis, we searched the Arabidopsis T-DNA database for insertions in the RidA (At3g20390) locus. A line (SK15304) with an insertion in the third exon was identified. Homozygous individuals were selected from the population, multiplied, and analyzed to verify the insertion location and to measure RidA transcript level (Supplemental Figure 4). No RidA transcript was detected in homozygous mutant plants. This loss of transcript, and an insertion that would truncate the RidA protein upstream of the catalytically essential Arg (Figure 2A), indicate that the SK15304 insertion completely ablates RidA activity.
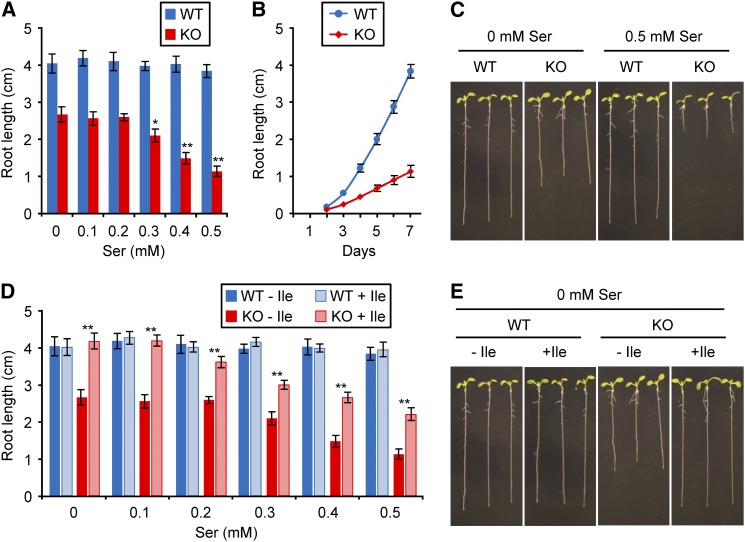
Given that S. enterica ridA- strains exhibit Ser-induced Ile deficiency (Christopherson et al., 2008), that plant RidA proteins complement this phenotype (Figure 2B), and that plant RidA proteins preempt damage to branched-chain aminotransferase in vitro (Figure 4), the Arabidopsis RidA knockout is predicted, particularly when exposed to Ser, to show Ile-correctable growth defects. Also, since partial Ile deficiency in Arabidopsis is known to affect root growth rather than shoot growth (Yu et al., 2013), the effects of ablating RidA are predicted to be most evident in roots. These predictions were matched by our observations. Thus, root growth of the RidA knockout was significantly less than the wild type when cultured on half-strength Murashige and Skoog (MS) medium alone and was more sensitive to added Ser (Figures 6A and 6C). For instance, 0.5 mM Ser reduced root length by 57% in the knockout but did not significantly affect growth in the wild type (Figure 6A). A time-course study showed that the knockout phenotype was due to a low growth rate, not to growth arrest at a particular stage (Figure 6B). The growth defect in the absence of Ser was fully overcome by adding 0.05 mM Ile but not 0.05 mM Val or Leu (Figures 6D and 6E). Similarly, when various concentrations of Ser were added, 0.05 mM Ile overcame the defect fully at the lowest Ser concentration tested (0.1 mM) but only partially at higher concentrations (Figure 6D), possibly due to imine damage other than that to BCAT3 (Flynn and Downs, 2013; Flynn et al., 2013). The growth defect in the absence of Ser was also fully overcome by adding 0.3 mM Thr, the precursor of Ile (Supplemental Figure 5). Collectively, these data indicate that the root growth defect of the RidA mutant is due to functional Ile deficiency. Shoot growth of the RidA knockout did not differ appreciably from that of the wild type in plantlets cultured on half-strength MS medium (Figures 6C and 6E) or in plants grown to maturity in soil.
Figure 6.
Impaired Root Growth of the Arabidopsis RidA Knockout, Its Exacerbation by Ser, and Reversal by Ile.
(A) Root growth of Col-0 (WT) and the RidA knockout (KO) after 7 d on half-strength MS medium containing 1% sucrose and various concentrations of Ser. Data are means and se for 10 to 18 replicate plants. For each strain, significant differences (Student’s t test) from growth without Ser are indicated by asterisks: *P < 0.05; **P < 0.01.
(B) Time course showing root growth of wild-type and knockout plants grown with 0.5 mM Ser. Data are means and se for 11 to 12 replicate plants.
(C) Images of wild-type and knockout plants grown for 7 d with or without 0.5 mM Ser.
(D) Reversal of growth phenotypes by Ile. Plants were cultured for 7 d in the presence of the indicated concentrations of Ser, with or without 0.05 mM Ile. Data are means and se for 10 to 18 replicate plants. Significant differences between plants grown with or without Ile are indicated as above. The growth defect in the absence of Ser was not overcome by 0.05 mM Val or Leu.
(E) Images of wild-type and knockout plants grown for 7 d with or without 0.05 mM Ile.
As the above root growth data imply a shortage of Ile in the knockout roots, particularly when exposed to Ser, we performed targeted analyses of branched-chain amino acids, Thr, Ser, and the corresponding 2-oxoacids in 14-d cultured roots that were untreated or treated with 0.5 mM Ser for 4 h before collection (Table 1). Knockout roots showed significant accumulation (6.4-fold) of 2-oxobutanoate, the 2-oxoacid product of Thr dehydratase, but no significant changes in Ile or any of the other metabolites profiled. After treatment with Ser, the accumulation of 2-oxobutanoate in knockout roots became much more pronounced (21.5-fold). Ser treatment also appeared to cause a general, ∼2-fold, accumulation of 2-oxoacids, although only that of 4-methyl-2-oxopentanoate (the 2-oxoacid of Leu) reached the P ≤ 0.05 level of significance. As in untreated roots, knockout leaves showed a modest (1.3-fold) but significant accumulation of 2-oxobutanoate and no other changes (Supplemental Table 3). These results are consistent with increased activity of Thr dehydratase and inhibition of BCAT3, as further discussed below.
