
Keywords: Arctic, Biodiversity, Climate change, Disease emergence, Historical biogeography, Metabolic Theory of Ecology, Ovibos, Rangifer
Highlights
-
•
Climate change is altering host–parasite interactions in the Arctic.
-
•
Changing ecological barriers reflect climate warming.
-
•
Metabolic Theory of Ecology advances understanding of host–parasite interactions.
-
•
Diversity emerges from host/parasite biogeographic/ecologic history.
-
•
Insights gained from the Arctic apply to more complex systems.
Abstract
Climate change is occurring very rapidly in the Arctic, and the processes that have taken millions of years to evolve in this very extreme environment are now changing on timescales as short as decades. These changes are dramatic, subtle and non-linear. In this article, we discuss the evolving insights into host–parasite interactions for wild ungulate species, specifically, muskoxen and caribou, in the North American Arctic. These interactions occur in an environment that is characterized by extremes in temperature, high seasonality, and low host species abundance and diversity. We believe that lessons learned in this system can guide wildlife management and conservation throughout the Arctic, and can also be generalized to more broadly understand host–parasite interactions elsewhere. We specifically examine the impacts of climate change on host–parasite interactions and focus on: (I) the direct temperature effects on parasites; (II) the importance of considering the intricacies of host and parasite ecology for anticipating climate change impacts; and (III) the effect of shifting ecological barriers and corridors. Insights gained from studying the history and ecology of host–parasite systems in the Arctic will be central to understanding the role that climate change is playing in these more complex systems.
1. Introduction
The Arctic is a vast, sparsely populated region that has captured the imagination and interest of explorers and scientists for generations. It is an environment of extremes and has been considered by many an inhospitable landscape and the last frontier to be explored. Despite the ‘hardships’ imposed by the environment, interconnected communities of wildlife, people, and pathogens have thrived in this dynamically changing ecosystem for millennia (Pitulko et al., 2004; Goebel et al., 2008; Stanford and Bradley, 2012). The current accelerating changes to the arctic climate are, however, unprecedented historically and are having profound impacts on the daily lives of the Arctic’s indigenous peoples and the structure and function of arctic ecosystems. The recently released IPCC report highlights the dramatic abiotic impacts that climate warming is having across the Arctic: shrinking sea-ice and ice caps, melting permafrost, erosion, and an increased frequency in extreme weather events (IPCC, 2013). Consequences of these changes include shifting plant and animal communities and changing food webs (Post et al., 2013), altered animal behaviour and phenology (Post and Forchhammer, 2008; Post et al., 2009), increased heat stress (Ytrehus et al., 2008; van Beest and Milner, 2013), and changes in pathogen diversity and patterns of exposure for people and animals (Kutz et al., 2005, 2009; Burek et al., 2008; Hoberg et al., 2008, 2013).
Wildlife is an integral part of the lives of northerners, and recent changes in the health and behaviour of populations of fishes, birds, and mammals are negatively influencing the physical, social, cultural and economic health of many human communities. For example, recent widespread and steep declines of barren-ground caribou populations have led to hunting quotas being imposed on indigenous peoples in many regions of northern Canada. As caribou hunting is considered an aboriginal right, these new regulations have resulted in dissent and political backlash (Gunn et al., 2009; Festa-Bianchet et al., 2011; Nesbitt and Adamczewski, 2013). Die-offs of muskoxen and population declines in western parts of the Arctic Archipelago over the last 5 years have also led to considerable concern about the future of subsistence and commercial hunting of these animals in the Canadian Arctic. Several recent mortality events in muskoxen have been attributed to the zoonotic bacterium, Erysipelothrix rhusiopathiae (promedmail.org 2012; Canadian Cooperative Wildlife Health Centre www.ccwhc.ca), exacerbating concerns among people about the safety of these ‘country foods’. More generally, with increasing awareness of emerging infectious diseases, northern residents are understandably expressing concern about the safety of foods that have historically been the cornerstone of their diets (Kutz, 2007; Brook et al., 2009; Meakin and Kurvits, 2009; Curry, 2012).
Demonstrating the biotic and abiotic linkages between climate-related drivers and disease emergence is complex (e.g., Brooks and Hoberg, 2006, 2007; Lafferty, 2009; Rohr et al., 2011; Brooks and Hoberg, 2013), but arctic ecosystems provide an intimate arena for understanding the impacts of climate change on host–parasite interactions (Kutz et al., 2005, 2009; Hoberg et al., 2008, 2013; Brooks and Hoberg, 2013). When compared to temperate and tropical regions, arctic ecosystems are characterized by relatively low species diversity (e.g., Callaghan et al., 2004a,b; Meltofte et al., 2013). Currently, anthropogenic disturbances, such as landscape change and introduced species, are also minimal. This allows scientists to track the impacts of climate change on a variety of species and ecological processes with a minimum of confounding factors.
There has been considerable progress in understanding host–parasite interactions in the Arctic over the last two decades. This has been fueled by a critical mass of scientists, wildlife managers, and concerned northern residents. The resulting integration of local knowledge, field biology, classical and advanced DNA-based diagnostic techniques in parasitology, new approaches in ecological modeling, and explorations of historical processes, have generated substantial baselines for parasite biodiversity and new ecological knowledge on host–parasite interactions, particularly for ungulates (e.g., Hoberg et al., 2008, 2013; Kutz et al., 2012; Verocai et al., 2012; Molnár et al., 2013a,b). Increasingly, the ecological, physiological and evolutionary mechanisms that determine the host–parasite interface are being examined and interpreted within historical biogeographic and phylogeographic frameworks (Waltari et al., 2007; Hoberg and Brooks, 2008, 2010; Galbreath and Hoberg, 2012; Hoberg et al., 2012). This synergy of ecology and history ultimately provides deeper insights into the historical and ongoing patterns and processes of faunal assembly that can help us anticipate and predict future impacts of climate change in the north.
In this article, we discuss the evolving insights into host–parasite interactions for wild ungulate species, specifically, muskoxen and caribou, in the North American Arctic. These interactions occur in an environment that is characterized by extremes in temperature, high seasonality, and low host species diversity and abundance. We believe that lessons learned in this system can guide wildlife management and conservation throughout the Arctic, and can also be generalized to provide insights into host–parasite interactions globally. We specifically examine the impacts of climate change on host–parasite interactions and focus on: (I) ‘Its Hotter than you Think’, the direct temperature effects on parasites; (II) ‘Evasions and Invasions’ the importance of considering the intricacies of host and parasite ecology for anticipating climate change impacts; and (III) ‘The Times They are a-Changin’, the effect of shifting ecological barriers and corridors (Fig 1).