Table 1. Relative Levels of Metabolites in 14-d-Old RidA Knockout and Wild-Type Arabidopsis Roots Either Untreated or Treated with 0.5 mM Ser for 4 h Prior to Collection.
| Untreated |
0.5 mM Ser, 4 h |
|||
|---|---|---|---|---|
| Metabolite | Fold Change (KO/WT) | P Value | Fold Change (KO/WT) | P Value |
| Thr | 0.8 | 0.284 | 1.2 | 0.454 |
| 2-Oxobutanoate | 6.4a | 0.009 | 21.5 | 0.003 |
| Ser | 0.9 | 0.455 | 1.2 | 0.470 |
| Pyruvate | 1.0 | 0.894 | 2.3 | 0.101 |
| Ile | 0.9 | 0.718 | 1.5 | 0.133 |
| 3-Methyl-2-oxopentanoate | 0.9 | 0.629 | 2.5 | 0.053 |
| Leu | 1.0 | 0.904 | 1.6 | 0.111 |
| 4-Methyl-2-oxopentanoate | 1.0 | 0.818 | 2.3 | 0.033 |
| Val | 0.9 | 0.475 | 1.3 | 0.308 |
| 2-Oxoisovalerate | 1.2 | 0.651 | 2.4 | 0.140 |
Data represent the mean of six biological replicates, normalized to internal standards. KO, knockout; WT, wild type.
Significant fold changes (P ≤ 0.05) are in bold.
DISCUSSION
Plant RidA Proteins Are Plastidial Damage Preemption Enzymes
Our work establishes a subcellular location, a biochemical activity, and a physiological function for plant RidA family proteins: They are plastidial proteins that hydrolyze the reactive and toxic imines formed by Thr dehydratase and thereby preempt the damage that the Ser-derived imine would otherwise do to BCAT3 and Ile biosynthesis. We accordingly propose the name RIDA (Reactive Intermediate Deaminase A) for plant genes encoding RidA family proteins, following the precedent in S. enterica (Lambrecht et al., 2012). As the evidence for RidA location, activity, and function comes from both Arabidopsis and maize, the functional annotations of RIDA genes in these model plants can be reliably propagated to orthologous genes in other eudicots and monocots. Our data are consistent with earlier reports that cucumber and tomato RidA proteins are plastid-localized (Leitner-Dagan et al., 2006), that suppressing tomato RidA expression caused plastid defects (Leitner-Dagan et al., 2006), and that cucumber RidA protected S. enterica IlvA from imine damage in vitro in the same way as S. enterica RidA (Lambrecht et al., 2012, 2013). Our data also fit with the finding that ablating a yeast RidA gene (MMF1) causes Ile auxotrophy and loss of Ile aminotransferase activity (Kim et al., 2001). Plant RidA proteins can thus now be added to the growing list of validated, cross-kingdom damage preemption or repair enzymes (Linster et al., 2013).
RidA Preempts Damage from 2-Iminopropanoate, a Side-Product of Thr Dehydratase
Although plant RidA efficiently hydrolyzed the imines formed by Thr dehydratase from Thr or Ser, only the Ser-derived imine (2-iminopropanoate; or its cognate enamine) caused damage to BCAT3 in our biochemical assay. Similarly, Ile synthesis and growth of RidA knockout roots were inhibited by Ser but not Thr. These in vitro and in vivo data collectively indicate (1) that 2-iminopropanoate is far more toxic than the Thr-derived imine 2-iminobutanoate, (2) that 2-iminopropanoate is the primary physiological target for RidA action, and, therefore, (3) that Ser is a significant substrate for Thr dehydratase in planta. There is ample evidence for the last inference. First, like the biosynthetic Thr dehydratases of microorganisms, plant Thr dehydratases prefer Thr but act also on Ser in vitro (Figures 3B and 3C; Szamosi et al., 1993). Second, levels of Ser are at least equal to those of Thr in both green and non-green plastids and can be far higher (Farré et al., 2001; Szecowka et al., 2013), so that Ser should be abundantly available as a substrate for Thr dehydratase in organello. Although these lines of evidence, and our RidA results, indicate that Thr dehydratase acts on Ser as well as on Thr in vivo, they shed no light on whether the activity against Ser is a physiologically important source of pyruvate for branched-chain amino acid biosynthesis, or simply a promiscuous one. Studies of enzyme evolution suggest that activity against Ser could be promiscuous inasmuch as it is also a secondary activity of related PLP-dependent enzymes. Thus, Thr synthase and Trp synthase appear to have evolved from the same ancestor as Thr dehydratase, and, despite their diverse main activities, retain Ser dehydratase side-activities (Skarstedt and Greer, 1973; Parsot, 1987; O’Brien and Herschlag, 1999). If the Ser dehydratase activity is indeed an unwanted side-reaction of Thr dehydratase, then RidA is an add-on solution to this enzyme’s lack of specificity. Whatever the case, RidA is clearly a crucial adjunct to Thr dehydratase.
2-Iminopropanoate Damages Plastidial Branched-Chain Aminotransferase
The damage inflicted by the Ser-derived imine 2-iminopropanoate is apparently specific in two respects. First, although 2-iminopropanoate could in principle attack various PLP enzymes (Flynn and Downs, 2013), eliminating RidA, i.e., unleashing 2-iminopropanoate, affects the branched-chain aminotransferase BCAT3 but not, for instance, aminotransferases that act on other substrates. Furthermore, only Ile biosynthesis appears to be affected even though branched-chain aminotransferase activity is also needed to make Val and Leu (Diebold et al., 2002). Second, although RidA and Thr dehydratase expression is similar in roots and leaves (Supplemental Figures 1A and 1B), the metabolic and growth consequences of ablating RidA are largely confined to roots.
The enzyme specificity of 2-iminopropanoate damage is paralleled in S. enterica and yeast, in both of which inactivating RidA gives rise not to a general inhibition of PLP enzymes, but specifically to loss of branched-chain aminotransferase activity, particularly toward Ile (Kim et al., 2001; Christopherson et al., 2008; Lambrecht et al., 2013). This specificity could be explained if the 2-iminopropanoate formed by Thr dehydratase has privileged access to branched-chain aminotransferase because both enzymes are in a multienzyme branched-chain amino acid synthesis complex in which intermediates are channeled. Such complexes have been reported in all kingdoms of life for the degradation of branched-chain amino acids (Heath et al., 2007) but not so far for their biosynthesis. There is nonetheless much support for biosynthesis complexes from high-throughput proteomic and genetic studies of protein-protein interactions in bacteria, yeast, and Arabidopsis. These interactions link each enzyme of branched-chain amino acid biosynthesis (except for those specific to Leu synthesis) with at least three of the others and also link RidA to Thr synthase (Supplemental Figure 6). Such proteomic and genetic interactions typically reflect the functional integration of metabolic pathways and protein complexes (Costanzo et al., 2010).
Comparative genomic evidence associates RidA with BCAT3, and our in vitro evidence for 2-iminopropanoate-driven aminotransferase inactivation was obtained with this enzyme. However, Arabidopsis plastids contain two other branched-chain aminotransferases, BCAT2 and BCAT5, that can both mediate biosynthesis of all three branched-chain amino acids in yeast (Diebold et al., 2002). Neither BCAT2 nor BCAT5 has been kinetically characterized, but BCAT3 has been shown to strongly prefer the 2-oxoacids corresponding to Ile and Leu (3-methyl-2-oxopentanoate and 4-methyl-2-oxopentanoate) over that corresponding to Val (2-oxoisovalerate) (Knill et al., 2008). This suggests that BCAT2 or BCAT3 might show an opposite specificity, i.e., a preference for 2-oxoisovalerate. Supporting this possibility, aminotransferases specific for either 3-methyl-2-oxopentanoate and 4-methyl-2-oxopentanoate or 2-oxoisovalerate have been isolated from spinach (Spinacia oleracea) chloroplasts (Hagelstein et al.,1997). If, as the comparative genomic data suggest, BCAT3 is the main in vivo target of 2-iminopropanoate, and if BCAT2 or BCAT5 prefers Val, the immunity of Val biosynthesis to RidA ablation would be explained.
RidA Plays a Role in Ile Biosynthesis in Plants
The Arabidopsis lib (low Ile biosynthesis) mutant, which is partially Ile-deficient due to a Thr dehydratase defect, shows normal shoot growth but slow root growth that can be overcome by supplying Ile (Yu et al., 2013). Identical phenotypes are observed in the RidA knockout in the absence of Ser. Thus, while it remains to be determined why Ile deficiency affects roots and not shoots, at least it is clear that the effect of ablating RidA can be attributed to Ile deficiency. The reversion of the RidA root growth phenotype by Thr, which can be converted to Ile, is further evidence for an Ile deficiency. That this deficiency is due to a BCAT3 defect is supported by the observation that Arabidopsis BCAT3 knockout plants (Knill et al., 2008) also show slow root growth (Kurt Lächler and Stefan Binder, personal communication).
Although the above evidence indicates that Arabidopsis RidA knockout plants experience functional Ile deficiency, the Ile levels in root or shoot extracts of Arabidopsis knockouts were not reduced. Arabidopsis BCAT3 knockouts likewise showed no significant change in Ile level (Knill et al., 2008). Because Thr dehydratase, BCAT3, and RidA are all located in plastids, it is possible that only the plastidial Ile pool is affected in RidA knockouts. If so, given that this pool may be just 10 to 30% of total Ile (Farré et al., 2001; Szecowka et al., 2013), the deficiency would be hard to detect in whole-tissue extracts. Ile biosynthesis in Arabidopsis is known to be regulated via feedback inhibition of Thr dehydratase by Ile (Wessel et al., 2000). If the RidA knockout indeed has a reduced plastidial Ile level due to BCAT3 inactivation, then (1) Thr dehydratase activity would be expected to rise due to relief of feedback inhibition, and (2) intermediates upstream of Ile would be expected to accumulate. This scenario could explain the elevated levels of 2-oxobutanoate (the product of Thr dehydratase) in roots and leaves of ridA mutants. Furthermore, as the Ser-derived imine 2-iminopropanoate damages BCAT3 (Figure 4), the metabolic effects of RidA deficiency are predicted to be exacerbated by Ser treatment. In agreement with this prediction, Ser-treated knockout roots have far higher 2-oxobutanoate levels than untreated roots.
Because the 2-oxobutanoate data suggest that Ile biosynthesis is perturbed in both roots and leaves of RidA knockouts, it seems surprising that only roots show a growth phenotype. This might be explained by translocation of Ile from roots to leaves, there being much evidence for inter-organ traffic in amino acids (Okumoto and Pilot, 2011). It is also possible that an Ile deficiency, or a consequence thereof such as 2-oxobutanoate accumulation, affects signaling pathways that control root growth. In this context, it may be noted that the Ile conjugate of jasmonic acid is a translocated signaling compound (Shah, 2009).
METHODS
Bioinformatics
Protein sequences were from GenBank and MaizeSeqence.org. Arabidopsis thaliana gene expression data were taken from CSB.DB (Steinhauser et al., 2004), which aggregates microarray results. Maize (Zea mays) gene expression data were from qTeller (http://qteller.com/), which aggregates published RNaseq data sets (Jia et al., 2009; Wang et al., 2009; Li et al., 2010; Davidson et al., 2011; Waters et al., 2011) and some unpublished ones. Arabidopsis gene networks were drawn with the NetworkDrawer tool at ATTED-II (Obayashi et al., 2009). Protein-protein interaction data sets were accessed via ATTED-II, STRING (Franceschini et al., 2013), and BioGRID (Chatr-Aryamontri et al., 2013).
Chemicals
[3H]Leu (115.8 Ci/mmol) was from Perkin-Elmer. Phusion High-Fidelity DNA polymerase (New England Biolabs) was used for PCR reactions. All other chemicals were obtained from Sigma-Aldrich unless otherwise stated.
cDNAs and Expression Constructs
Primer sequences are listed in Supplemental Table 4. All constructs were sequence verified. Full-length cDNAs for Arabidopsis RidA and BCAT3 were from the ABRC (clones U12465 and U21141, respectively); the Arabidopsis Thr dehydratase cDNA was from the Riken BioResource Center (clone RAFL09-97-N03). The maize RidA cDNA was from the Arizona Genomics Institute (clone ZM_BFc0033I17). Salmonella RidA was amplified from genomic DNA of Salmonella enterica subsp enterica serovar Typhimurium str. LT2.
For functional complementation experiments, sequences encoding predicted mature proteins were PCR amplified (Arabidopsis RidA, primers 1 and 2; maize RidA, primers 3 and 4). Amplicons were digested with NcoI and XbaI and ligated into the matching sites of pBAD24. Constructs in which the conserved Arg codon was changed to Ala were created with the QuikChange site-directed mutagenesis kit (Stratagene) using Arabidopsis RidA-pBAD24 or maize RidA-pBAD24 as templates and primers 5 and 6, or 7 and 8, respectively. The Salmonella RidA-pBAD24 construct was as previously reported (Lambrecht et al., 2013).