Fig. 1.

The parasite fauna of Arctic ungulates has been shaped by historical and contemporary processes. Today, the Arctic today is characterized by extremes in temperature, high seasonality, and low host species diversity and abundance. Rapid climate warming is now a dominant feature that is altering host–parasite interactions in several ways. Temperatures directly affect parasite development and survival in the environment and in ectotherm hosts, and although warming temperatures may initially accelerate transmission, they may quickly exceed the upper thermal tolerance limits for some arctic parasites. Using the Metabolic Theory of Ecology, temperature dependencies can be modeled and generalized to provide broader insights across genera and ecological regions. Climate changes may also alter both host and parasite life-history strategies and phenology, including migration patterns, leading to non-linear changes and tipping points in transmission ecology. Climate warming and associated changes in the cryosphere also alters ecological barriers and corridors, leading to range shifts and new contact zones.
2. The hosts
An appreciation of the biogeographic history of the hosts is central to our current and future understanding of parasite diversity and host–parasite interactions. Caribou (Rangifer tarandus sspp.) and muskoxen (Ovibos moschatus sspp.) are the dominant and most abundant ungulates across the Arctic. The diversification, population structure, and current geographic ranges for these ungulates and their parasites was largely determined by historical processes of episodic expansion and isolation in response to climate variation and alternating glacial-interglacial stages during the last 3 million years. In the late Pliocene (3 Ma; million years before present) and through the Pleistocene (2.6 Ma to 10 Ka; 1000 years before present), ungulate populations in Eurasia and North America were intermittently linked across the Bering Land Bridge (Hoberg et al., 2012); within North America, populations and species were episodically isolated in refugial zones of varying extent and duration, north (Beringia) and south of the continental Laurentide–Cordillera glaciers (e.g., Shafer et al., 2010) (Fig. 2). During the late Pleistocene, muskoxen and caribou were important species in a highly diverse and largely sympatric assemblage of ungulates in Beringia (e.g., Guthrie, 1982). The relative sympatry, diversity and vagility of these ungulates, and their patterns of geographic colonization during episodic periods of climate change in the late Pleistocene, were important in shaping the parasite fauna of caribou and muskoxen (e.g., Hoberg et al., 1999, 2012; Shafer et al., 2010; Hoberg and Brooks, 2013).
Fig. 2.
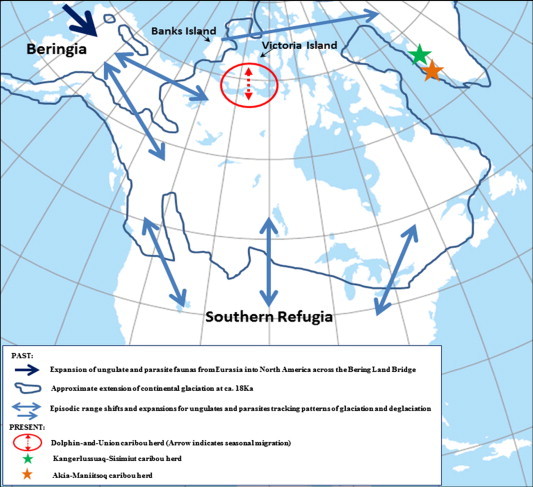
Representation of historical drivers for host and parasite distributions across North America during the Last Glacial Maximum and the post-Pleistocene. The map depicts the current geography of the continent showing an overlay of the maximum extent of past glaciations, pathways for expansion and episodic range shifts by ungulates and parasitic nematodes, and the contemporary distributions of caribou of the migratory Dolphin and Union herd, and of the sedentary Kangerlussuaq-Sisimiut and Akia-Maniitsoq herds of West Greenland.
Today, subspecies of R. tarandus (caribou in North America, reindeer in Eurasia) occur in a near continuous circum-Arctic distribution north of the Arctic Circle. Importantly, with respect to understanding North American parasite faunas, the Peary (R. t. pearyi), Grant’s (R. t. granti) and barren-ground (R. t. groenlandicus) populations are of ‘Beringian’ origin and have closer genetic affinities with Eurasian reindeer than they do with the woodland caribou (R. t. caribou) of boreal North America (e.g., Flagstad and Røed, 2003; McDevitt et al., 2009; Festa-Bianchet et al., 2011; Weckworth et al., 2012; Yannic et al., 2014). These genetic patterns reflect distributions during the Last Glacial Maximum (25–13 Ka) when the continental glaciers in North America separated barren-ground (tundra) and woodland ecotypes. During this time, there were continuous connections for the tundra ecotypes across Beringia into Eurasia, whereas the woodland ecotypes were isolated within multiple refugia south of the continental glaciers (Klütsch et al., 2012) (Fig. 2). Substantial post-Pleistocene expansion of the barren-ground and woodland ecotypes, tracking the de-glaciation of North America, resulted in secondary contact during the Holocene (McDevitt et al., 2009; Weckworth et al., 2012; Yannic et al., 2014). Wild populations of North America and Eurasia have been geographically isolated since the termination of the Pleistocene and the coincidental sea level rise of the Bering Strait about 13–10 Ka. Since the late 1800’s, periodic introductions of Eurasian reindeer into Alaska and Canada for domestic food production (Scotter, 1972; Godkin, 1986; Lankester and Fong, 1989; Finstad et al., 2006) lead to confirmed and suspected translocation, establishment and exchange of some pathogens among the subspecies (Tessaro and Forbes, 1986; Madubata et al., 2012; Verocai et al., 2013). The parasite fauna of caribou thus reflects a mosaic of Eurasian tundra forms and North American woodland forms resulting from both recurrent historical episodes of expansion and isolation and more recent contact among populations (e.g., Hoberg et al., 1999, 2012; Kutz et al., 2012).