For expression of His-tagged proteins in Escherichia coli, sequences encoding predicted mature proteins were PCR amplified (Arabidopsis RidA, primers 9 and 10; maize RidA, primers 11 and 12; Salmonella RidA, primers 13 and 14; Thr dehydratase, primers 15 and 16; BCAT3, primers 17 and 18). Amplicons were digested with NcoI and XhoI (Arabidopsis, maize, and Salmonella RidA, C-terminal His-tag), NcoI and NotI (Thr dehydratase, C-terminal His-tag), or NdeI and XhoI (BCAT3, N-terminal His-tag) and ligated into the matching sites of pET28b.
For chloroplast import assays, full-length (FL) and predicted mature (T) coding sequences were PCR amplified (Arabidopsis RidA-FL, primers 19 and 20; Arabidopsis RidA-T, primers 21 and 20; maize RidA-FL, primers 22 and 23; maize RidA-T, primers 24 and 23; Thr dehydratase-FL, primers 25 and 26; Thr dehydratase-T, primers 27 and 26). Amplicons were digested with EcoRI and HindIII (RidA), XmaI and XbaI (Thr dehydratase-FL), or EcoRI and XbaI (Thr dehydratase-T) and ligated into the matching sites of pGEM4z.
For localization studies, full-length coding sequences were PCR amplified (Arabidopsis RidA, primers 28 and 29; Thr dehydratase, primers 30 and 31; BCAT3, primers 32 and 33). Amplicons were digested with XmaI and NheI and ligated into the matching sites of pUC18-MCS-mGFP (RidA and Thr dehydratase) or pRTL2-mCherry (BCAT3). For cloning into binary vectors, full-length coding sequences of proteins C-terminally fused to mGFP were PCR amplified from the pUC18-MCS-mGFP constructs (Arabidopsis RidA, primers 34 and 36; Thr dehydratase, primers 35 and 36). The amplicons were Gateway cloned (Life Technologies) into binary vector pMDC32.
Functional Complementation
S. enterica ridA knockouts (Lambrecht et al., 2013) harboring each complementation construct were grown overnight in Luria-Bertani medium with 150 mg/L ampicillin and subcultured into M9 minimal medium with 15 mg/L ampicillin and 0.2% (w/v) arabinose or minimal medium with ampicillin, arabinose, and 5 mM L-Ser, and then grown at 37°C with shaking. Absorbance at 650 nm was measured.
Protein Production and Isolation
E. coli strain BL21 (DE3) RIPL harboring each expression construct was grown in 200 mL Luria-Bertani medium with 50 mg/L kanamycin at 37°C until OD at 600 nm reached 0.8. Cultures were then cooled to 22°C, and isopropyl-3-d-thiogalactoside and ethanol were added to final concentrations of 0.5 mM and 4% (v/v), respectively. Cultures were incubated for a further 20 h at 22°C; cells were then collected by centrifugation and stored at −80°C. Pellets were resuspended in 7 mL ice-cold lysis buffer containing 50 mM potassium phosphate, pH 8.0, 300 mM NaCl, and 10 mM imidazole and sonicated (Fisher Scientific Ultrasonic Dismembrator, model 150E) using seven 15-s pulses at 70% power, cooling on ice for 2 min between pulses. Lysates were centrifuged (10,000g, 10 min) and the supernatant was applied to 1.0 mL Ni-NTA superflow resin columns (Qiagen), from which proteins were purified using the manufacturer’s protocol. Proteins were passed through PD-10 columns (GE Healthcare) equilibrated with 5 mM triethanolamine, pH 7.6, 10% (v/v) glycerol (RidA proteins), 50 mM potassium phosphate, pH 7.7, 100 mM NaCl, 10% (v/v) glycerol (BCAT3), or 50 mM HEPES, pH 7.5, 1 mM EDTA, 1 mM DTT, 1 mM l-Ile, 10% (v/v) glycerol (Thr dehydratase), and then concentrated to 20 to 100 mg/mL with Amicon Ultra-4 3000 NMWL (RidA) or 10,000 NMWL centrifugal filters (Millipore). Aliquots (5 to 10 μL) were snap frozen in liquid nitrogen and stored at −80°C. All buffers used to purify Thr dehydratase contained 1 mM l-Ile.
Enzyme Assays
To assess the ability of RidA to hydrolyze imines, assays (100 μL) contained 50 mM potassium pyrophosphate, pH 8.7, 10 mM semicarbazide HCl (neutralized), 0.5 μM (l-Thr) or 2.5 μM (l-Ser) Thr dehydratase and the indicated amount of Arabidopsis or maize RidA. Reactions were started by adding l-Ser or l-Thr to a final concentration of 2 mM, and absorbance at 248 nm was monitored at 22°C. To measure 2-oxoacid formation in these reactions, assays were performed as above without or with the indicated amount of Arabidopsis RidA. After 5 min, 75 μL was withdrawn and mixed with 75 μL ice-cold buffer containing 20 mM Tris-HCl, pH 8.0, and 20 mM l-Ile to stop the reaction. The stopped reactions were then held on ice for up to 15 min. For enzymatic determination of 2-oxoacids, aliquots (20 to 50 μL) of the stopped reactions were added to assays (100 μL) containing 50 mM Tris-HCl, pH 8.0, 150 μM NADH, 5 units (l-Thr) or 1 unit (l-Ser) of rabbit muscle l-lactic dehydrogenase (Sigma-Aldrich), and incubated for 10 min (l-Thr) or 5 min (l-Ser) at 22°C while monitoring absorbance at 340 nm. Controls showed that lactic dehydrogenase activity was unaffected by 2.5 mM semicarbazide, that no further 2-oxoacid formation occurred after reactions had been stopped, and that the semicarbazones were stable for at least 15 min in stopped reactions.
To measure Arabidopsis BCAT3 inactivation, preincubations (50 μL) were set up that contained 60 mM Tris-HCl, pH 8.8, 10 μM Arabidopsis Thr dehydratase, 10 or 20 μM Arabidopsis BCAT3, the indicated concentration of Arabidopsis or maize RidA, and 160 mM Ser or Thr and were incubated at 22°C for 1 h. BCAT activity was then assayed spectrophotometrically in continuous coupled assays as described (Schadewaldt and Adelmeyer, 1996). BCAT assays (100 μL) contained 100 mM Tris-HCl, pH 8.3, 5 mM 3-methy-2-oxopentanoic acid (the 2-oxoacid corresponding to Leu), 150 mM glutamic acid, 100 mM aspartic acid, 100 μM PLP, 200 μM NADH, 2 units glutamate aminotransferase (Sigma-Aldrich), and 8.5 units malate dehydrogenase (Sigma-Aldrich) and were started by adding 5 μL preincubation reaction while monitoring OD 340 nm at 22°C.
Subcellular Localization
For chloroplast import assays, in vitro transcription-translation was performed using a TNT-coupled wheat germ extract system (Promega) with [3H]Leu as label, according to the manufacturer’s instructions. Import assays with isolated pea (Pisum sativum) chloroplasts were as described; Arabidopsis YgfZ served as positive control (Rudhe et al., 2002; Frelin et al., 2012). Reaction time was 20 min.
Stable transgenic lines of Arabidopsis (Columbia-0 ecotype) expressing GFP fusion proteins were generated using Agrobacterium tumefaciens (strain GV3101)-mediated floral dip transformation (Clough and Bent, 1998). Confocal microscopic analysis of ∼14-d-old transgenic Arabidopsis seedling leaf cells (and infiltrated Nicotiana benthamiana leaf cells; see below) was performed using a Leica DM RBE microscope (Leica). Fluorophore emissions were collected sequentially; single-labeling experiments showed no detectable crossover for GFP and chlorophyll at the settings used for data collection. Confocal images were acquired as single optical sections and all images of Arabidopsis seedling leaf cells shown in figures are representative of >20 cells from at least two independent lines. None of the transgenic lines displayed obvious growth abnormalities.
For transient expression of mGFP fusion proteins in N. benthamiana, leaves of 4-week-old plants were infiltrated with cultures of Agrobacterium (strain LBA4404) harboring the appropriate binary vector. Procedures for Agrobacterium growth, transformation, infiltration, processing of leaf material for confocal microscopy, and the growth conditions for N. benthamiana plants are described (McCartney et al., 2005). Biolistic bombardment of Nicotiana tabacum BY-2 suspension-cultured cells and epifluorescence microscopic analysis of coexpressed mGFP and mCherry fusion proteins in BY-2 cells were performed as described (Frelin et al., 2012). All fluorescence images of BY-2 cells (and N. benthamiana leaf cells) shown in the figures are representative of >25 transformed cells from at least two independent (co)transformation experiments.
Arabidopsis RidA Knockout Lines
One Arabidopsis line containing a T-DNA insertion in exon three of the gene encoding RidA was identified in the Saskatoon collection (SK15304; Supplemental Figure 4). Genomic DNA was isolated from young leaves of SK15304 and wild-type Columbia-4 plants, and PCR reactions were performed with wild-type allele primers (37 and 38) or T-DNA insertion allele primers (38 and 39); amplicons were analyzed by agarose-gel electrophoresis. All SK15304 plants tested were homozygous for the T-DNA insertion allele. The amplicon obtained with the T-DNA insertion allele primers was T/A cloned into pGEM-T easy (Promega) and sequenced to confirm the insertion site. Total RNA was isolated from homozygous plants using the RNeasy plant mini kit (Qiagen) with on-column DNase treatment. First-strand cDNA was created with the Superscript III First-Strand Synthesis System (Life Technologies). To detect transcripts, PCR reactions were performed with primers designed to amplify the coding sequence of the RidA transcript (40 and 41) or a fragment of the ACTIN-7 transcript (42 and 43); amplicons were analyzed by agarose-gel electrophoresis.
Growth Experiments with Arabidopsis RidA Knockout Plants
Seeds from wild-type and RidA knockout plants grown under identical conditions were surface-sterilized, suspended in water, and held at 4°C for 3 d before beginning all experiments. For root growth experiments, seeds were plated on half-strength MS medium containing 1% (w/v) sucrose, 0.1% (w/v) MES, pH 5.7, 0.6% (w/v) phytagel, and the indicated amount of amino acid(s). Plates were placed vertically under fluorescent lights (photosynthetic photon flux of 100 to 150 μmol photons m−2 s−1) on a 12:12-h light/dark cycle at 22°C. Images were captured every 24 h or after 7 d, and root length was determined with ImageJ software. To obtain leaf samples for metabolomics analysis, seeds were plated on half-strength MS medium, pH 5.7, containing 1% sucrose and 0.5% phytagel and grown as above for 21 d before collecting 100-mg leaf samples. Samples were frozen in liquid nitrogen and stored at −80°C in 1.5-mL Eppendorf tubes. To obtain root samples for metabolomics analysis, 15 to 20 seeds were placed in 125-mL Erlenmeyer flasks containing 50 mL half-strength MS medium plus 1% sucrose and 0.1% MES, pH 5.7. Flasks were incubated for 14 d in continuous fluorescent light (photosynthetic photon flux of 100 to 150 μmol photons m−2 s−1) at 22°C with shaking at 150 rpm. Ser (final concentration 0.5 mM) was added 4 h before harvest; control samples received no Ser. Excess medium was removed from harvested roots by blotting immediately before collecting 100-mg samples. Samples were frozen and stored as above.
Metabolomic Analysis
Samples were extracted by adding 1 mL of methanol:chloroform:water (5:2:2, v/v/v) to the Eppendorf tubes and homogenizing with a SPEX SamplePrep Geno/Grinder 2010 (SPEX SamplePrep) for 2 min at 1350 rpm with 1.6-mm steel balls. Extracts were briefly vortexed, then centrifuged for 2 min at 14,000g. A 500-μL aliquot of the resulting supernatant was transferred to new 1.5-mL Eppendorf tubes containing internal standard (alanine-2,3,3,3-d4; Sigma-Aldrich) and evaporated to dryness in a Labconco CentriVap Concentrator (Labconco). The dried material was derivatized with methoxyamine hydrochloride in pyridine followed by N-tert-butyldimethylsilyl-N-methyltrifluoracetamide (Sigma-Aldrich). Chromatography was performed on an Agilent 7890A GC system (Agilent Technologies) controlled by Agilent Mass Hunter B.06.00 software. Samples (1 μL) were injected in splitless mode onto an Agilent Technologies DB-5MS-UI 30 m × 0.25-mm GC column, 0.25-mm film thickness, with helium carrier gas at 1.1 mL/min. Inlet temperature was set to 250°C, and initial oven temperature was held at 60°C for 0.5 min then ramped 10°C/min to 325°C and held for 10 min; total run time was 37 min. Metabolites were detected with an Agilent 5977 MSD equipped with an Agilent EI source (70 eV). The MS source temperature and quadrupole temperature were set to 230 and 150°C, respectively. MS data were acquired after an 8.0 min solvent delay in selective ion monitoring/scan mode with an electron multiplier gain set to 2.0 and scanning range of m/z 50.0 to 400. Four selective ion monitoring groups were monitored with the following start time and mass (m/z)/dwell times (ms): group 1: 8 min, 89/50, 174/75, 188/75, 202/75, 216/75, and 230/75; group 2: 10.2 min, 216/100 and 258/100; group 3: 14 min, 130/50, 186/50, 200/75, 260/60, 274/60, and 347/60; group 4: 17 min, 288/50, 303/50, 362/50, 376/50, 390/50, and 404/50.