Migratory caribou and reindeer are now found across the tundra regions of North America and Eurasia, moving hundreds to thousands of kilometres annually between their summer and winter ranges. They are a keystone species across the Arctic, major consumers of primary productivity and serve as important sources of food for people and predators. Widespread declines have been documented over the last 10 years (e.g., Vors and Boyce, 2009). In some regions these declines are partially explained by intrinsic cyclical variation in caribou population sizes, but population declines have been steep, and may be linked to the cumulative impacts of climate, increased human access, disturbance, and parasitism and disease (Johnson et al., 2005; Nesbitt and Adamczewski, 2013).
Muskoxen originated in Eurasia and expanded into North America at the Beringian nexus about 900–700 Ka. During the late Pleistocene and the Last Glacial Maximum, muskoxen (as O. moschatus) were present in peri-glacial habitats in Beringia and south of the Laurentide ice sheet. They expanded their range from Beringia to the central Canadian Arctic during the Holocene, colonizing Greenland, about 4.5 Ka (Campos et al., 2010). Their late arrival in Greenland contrasts with the history of caribou, which were already present in refugial habitats at high latitudes during the last interglacial stage prior to 110 Ka (Campos et al., 2010).
Muskox distribution today is greatly reduced compared to the historical range. They became extinct from Eurasia by the late Holocene, and were extirpated across large regions of North America in the late 19th and early 20th century (Lent, 1999; Campos et al., 2010). Two extant subspecies of muskoxen are recognized, O. moschatus moschatus and O. m. wardi. The former is distributed on the North American mainland and the latter, the “white-faced muskox”, is found naturally in the Canadian Arctic Archipelago and Greenland. Introduced populations of muskoxen in Norway, Sweden, Russia, northern Quebec and Alaska all originated from translocations of O. m. wardi from East Greenland and Ellesmere Island, Canada. As with caribou and reindeer, the potential for widespread dissemination of parasites and other pathogens with such translocations is apparent (e.g., Kutz et al., 2012; Hoberg et al., 2013).
Muskoxen may be found as solitary males or small bachelor groups, or in larger mixed groups ranging in size from 3 to 50 (Gunn and Adamczewski, 1998; Lent, 1999). They are relatively sedentary with limited to no seasonal migration, although sporadic dispersal such as that between the Canadian mainland and the low arctic islands, and expansion across the Arctic Coastal Plain in Alaska has occurred (Gunn et al., 1991; Reynolds, 1998). Muskoxen are highly susceptible to a wide range of pathogens (Alendal and Helle, 1983; Kutz et al., 2012) and are particularly sensitive to the interacting effects of heat, humidity, disturbance and infectious diseases (Ytrehus et al., 2008).
3. It’s hotter than you think
3.1. Life at the tundra surface
Arctic ecosystems have typically been considered cold, harsh and inhospitable, principally because low temperatures are a major limiting factor for many biological processes. However, recent work by Hoar et al. (2012) suggests that tundra temperatures may exceed the thermal tolerance of at least one parasite, paradoxically reducing transmission potential during the hottest times of the summer. Studying the life history of Ostertagia gruehneri, a common and pathogenic abomasal nematode of caribou, and to a lesser extent, muskoxen, these authors initially hypothesized that tundra temperatures were too cool for larvae to develop from freshly deposited eggs in feces to the infective third larval stage in one Arctic summer. They established paired parasite plots under normal and warmed conditions on the tundra, and monitored temperatures as well as parasite development and survival. This work resulted in several novel findings. First, under both warmed and natural conditions, soil surface temperatures (those that determine the microclimate of the parasites), were considerably warmer than air temperatures, with daily maximums frequently exceeding 30 °C in mid-summer. Second, infective larvae developed in as few as 3 weeks in early summer and would thus be available to calves-of-the-year on the calving grounds. Third, during the hottest part of the summer, the development of parasites on warmed plots was substantially slower than that on non-warmed plots (7 weeks vs. 3 weeks). Linking these results to those from laboratory experiments, several new insights have emerged.
3.1.1. It is warm! A paradigm shift
Ostertagia gruehneri developed more rapidly than might have been expected based on air temperatures alone. This finding is consistent with previous arctic field studies on protostrongylid nematodes where parasite development rates were most accurately predicted by temperatures at the soil surface, while air temperatures consistently underestimated development rates (Kutz et al., 2002; Jenkins et al., 2006a,b). With 24 h of daylight (more often than not manifested as full sunlight) throughout much of the summer, and with minimal shade, tundra soil surface temperatures can get extremely warm (Kutz et al., 2002). Thus, the temperatures experienced by pathogens and their ectothermic intermediate hosts and vectors are often substantially warmer than air temperatures suggest, resulting in more rapid metabolic processes and development occurring faster than expected. These warm temperatures at the level of the parasite microhabitat have striking consequences for pathogen transmission, invasion, and range expansion into tundra ecosystems. Conceivably, tundra surface temperatures may frequently exceed those of the shaded boreal forest to the south, facilitating invasion and establishment of insects and parasites from lower latitudes. One example where this observation becomes immediately relevant is the winter tick, Dermacentor albipictus. Winter ticks can infest and cause significant clinical disease in moose (Alces americanus) and Rangifer subspecies (Welch et al., 1990). Historically limited by temperature to areas far south of the arctic tundra (Wilkinson, 1967), the winter tick substantially expanded its geographic range from 2000 to 2012 and is now approaching the tundra-boreal forest ecotone (Kutz et al., 2009; Kashivakura, 2013). Based on Hoar et al. (2012)’s findings, tundra microhabitat temperatures may be more than adequate for egg development to questing larvae in the short Arctic summer. The potential of winter ticks to establish and amplify in the tundra ecosystem on migratory tundra caribou is thus of considerable concern, in particular because of the significant health impacts on these hosts (Kutz et al., 2009).
3.1.2. Is it too warm?
The reduced development rates of O. gruehneri in warmed plots were at first unexpected. Warmed plots were on average only 1–2 °C warmer than the control plots; however, they had significantly more days where temperatures exceeded 30 °C. Under laboratory conditions, development of O. gruehneri ceases at temperatures greater than 31–34 °C and mortality is 100% at 35 °C or higher. It was thus concluded that these extremes in high temperature were likely the cause of the reduced development rates in the field (Hoar et al., 2012). Interestingly, this upper development threshold of O. gruehneri is lower than that for related nematodes, Ostertagia ostertagi and Teladorsagia circumcincta, parasites of domestic livestock occurring in temperate climates. Larvae of these parasites continue to develop at temperatures up to 35 and 40 °C, respectively. Additionally, although development rates of O. gruehneri were similar to those of O. ostertagi and T. circumcincta at 5–15 °C, at temperatures of 20–30 °C these temperate parasites developed much more rapidly than O. gruehneri (Hoar, 2012).