Accession Numbers
Arabidopsis Genome Initiative or maize locus identifiers and GenBank accession numbers for the proteins mentioned in this article are as follows: Arabidopsis RidA, At3g20390 (NP_188674); Arabidopsis Thr dehydratase, At3g10050 (NP_187616); Arabidopsis BCAT3, At3g49680 (NP_566923); and maize RidA, GRMZM2G117642 (ACF84444).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Transcriptomic Evidence Connecting Arabidopsis and Maize RidA with BCAT3 and Thr dehydratase.
Supplemental Figure 2. Recombinant Proteins Used in This Study Were Purified to Near Homogeneity.
Supplemental Figure 3. Arabidopsis RidA and Thr Dehydratase GFP Fusions Are Targeted to Chloroplasts in N. benthamiana Leaf Mesophyll Cells and to Leucoplasts in N. tabacum BY-2 Cells.
Supplemental Figure 4. Confirmation That the Arabidopsis RidA T-DNA Insertion Line Is a Knockout.
Supplemental Figure 5. Restoration by Thr of Root Growth of the Arabidopsis RidA Knockout.
Supplemental Figure 6. Evidence for Protein Complex Formation among Enzymes of Branched-Chain Amino Acid Biosynthesis.
Supplemental Table 1. RidA Family Proteins Used as Query Sequences to Search for Homologs in Arabidopsis and Maize.
Supplemental Table 2. Predicted Localization of Arabidopsis and Maize RidA, Thr Dehydratase, and BCAT Proteins.
Supplemental Table 3. Targeted Metabolomics Analysis of Relative Levels of Thr, Ser, Branched-Chain Amino Acids, and 2-Oxo Acids in RidA Knockout and Wild-Type Arabidopsis Leaves.
Supplemental Table 4. Oligonucleotide Primers Used in This Study.
Supplementary Material
Acknowledgments
This research was supported by U.S. National Science Foundation Awards MCB-1153413 and IOS-1025398 (to A.D.H.) and MCB-1153491 (to O.F.), by National Sciences and Engineering Research Council of Canada Award 217291 (to R.T.M.), and by an endowment from the C.V. Griffin Sr. Foundation. R.T.M. holds a University of Guelph Research Chair. We thank Kenneth Cline for help with chloroplast import assays, Anna-Lisa Paul for advice on root growth experiments, and Kurt Lächler and Stefan Binder for sharing their data on the Arabidopsis BCAT3 knockout.
AUTHOR CONTRIBUTIONS
T.D.N., R.T.M., D.M.D., A.J.L.C., O.F., and A.D.H. designed research. T.D.N., T.N.D.N., S.K.G., M.E.-S., J.A.L., D.R.M., and A.D.H. performed research. T.D.N., M.E.-S., O.F., R.T.M., and A.D.H. analyzed data. T.D.N. and A.D.H. wrote the article.
Glossary
- PLP
pyridoxal 5′-phosphate
- BCAT
branched-chain aminotransferase
- MS
Murashige and Skoog
- BY-2
Bright Yellow-2
Footnotes
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Bessman M.J., Frick D.N., O’Handley S.F. (1996). The MutT proteins or “Nudix” hydrolases, a family of versatile, widely distributed, “housecleaning” enzymes. J. Biol. Chem. 271: 25059–25062. [DOI] [PubMed] [Google Scholar]
- Burman J.D., Stevenson C.E., Sawers R.G., Lawson D.M. (2007). The crystal structure of Escherichia coli TdcF, a member of the highly conserved YjgF/YER057c/UK114 family. BMC Struct. Biol. 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chargaff E., Sprinson D.B. (1943). Studies on the mechanism of deamination of serine and threonine in biological systems. J. Biol. Chem. 151: 273–280. [Google Scholar]
- Chatr-Aryamontri A., et al. (2013). The BioGRID interaction database: 2013 update. Nucleic Acids Res. 41: D816–D823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson M.R., Lambrecht J.A., Downs D., Downs D.M. (2012). Suppressor analyses identify threonine as a modulator of ridA mutant phenotypes in Salmonella enterica. PLoS ONE 7: e43082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson M.R., Schmitz G.E., Downs D.M. (2008). YjgF is required for isoleucine biosynthesis when Salmonella enterica is grown on pyruvate medium. J. Bacteriol. 190: 3057–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Colabroy K.L., Begley T.P. (2005). Tryptophan catabolism: identification and characterization of a new degradative pathway. J. Bacteriol. 187: 7866–7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M., et al. (2010). The genetic landscape of a cell. Science 327: 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ari R., Casadesús J. (1998). Underground metabolism. BioEssays 20: 181–186. [DOI] [PubMed] [Google Scholar]
- Datta P., Bhadra R. (1978). Biodegradative threonine dehydratase. Reduction of ferricyanide by an intermediate of the enzyme-catalyzed reaction. Eur. J. Biochem. 91: 527–532. [DOI] [PubMed] [Google Scholar]
- Davidson R.M., Hansey C.N., Gowda M., Childs K.L., Lin H., Vaillancourt B., Sekhon R.S., de Leon N., Kaeppler S.M., Jiang N., Buell C.R. (2011). Utility of RNA sequencing for analysis of maize reproductive transcriptomes. Plant Genome 4: 191–203. [Google Scholar]
- Diebold R., Schuster J., Däschner K., Binder S. (2002). The branched-chain amino acid transaminase gene family in Arabidopsis encodes plastid and mitochondrial proteins. Plant Physiol. 129: 540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enos-Berlage J.L., Langendorf M.J., Downs D.M. (1998). Complex metabolic phenotypes caused by a mutation in yjgF, encoding a member of the highly conserved YER057c/YjgF family of proteins. J. Bacteriol. 180: 6519–6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farré E.M., Tiessen A., Roessner U., Geigenberger P., Trethewey R.N., Willmitzer L. (2001). Analysis of the compartmentation of glycolytic intermediates, nucleotides, sugars, organic acids, amino acids, and sugar alcohols in potato tubers using a nonaqueous fractionation method. Plant Physiol. 127: 685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J.M., Christopherson M.R., Downs D.M. (2013). Decreased coenzyme A levels in ridA mutant strains of Salmonella enterica result from inactivated serine hydroxymethyltransferase. Mol. Microbiol. 89: 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J.M., Downs D.M. (2013). In the absence of RidA, endogenous 2-aminoacrylate inactivates alanine racemases by modifying the pyridoxal 5′-phosphate cofactor. J. Bacteriol. 195: 3603–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini A., Szklarczyk D., Frankild S., Kuhn M., Simonovic M., Roth A., Lin J., Minguez P., Bork P., von Mering C., Jensen L.J. (2013). STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 41: D808–D815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelin O., Agrimi G., Laera V.L., Castegna A., Richardson L.G., Mullen R.T., Lerma-Ortiz C., Palmieri F., Hanson A.D. (2012). Identification of mitochondrial thiamin diphosphate carriers from Arabidopsis and maize. Funct. Integr. Genomics 12: 317–326. [DOI] [PubMed] [Google Scholar]
- Galperin M.Y., Moroz O.V., Wilson K.S., Murzin A.G. (2006). House cleaning, a part of good housekeeping. Mol. Microbiol. 59: 5–19. [DOI] [PubMed] [Google Scholar]
- Golubev A.G. (1996). The other side of metabolism: a review. Biochemistry (Mosc.) 61: 2018–2039. [PubMed] [Google Scholar]
- Hafner E.W., Wellner D. (1979). Reactivity of the imino acids formed in the amino acid oxidase reaction. Biochemistry 18: 411–417. [DOI] [PubMed] [Google Scholar]
- Hagelstein P., Sieve B., Klein M., Jans H., Schultz G. (1997). Leucine synthesis in chloroplasts: Leucine/isoleucine aminotransferase and valine aminotransferase are different enzymes in spinach chloroplasts. J. Plant Physiol. 150: 23–30. [Google Scholar]
- Heath C., Posner M.G., Aass H.C., Upadhyay A., Scott D.J., Hough D.W., Danson M.J. (2007). The 2-oxoacid dehydrogenase multi-enzyme complex of the archaeon Thermoplasma acidophilum - recombinant expression, assembly and characterization. FEBS J. 274: 5406–5415. [DOI] [PubMed] [Google Scholar]
- Hillebrand G.G., Dye J.L., Suelter C.H. (1979). Formation of an intermediate and its rate of conversion to pyruvate during the tryptophanase-catalyzed degradation of S-o-nitrophenyl-L-cysteine. Biochemistry 18: 1751–1755. [DOI] [PubMed] [Google Scholar]
- Jia Y., Lisch D.R., Ohtsu K., Scanlon M.J., Nettleton D., Schnable P.S. (2009). Loss of RNA-dependent RNA polymerase 2 (RDR2) function causes widespread and unexpected changes in the expression of transposons, genes, and 24-nt small RNAs. PLoS Genet. 5: e1000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler F., Schnell D. (2009). Chloroplast biogenesis: diversity and regulation of the protein import apparatus. Curr. Opin. Cell Biol. 21: 494–500. [DOI] [PubMed] [Google Scholar]
- Kim J.M., Yoshikawa H., Shirahige K. (2001). A member of the YER057c/yjgf/Uk114 family links isoleucine biosynthesis and intact mitochondria maintenance in Saccharomyces cerevisiae. Genes Cells 6: 507–517. [DOI] [PubMed] [Google Scholar]
- Kim K.S., Pelton J.G., Inwood W.B., Andersen U., Kustu S., Wemmer D.E. (2010). The Rut pathway for pyrimidine degradation: novel chemistry and toxicity problems. J. Bacteriol. 192: 4089–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knill T., Schuster J., Reichelt M., Gershenzon J., Binder S. (2008). Arabidopsis branched-chain aminotransferase 3 functions in both amino acid and glucosinolate biosynthesis. Plant Physiol. 146: 1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht J.A., Browne B.A., Downs D.M. (2010). Members of the YjgF/YER057c/UK114 family of proteins inhibit phosphoribosylamine synthesis in vitro. J. Biol. Chem. 285: 34401–34407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht J.A., Flynn J.M., Downs D.M. (2012). Conserved YjgF protein family deaminates reactive enamine/imine intermediates of pyridoxal 5′-phosphate (PLP)-dependent enzyme reactions. J. Biol. Chem. 287: 3454–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht J.A., Schmitz G.E., Downs D.M. (2013). RidA proteins prevent metabolic damage inflicted by PLP-dependent dehydratases in all domains of life. MBio 4: e00033–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner-Dagan Y., Ovadis M., Zuker A., Shklarman E., Ohad I., Tzfira T., Vainstein A. (2006). CHRD, a plant member of the evolutionarily conserved YjgF family, influences photosynthesis and chromoplastogenesis. Planta 225: 89–102. [DOI] [PubMed] [Google Scholar]
- Li P., et al. (2010). The developmental dynamics of the maize leaf transcriptome. Nat. Genet. 42: 1060–1067. [DOI] [PubMed] [Google Scholar]
- Libal-Weksler Y., Vishnevetsky M., Ovadis M., Vainstein A. (1997). Isolation and regulation of accumulation of a minor chromoplast-specific protein from cucumber corollas. Plant Physiol. 113: 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C.L., Van Schaftingen E., Hanson A.D. (2013). Metabolite damage and its repair or pre-emption. Nat. Chem. Biol. 9: 72–80. [DOI] [PubMed] [Google Scholar]
- Manjasetty B.A., et al. (2004). Crystal structure of Homo sapiens protein hp14.5. Proteins 54: 797–800. [DOI] [PubMed] [Google Scholar]