These data suggest that O. gruehneri has been selected to persist under cooler climatic conditions, has a relatively low upper thermal threshold, and may have reduced fitness under warmer climatic conditions. Ostertagia gruehneri is widespread and abundant in Rangifer across almost their entire range, suggesting that it initially radiated in this host in the amphi-Beringian regions after the inception of the first Northern Hemisphere glacial cycles in the early Pleistocene (after 2.6 Ma) (Hoberg et al., 2012). Further, O. gruehneri is the only species of Ostertagia with a Holarctic distribution; all other species within this genus are either endemic to the Palearctic or Nearctic, but not both, indicating an extended evolutionary association with caribou for this ostertagiine. In this genus, developmental thresholds, thermal tolerances and narrow resilience appear to have strongly influenced biogeographic patterns and parasite diversity between Eurasia and the Nearctic over the late Pliocene and Quaternary (Hoberg et al., 2012). Increasingly cool, xeric, and rigorous environments associated with hemispheric cooling following the late Pliocene were likely drivers for selection and development of specific adaptations for persistence of O. gruehneri in caribou and at high latitudes. If O. gruehneri is narrowly adapted to cooler climate regimes, how will it respond to accelerated warming? Will this new selection pressure predominantly influence development, mortality, or other life history features of this parasite that are central in determining its persistence and geographic distribution?
3.2. Incorporating metabolic theory into predictive models
Hoar et al.’s (2012) work on O. gruehneri suggested an upper thermal threshold for larval development, above which development slows or stops. These developmental restrictions can lead to a mid-summer trough in L3 (third-stage larvae) availability, and a decrease in infection pressure on hosts under warm conditions. These insights stand in contrast to prevailing views that climate warming would simply ‘speed up processes’ and lead to increased infection pressures and increased frequency and severity of disease (e.g., Harvell et al., 2002). Challenges associated with field logistics and small sample sizes, however, prevented a more detailed view on the impacts of warming on host–parasite interactions. Recent application of the Metabolic Theory of Ecology (MTE) to this system has since supported the field studies, and provided a deeper understanding of the consequences of the temperature-dependencies in development, mortality and other parasite life history components on the outcome of dynamic host–parasite interactions under climate change (Molnár et al., 2013b). Moreover, the application of MTE to host–parasite systems, albeit still in its infancy, has already provided a number of tools for generalizing and comparing the impacts of temperature on host–parasite interactions across taxa and ecological regions (Hechinger et al., 2011; Molnár et al., 2013a,b).
The MTE is based on the concept that metabolism scales allometrically with body size and according to the Arrhenius relationship with temperature, or specifically, , where I represents metabolic rate, M is body mass, E is the average activation energy of respiration, T is temperature in degrees Kelvin, and k is Boltzmann’s constant (Gillooly et al., 2001). As metabolism and energy use can be considered one of the fundamental mechanisms underlying all ecological processes, MTE further suggests that the same scaling relationships transfer to processes and patterns across all levels of biological organization (Brown et al., 2004), and in particular to the parasite life history parameters of interest here: development, mortality, reproduction, and parasite uptake. Moreover, while the Arrhenius relationship is only expected to hold at intermediate temperatures (Brown et al., 2004; Dell et al., 2011), the models suggested by MTE can easily be extended to incorporate thermal thresholds in any parasite life history component (Molnár et al., 2013b). Critically, MTE also suggests that the activation energy E will centre around 0.65 eV in most species, or would vary systematically around this value with covariates such as latitude and phylogenetic association (Gillooly et al., 2001; Brown et al., 2004; Irlich et al., 2009; Dell et al., 2011), thereby making temperature effects on parasites inherently predictable even if species-specific data are lacking (Molnár et al., 2013a,b).
Indeed, application of MTE to O. gruehneri showed that the metabolic models were able to capture laboratory data on the temperature-dependencies of parasite development and mortality accurately (Molnár et al., 2013b). Moreover, the metabolic models of Molnár et al. (2013b) also closely matched the patterns observed by Hoar et al. (2012) in the field, in particular with regards to the summer trough in the availability of O. gruehneri L3 under warmed conditions. The metabolic models in particular showed that this trough arises not only because of the slowed development above the upper thermal development threshold but also because larval mortality is substantially increased under warm conditions. Overall, MTE-parameterized host–parasite models focus on the fundamental mechanisms by which temperature changes affect host–parasite systems and also seem to capture the observed parasite dynamics of species where data exist. As such, the approach provides a promising candidate for a predictive framework for the impacts of climate change on host–parasite systems globally (Altizer et al., 2013; Molnár et al., 2013b). The approach allows quantitative predictions for any characteristics describing host–parasite dynamics, such as parasite fitness (as measured through R0), parasite burden and prevalence in hosts, or the stability of host–parasite equilibria and limit cycles, under yet-to-be-observed environmental conditions. Moreover, the approach is particularly suited to understand and predict changes in the temporal and spatial dynamics of host–parasite systems, such as likely range shifts in parasites and phenological changes in parasite transmission. In the O. gruehneri-caribou system, for example, the metabolic models predicted that climate warming would extend the season where parasites are able to survive development to the infective stage towards earlier in spring and later in fall (Molnár et al., 2013b). Furthermore, the models showed that climate warming could split a previously continuous spring-to-fall transmission season into two separate seasons (Molnár et al., 2013b). Whether or not these changes would lead to increased or decreased parasite burdens in caribou is unclear to date, but this could also be evaluated within the energetic framework. Critically, the metabolic approach offers a detailed view on the mechanisms by which climate change impacts host–parasite systems and allows integrating multiple complex and interactive effects within a single framework. In particular, it provides a pathway to understand how environmental factors may either facilitate or limit the potential for range expansions and the establishment of parasites in space and time, making the approach integral to understanding the dynamics of faunal assembly and persistence in both the past and the future (e.g., Hoberg et al., 2012; Hoberg and Brooks, 2013).
3.3. The shelter effect
Another key point that is re-emphasized by the metabolic approach is that it is not the ambient temperature that is critical for host–parasite dynamics, but rather the temperature that parasite larvae experience in their microhabitat (Molnár et al., 2013a). Kutz et al. (2009), for example, suggested that larvae within intermediate hosts would be better able to avoid high temperature extremes on the tundra surface than larvae of parasites with a direct life cycle. This is because free-living larvae are relatively limited in their mobility, whereas larvae within intermediate hosts can benefit from the behavioural thermoregulation of these hosts. Larvae of the muskox lungworm Umingmakstrongylus pallikuukensis, for example, utilize the gastropod host Deroceras laeve for development to the infective stage, and this host seeks shelter if surface temperatures rise above 21 °C (Kutz et al., 2002, 2005). Unlike the free-living larvae of direct life cycle parasites, U. pallikuukensis larvae will, therefore, rarely be exposed to high temperature extremes, and this shelter effect allows them to escape high-temperature-induced decreases in survival and/or development without effort. While direct life cycle parasites generally possess a fitness advantage at low temperatures, the shelter effect can reverse this advantage at high temperatures (Molnár et al., 2013a). As a direct consequence of this, it is conceivable that in seasonal environments climate warming would create a temporal niche in mid-summer that excludes parasites with a direct life cycle, but allows those with an indirect life cycle to persist (Molnár et al., 2013a). These hypotheses, derived from first principles, challenge the conventional assumption (e.g., Harvell et al., 2002; Poulin and Morand, 2004; Dobson et al., 2008) that parasites with an indirect life cycle would be more vulnerable to climate warming than parasites with a direct life cycle.
4. Evasions and invasions: a rolling stone gathers no parasites, especially in the snow – migration, seasonality, and invasions
4.1. Migration to avoid parasitism – arrested development to maximize fitness
Migration is a key adaptation that allows arctic species to cope with winter conditions. Most barren-ground caribou populations undergo lengthy migrations between their summer range on the tundra and their winter ranges below the tree line. Additionally, migration may also help animals to avoid their parasites temporally and may further influence the microevolutionary interactions between hosts and pathogens (Dobson, 1988; Gunn and Irvine, 2003). Two hypotheses of how migration may reduce parasitism in hosts have been advanced: First, migration may allow hosts to separate themselves spatially from parasites, a hypothesis known as ‘migratory escape’. Second, heavily infected animals may have reduced fitness and may not survive the combined energetic costs of parasitism and migration. Such ‘migratory culling’ would effectively remove those parasites from the population (Altizer et al., 2011).
In the barren-ground caribou-Ostertagia system, migratory escape appears to occur, effectively separating caribou from infective larvae in the winter, and perhaps in some areas in the summer range, as well (Hoar, 2012). Eggs of O. gruehneri are shed from spring to fall, but are highly vulnerable to freezing, so if shed too early or too late in the year, the egg mortality approaches 100% (deBruyn, 2010; Hoar et al., 2012). Eggs shed early- to mid-summer can develop to the infective L3 within 3 weeks under optimal conditions, but directional movement of caribou away from the calving grounds to post-calving aggregations may limit their exposure to infective L3 in early- and mid-summer. In contrast, non-directional movement in August–October may increase exposure to parasites in late-summer and early-fall. Subsequently, in late fall, caribou migrate hundreds of kilometres south to the wintering grounds where exposure to O. gruehneri is negligible at best as eggs shed in this range during the winter are unlikely to survive.
The parasitism-reducing effects of host migratory behaviour may, in a sense, be counteracted by the parasites through winter-hardy infectious larval stages in the environment and hypobiosis. The infective L3 larvae (and possibly also the pre-infective L2 stage) of O. gruehneri are characterized by high overwinter survival approaching 100% in experimental plots on the tundra. Thus, although caribou spatially separate themselves from infective larvae in the winter, they may be exposed to two generations of larvae upon their return to the summer range – those shed the previous year and those shed in the current year. Hypobiosis is another prominent feature of O. gruehneri in migratory tundra caribou that may further optimize the parasite’s chances of success. Field post mortems of caribou and experimental infections of captive reindeer have both demonstrated that, regardless of how early or late in the summer an animal is exposed to L3, these larvae do not mature to egg producing adults within that same summer (Hoar, 2012; Hoar et al., 2012). This apparent obligate hypobiosis results in a synchronous emergence of larvae from the abomasal mucosa in the spring, after which they mature to adults and produce high numbers of eggs from May through to August. Under suitable conditions, the large numbers of eggs deposited on the calving grounds may mature to L3 and infect young naïve calves before they leave the calving grounds a month later. These larvae enter hypobiosis as arrested fourth stage larvae in the abomasal mucosa, emerging and maturing to reproductively active adults the following spring. Hypobiosis thus maximizes the parasite’s fitness – under historical and current climatic conditions, development to mature worms immediately following ingestion in the summer would be risky as the time to winter would be short. Eggs deposited late in the summer would be unlikely to develop to the infective stage before winter resulting in high mortality and wasted reproductive effort. A key limitation of this hypobiosis strategy, however, is that the parasite’s generation time is 1 year under the best conditions, and may frequently, in the case of overwintered larvae, be 2 years. However, the propensity for hypobiosis is likely to change under warming conditions and/or with changes in spatial and/or temporal patterns of caribou migration. For example, in the relatively temperate and maritime climate of South Georgia island in the sub-Antarctic, O. gruehneri consistently develops patent infections within a single summer (Leader-Williams, 1980), and related species of Ostertagia and Haemonchus are known to change their propensity to arrest within a few generations when translocated to substantially different ecological regions (Gibbs, 1986; Waller et al., 2004). Consequently, hypobiosis, that has been historically present in an ostertagiine (or more broadly trichostrongyline) lineage over evolutionary timeframes, may be secondarily influenced by selection related to ambient environments or host immunological status (Eysker, 1993); this may also reflect epigenetic effects related to which biochemical pathways are up or down regulated under different prevailing conditions. Thus, hypobiosis may not be a specific adaptation selected in a migration regime, but may be a more general pattern of development and life history enhancing persistence in extreme or variable environments – whether this reflects cold or hot climates on latitudinal gradients (Gibbs, 1986; Hoar, 2012).