- McCartney A.W., Greenwood J.S., Fabian M.R., White K.A., Mullen R.T. (2005). Localization of the tomato bushy stunt virus replication protein p33 reveals a peroxisome-to-endoplasmic reticulum sorting pathway. Plant Cell 17: 3513–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A. (1950). Reduction of alpha gamma-diketo and alpha-keto acids catalyzed by muscle preparations and by crystalline lactic dehydrogenase. J. Biol. Chem. 184: 117–129. [PubMed] [Google Scholar]
- Morishita R., Kawagoshi A., Sawasaki T., Madin K., Ogasawara T., Oka T., Endo Y. (1999). Ribonuclease activity of rat liver perchloric acid-soluble protein, a potent inhibitor of protein synthesis. J. Biol. Chem. 274: 20688–20692. [DOI] [PubMed] [Google Scholar]
- Obayashi T., Hayashi S., Saeki M., Ohta H., Kinoshita K. (2009). ATTED-II provides coexpressed gene networks for Arabidopsis. Nucleic Acids Res. 37: D987–D991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien P.J., Herschlag D. (1999). Catalytic promiscuity and the evolution of new enzymatic activities. Chem. Biol. 6: R91–R105. [DOI] [PubMed] [Google Scholar]
- Okumoto S., Pilot G. (2011). Amino acid export in plants: a missing link in nitrogen cycling. Mol. Plant 4: 453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsot C. (1987). A common origin for enzymes involved in the terminal step of the threonine and tryptophan biosynthetic pathways. Proc. Natl. Acad. Sci. USA 84: 5207–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Y.G., Jiang Y.L., Ye X.D., Ma X.X., Guo P.C., Lian F.M., Teng Y.B., Chen Y., Zhou C.Z. (2011). Crystal structures and putative interface of Saccharomyces cerevisiae mitochondrial matrix proteins Mmf1 and Mam33. J. Struct. Biol. 175: 469–474. [DOI] [PubMed] [Google Scholar]
- Rudhe C., Chew O., Whelan J., Glaser E. (2002). A novel in vitro system for simultaneous import of precursor proteins into mitochondria and chloroplasts. Plant J. 30: 213–220. [DOI] [PubMed] [Google Scholar]
- Schadewaldt P., Adelmeyer F. (1996). Coupled enzymatic assay for estimation of branched-chain L-amino acid aminotransferase activity with 2-Oxo acid substrates. Anal. Biochem. 238: 65–71. [DOI] [PubMed] [Google Scholar]
- Shah J. (2009). Plants under attack: systemic signals in defence. Curr. Opin. Plant Biol. 12: 459–464. [DOI] [PubMed] [Google Scholar]
- Skarstedt M.T., Greer S.B. (1973). Threonine synthetase of Bacillus subtilis. The nature of an associated dehydratase activity. J. Biol. Chem. 248: 1032–1044. [PubMed] [Google Scholar]
- Steinhauser D., Usadel B., Luedemann A., Thimm O., Kopka J. (2004). CSB.DB: a comprehensive systems-biology database. Bioinformatics 20: 3647–3651. [DOI] [PubMed] [Google Scholar]
- Sun Q., Zybailov B., Majeran W., Friso G., Olinares P.D., van Wijk K.J. (2009). PPDB, the Plant Proteomics Database at Cornell. Nucleic Acids Res. 37: D969–D974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szamosi I., Shaner D.L., Singh B.K. (1993). Identification and characterization of a biodegradative form of threonine dehydratase in senescing tomato (Lycopersicon esculentum) leaf. Plant Physiol. 101: 999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szecowka M., Heise R., Tohge T., Nunes-Nesi A., Vosloh D., Huege J., Feil R., Lunn J., Nikoloski Z., Stitt M., Fernie A.R., Arrivault S. (2013). Metabolic fluxes in an illuminated Arabidopsis rosette. Plant Cell 25: 694–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka S., Murakami S., Kim Y.J., Aoki K. (2000). Complete nucleotide sequence and functional analysis of the genes for 2-aminophenol metabolism from Pseudomonas sp. AP-3. Arch. Microbiol. 174: 265–272. [DOI] [PubMed] [Google Scholar]
- Tawfik D.S. (2010). Messy biology and the origins of evolutionary innovations. Nat. Chem. Biol. 6: 692–696. [DOI] [PubMed] [Google Scholar]
- Teng Y.S., Chan P.T., Li H.M. (2012). Differential age-dependent import regulation by signal peptides. PLoS Biol. 10: e1001416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Rzem R., Marbaix A., Collard F., Veiga-da-Cunha M., Linster C.L. (2013). Metabolite proofreading, a neglected aspect of intermediary metabolism. J. Inherit. Metab. Dis. 36: 427–434. [DOI] [PubMed] [Google Scholar]
- Wang X., Elling A.A., Li X., Li N., Peng Z., He G., Sun H., Qi Y., Liu X.S., Deng X.W. (2009). Genome-wide and organ-specific landscapes of epigenetic modifications and their relationships to mRNA and small RNA transcriptomes in maize. Plant Cell 21: 1053–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters A.J., Makarevitch I., Eichten S.R., Swanson-Wagner R.A., Yeh C.T., Xu W., Schnable P.S., Vaughn M.W., Gehring M., Springer N.M. (2011). Parent-of-origin effects on gene expression and DNA methylation in the maize endosperm. Plant Cell 23: 4221–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel P.M., Graciet E., Douce R., Dumas R. (2000). Evidence for two distinct effector-binding sites in threonine deaminase by site-directed mutagenesis, kinetic, and binding experiments. Biochemistry 39: 15136–15143. [DOI] [PubMed] [Google Scholar]
- Yu H., Zhang F., Wang G., Liu Y., Liu D. (2013). Partial deficiency of isoleucine impairs root development and alters transcript levels of the genes involved in branched-chain amino acid and glucosinolate metabolism in Arabidopsis. J. Exp. Bot. 64: 599–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Wang J.Y., Wu J., Wang J., Poplawsky A., Lin S., Zhu B., Chang C., Zhou T., Zhang L.H., He Y.W. (2013). The diffusible factor synthase XanB2 is a bifunctional chorismatase that links the shikimate pathway to ubiquinone and xanthomonadins biosynthetic pathways. Mol. Microbiol. 87: 80–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.