The Dolphin and Union caribou herd in Nunavut, Canada, is an extreme example of migration aiding parasite avoidance. This herd migrates annually from its summer range on Victoria Island in the Arctic Archipelago to its wintering grounds on the mainland across the sea ice (Poole et al., 2010; Dumond and Lee, 2013) (Fig. 1), completely separating the herd temporally from the parasites shed on the island. Interestingly, the Dolphin and Union caribou herd historically remained on the island year round and only started migrating to the mainland 30–40 years ago, coincident with significantly increasing muskox populations (Poole et al., 2010). Muskoxen share all three endemic abomasal nematode species (O. gruehneri, Teladorsagia boreoarcticus, and M. marshalli) (Kutz et al., 2012) with caribou, and both M. marshalli and O. gruehneri are negatively associated with body condition and pregnancy rates in caribou (Steele, 2013; Stien et al., 2002). The high numbers of muskoxen on the island contribute substantially to the abundance of these parasites on the island, and migration of caribou may, at least for part of the year, reduce parasite-mediated competition with muskoxen (e.g., Hughes, 2006; Hughes et al., 2009).
The timing of the Dolphin and Union caribou population’s migration to the mainland is constrained by the freeze-up of the ocean channel separating Victoria Island from the mainland (Poole et al., 2010). Caribou stage on the south shore of the island in October and wait for the ice on Coronation Gulf to form. Climate change scenarios predict later freeze-up dates (IPCC, 2013), and this may have significant impacts on the parasite transmission dynamics. A later freeze-up would extend the time that the caribou are waiting to go to the mainland, thereby increasing the temporal exposure of caribou to parasites on the island.
4.2. Migration and dispersal as a mechanisms for parasite invasions
While the migration of the Dolphin and Union herd may help these caribou avoid parasites on the island, concomitantly, this migration and a stochastic dispersal of muskoxen between the mainland and the island – together with a warming climate that has become increasingly permissive for parasite development – may also be responsible for the introduction, establishment and dissemination of two protostrongylid parasites on Victoria island (Kutz et al., 2013). Protostrongylid lungworms, were previously unknown across the arctic islands, but in 2008, U. pallikuukensis, and in 2010, Varestrongylus sp., were detected for the first time on Victoria Island (Kutz et al., 2013). This family of nematodes have gastropod intermediate and ungulate definitive hosts.
Varestrongylus sp., is a cryptic lungworm found in caribou, muskoxen, and less often moose, across most of the mainland Arctic and Subarctic from Alaska to Labrador (Kutz et al., 2007; Verocai et al., 2011). Migratory Dolphin and Union caribou are thought to be responsible for the introduction of this parasite to Victoria Island, where recently permissive climatic conditions appear to have allowed the establishment and subsequent spread of this parasite (Kutz et al., 2013). Laboratory observations of emergence of infective L3 from gastropod intermediate hosts (Verocai, Sullivan, Kafle, Kutz unpubl. data) suggests that transmission for this parasite can occur during the winter, similar to the muskox lungworm, U. pallikuukensis (Kutz et al., 2000). Thus, parasites, acquired by migratory caribou during the winter on the mainland, have likely been introduced repeatedly on the island with the Dolphin and Union herd, over possibly decades. Cooler climatic conditions before the 2000s would have restricted establishment of the lungworm on the island both by limiting its development and influencing the population dynamics of the ectothermic gastropod intermediate hosts. More recent climate warming has likely relaxed constraints for establishment, and could further be driving the potential for host-switching from caribou to muskoxen in areas of habitat overlap. Presence, and apparently increasing prevalence and abundance, of Varestrongylus sp. in muskoxen on Victoria Island indicate that transmission is now occurring on the island, and that muskoxen may now be a mediator of maintenance and amplification of the parasite locally as well as for continued expansion (Kutz et al., 2013).
Umingmakstrongylus pallikuukensis was also recently detected on the island for the first time, but its mechanism for invasion, establishment and spread differs from that of Varestrongylus sp. (Kutz et al., 2013). This protostrongylid parasite is host-specific to muskoxen and has previously been reported only from the central Canadian Arctic mainland (Hoberg et al., 1995; Kutz et al., 2001). Similar to Varestrongylus sp., temperature constraints would have previously limited development of this parasite in gastropods on the island (e.g., Kutz et al., 2005). In contrast, the potential for invasion of U. pallikuukensis was always extremely low given the highly sedentary nature of muskoxen. However, sporadic dispersal of muskoxen from the mainland to the island has occurred historically (M. Dumond, pers. comm.). Converging conditions of a warming climate, a high prevalence and intensity of infection in adult muskoxen from the mainland, and extraordinary levels of production of extremely hardy first-stage larvae over the life spans of the long-lived gravid female U. pallikuukensis (Kutz et al., 2001; Hoberg et al., 2012;) would have facilitated the establishment following dispersal of muskoxen from the mainland to Victoria Island.
This contemporary long range invasion of two protostrongylids has occurred under contrasting mechanisms of recurrent migration (Varestrongylus sp. in caribou) versus sporadic dispersal (U. pallikuukensis in muskoxen). These ongoing invasions afford a unique opportunity to explore parasites with differing host specificity while addressing the specific mode and tempo of geographic colonization under differing mechanisms of dispersal that have served to structure species and faunas since the Pleistocene (Hewitt, 1996, 2004; Hoberg, 2005; Kutz et al., 2013). For example, the phalanx model for expansion, involving large numbers of animals on broad fronts, is consistent with processes influencing the occurrence of Varestrongylus sp.. In contrast, the pioneer model, linked to rapid, sporadic, expansion and small numbers of animals describes the current drivers for U. pallikuukensis. Climate warming, in both cases, is likely driving the successful establishment of the parasites on the island. These observations, now unfolding in the field, provide direct insights into the dynamic processes linking climate, parasite developmental biology, and host population ecology, with the invasion and establishment of macroparasites. Thus, lungworms on Victoria Island are a model for understanding the determinants, modes and history of geographic colonization of parasites at intercontinental and intracontinental scales (Hoberg, 2010; Hoberg et al., 2012).
5. The times they are a-changin’, and so are the barriers and the impacts!
5.1. Changes
Rapid and ongoing climate change is altering ecological barriers, parasite faunal diversity and host–parasite interactions in the Arctic (Hoberg et al., 2013; Kutz et al., 2013). Among arctic ungulates, most gastrointestinal, and many pulmonary and tissue parasites appear to be generalists, establishing with varying fitness in taxonomically diverse hosts such as caribou, moose, muskoxen, and Dall’s sheep where these species are sympatric. For example, Hoberg et al. (2002) reported the colonization of translocated muskoxen with Protostrongylus stilesi from Dall’s sheep and suggested that apparent host-specificity may simply be a result of historical ecological barriers (e.g., Hoberg and Brooks, 2008; Agosta et al., 2010; Brooks and Hoberg, 2013). Thus, shifting ecological conditions and the breakdown of barriers, have the potential to significantly influence host–parasite interactions.
Caribou of west Greenland provide an important example of how both historical and recent processes have shaped the parasite faunal diversity of two caribou populations and how that is likely to change under future scenarios of climate warming. The Kangerlussuaq and Akia-Maniitsoq caribou populations are located in western Greenland and separated by the Sukkertoppen glacier. The abomasal parasite fauna of these two populations differs completely, with only M. marshalli and T. boreoarcticus present in the Kangerlussuaq herd, and only O. gruehneri present in the Akia-Maniitsoq herd (Steele et al., 2013).
At first glance these diversity and geographic patterns are quite enigmatic: O. gruehneri is the most common abomasal parasite of Rangifer, approaching 100% prevalence within most herds, and is only known to be absent from one other herd on Earth – an introduced population in Iceland (Guðmundsdóttir, 2006). Absence of O. gruehneri from the Kangerlussuaq herd is particularly surprising as these caribou are R. t. groenlandicus, originating from the North American Arctic with a colonization history of the western Greenland coast from north to south in the post-Pleistocene. Although parasites were likely lost during geographic colonization (probably reflecting severe bottlenecks and a rapid dispersal of the hosts), the absence of O. gruehneri from Kangerlussuaq, and the presence in the Akia-Maniitsoq herd to the south, is unexpected as the latter herd likely originated from a stochastic dispersal event by a few individuals of the Kangerlussuaq herd (Jepsen et al., 2002). Similarly, the absence of M. marshalli and T. boreoarcticus from the Akia-Maniitsoq herd requires explanation, which may be sought in recent history (ecological time).
Anthropogenic movements of animals in the 20th century likely shaped the current parasite fauna of these caribou. First, muskoxen were translocated from east Greenland to the Kangerlussuaq region in 1962 and 1965 (Korsholm and Olesen, 1993). Both M. marshalli and T. boreoarcticus are common parasites of muskoxen and were likely introduced at that time and subsequently established in the Kangerlussuaq herd. Similarly, there have been at least two introduction events of Eurasian reindeer to the region of Akia-Maniitsoq for commercial farming. These reindeer were frequently sympatric with wild caribou and ultimately many were lost and/or released in this region and interbred with the caribou (Jepsen et al., 2002); O. gruehneri, common in Eurasian reindeer, was likely introduced at that time (Steele et al., 2013).
Thus, the fauna of these herds was most likely shaped initially by historical colonization events where the abomasal parasite fauna was left behind – “missed the boat”– or was extirpated following invasion “drowned on arrival” (Paterson et al., 1999, 2003; Torchin et al., 2003). Subsequent introductions of muskoxen and reindeer likely lead to spill-over of parasites into the respective sympatric caribou populations (Steele et al., 2013). This unique situation, with the divergent parasite faunas, offers a natural laboratory for investigating effects of different parasites in isolation on caribou fitness. Work by Steele (2013) has demonstrated significant impacts of both M. marshalli and O. gruehneri on body condition in these respective host herds; other authors have similarly identified negative impacts of O. gruehneri (Stien et al., 2002). Accelerated warming and deglaciation of the Greenland coastal zone suggests that these herds are not likely to remain isolated for long. Melting of the Sukkertoppen ice cap will allow movement of animals between populations and sharing of parasites with unknown consequences as these nematodes are exchanged between reciprocally naïve populations.
6. Conclusion: the past provides the context for the future – and the future is upon us
The Arctic is a vast landscape of extremes. It is home to a flora and fauna that thrive under these conditions, using a variety of life-history strategies that enable them to persist in this highly seasonal environment. Deep and recent historical processes have played a defining role in determining the structure and diversity of host–parasite assemblages in the Arctic (e.g., Hoberg et al., 1999, 2005). Dynamic variation in climate, operating on a variety of temporal and spatial scales across the landscape, has contributed to the development of significant faunal complexity in what is otherwise a “simple” system. Recognizing this past remains an important key to understanding the present and providing a pathway for potentially predicting how host–parasite systems may respond to a regime of accelerating climate change (Hoberg et al., 2012).
Today and in the future, changes in the cryosphere may create or remove ecological barriers. Extended summers and extremes in heat will alter habitat and host ecology (caribou migration phenology and landscape use), and may exceed thermal tolerances of some arctic adapted hosts (e.g., muskoxen) and parasites (e.g., O. gruehneri), while permitting invasions of others (winter ticks, protostrongylids). Changes in host–parasite interactions are inevitable. A viable pathway to anticipate faunal responses to rapid environmental change will blend robust baselines for diversity, historical biogeography, empirical data on contemporary processes, and quantitative modeling approaches based on the underlying mechanisms determining ecological and physiological processes to (also see Altizer et al., 2013; Hoberg et al., 2013).
Climate change is occurring very rapidly in the Arctic, and the processes that have taken millions of years to evolve in this extreme environment are now under accelerating pressure on timescales as short as decades. These changes are dramatic, subtle and non-linear. The insights we gain from understanding them in the relatively simple world of the Arctic will extrapolate to the temperate and tropical regions where climate warming may be slower but interacts synergistically with rapid rates of land-use change. Insights gained from studying the history and ecology of host–parasite systems in the Arctic will be central to understanding the role that climate change is playing in these more complex systems.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
Our synthesis was in part supported by grants from: the Natural Sciences and Engineering Research Council Government of Canada (to SK), Alberta Ingenuity and Alberta Innovates Health Solutions (SK, GV), the James S. McDonnell Foundation (AD & PM), the Integrated Inventory of Biomes of the Arctic, and funding from the National Science Foundation (DEB- Biodiversity Discovery and Analysis- 1258010) to J.A. Cook (University of New Mexico), EPH (US National Parasite Collection), K.E. Galbreath (Northern Michigan University) and E. Dechaine (Western Washington University).
References
- Agosta S.J., Janz N., Brooks D.R. How specialists can be generalists: resolving the “parasite paradox” and implications for emerging infectious disease. Zoologia (Curitiba) 2010;27:151–162. [Google Scholar]
- Alendal E., Helle O. Helminth parasites of muskoxen Ovibos moschatus in Norway including Spitsbergen and in Sweden, with a synopsis of parasites reported from this host. Fauna Nor. A. 1983;4:41–52. [Google Scholar]
- Altizer S., Bartel R., Han B.A. Animal migration and infectious disease risk. Science. 2011;331:296–302. doi: 10.1126/science.1194694. [DOI] [PubMed] [Google Scholar]
- Altizer S., Ostfeld R., Johnson P.T.J., Kutz S.J., Harvell C.D. Climate change and infectious diseases: from evidence to a predictive framework. Science. 2013;341:514–519. doi: 10.1126/science.1239401. [DOI] [PubMed] [Google Scholar]
- Brook R., Kutz S., Veitch A., Popko R., Elkin B., Guthrie G. Fostering community-based wildlife health monitoring and research in the Canadian North. EcoHealth. 2009;6:266–278. doi: 10.1007/s10393-009-0256-7. [DOI] [PubMed] [Google Scholar]
- Brooks D.R., Hoberg E.P. Systematics and emerging infectious diseases: from management to solution. J. Parasitol. 2006;92:426–429. doi: 10.1645/GE-711R.1. [DOI] [PubMed] [Google Scholar]
- Brooks D.R., Hoberg E.P. How will global climate change affect parasite–host assemblages? Trends Parasitol. 2007;23:571–574. doi: 10.1016/j.pt.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Brooks D.R., Hoberg E.P. The emerging infectious diseases crisis and pathogen pollution. In: Rohde K., editor. The Balance of Nature and Human Impact. Cambridge University Press; 2013. pp. 215–229. [Google Scholar]
- Brown J.H., Gillooly J.F., Allen A.P., Savage V.M., West G.B. Toward a metabolic theory of ecology. Ecol. Lett. 2004;85:1771–1789. [Google Scholar]
- Burek K.A., Gulland F.M.D., O’Hara T.M. Effects of climate change on Arctic marine mammal health. Ecol. Appl. 2008;18:S126–134. doi: 10.1890/06-0553.1. [DOI] [PubMed] [Google Scholar]
- Callaghan T.V., Björn L.O., Chernov Y., Chapin T., Christiansen T.R., Huntley B., Ims R.A., Johansson M., Jolly D., Jonasson S., Matveyeva N., Panikov N., Oechel W., Shaver G., Elster J., Henttonen H., Laine K., Taulavuori K., Taulavuori E., Zöckler C. Biodiversity, distributions and adaptations of Arctic species in the context of environmental change. Ambio. 2004;33:404–417. doi: 10.1579/0044-7447-33.7.404. [DOI] [PubMed] [Google Scholar]
- Callaghan T.V., Björn L.O., Chernov Y., Chapin T., Christiansen T.R., Huntley B., Ims R.A., Johansson M., Jolly D., Jonasson S., Matveyeva N., Panikov N., Oechel W., Shaver G., Henttonen H. Effects on the structure of Arctic ecosystems in the short- and long-term perspectives. Ambio. 2004;33:436–447. doi: 10.1579/0044-7447-33.7.436. [DOI] [PubMed] [Google Scholar]
- Campos P.F., Willerslev E., Sher A., Orlando L., Axelsson E., Tikohonov A., Aaris-Sørensen K., Greenwood A.D., Kahlke R.-D., Kosintsev P., Krakhmalnaya T., Kuznetsova T., Lemey P., MacPhee R., Norris C.A., Shepherd K.A., Suchard M.A., Zazula G.D., Shapiro B., Gilbert M.T.P. Ancient DNA analyses exclude humans as the driving force behind late Pleistocene musk ox (Ovibos moschatus) population dynamics. PNAS. 2010;107:5675–5680. doi: 10.1073/pnas.0907189107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry P.S. Department of Ecosystem and Public Health, University of Calgary; Calgary: 2012. Blood on Filter Paper for Monitoring Caribou Health: Efficacy, Community-Based Collection, and Disease Ecology in Circumpolar Herds. p. 308. [Google Scholar]
- deBruyn N.P. Department of Biological Sciences, University of Calgary; Calgary: 2010. Gastrointestinal nematodes of western Canadian cervids: molecular diagnostics, faunal baselines and management considerations. [Google Scholar]
- Dell A.I., Pawar S., Savage V.M. Systematic variation in the temperature dependence of physiological and ecological traits. PNAS. 2011;108:10591–10596. doi: 10.1073/pnas.1015178108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson A.P. The population biology of parasite-induced changes in host behavior. Q. Rev. Biol. 1988;63:139–165. doi: 10.1086/415837. [DOI] [PubMed] [Google Scholar]
- Dobson A., Lafferty K.D., Kuris A.M., Hechinger R.F., Jetz W. Homage to Linnaeus: how many parasites? How many hosts? PNAS. 2008;105:11482–11489. doi: 10.1073/pnas.0803232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumond M., Lee D.S. Dolphin and Union caribou herd status and trends. Arctic. 2013;66:132–137. [Google Scholar]
- Eysker M. The role of inhibited development in the epidemiology of Ostertagia infections. Vet. Parasitol. 1993;46:259–269. doi: 10.1016/0304-4017(93)90063-s. [DOI] [PubMed] [Google Scholar]
- Festa-Bianchet M., Ray J.C., Boutin S., Côté S.D., Gunn A. Conservation of caribou (Rangifer tarandus) in Canada: an uncertain future. Can. J. Zool. 2011;89:419–434. [Google Scholar]
- Finstad G.L., Kielland K.K., Schneider W.S. Reindeer herding in transition: Historical and modern day challenges for Alaskan reindeer herders. Nomadic Peoples. 2006;10:31–49. [Google Scholar]
- Flagstad O., Røed K.H. Refugial origins of reindeer (Rangifer tarandus L.) inferred from mitochondrial DNA sequences. Evolution. 2003;57:658–670. doi: 10.1111/j.0014-3820.2003.tb01557.x. [DOI] [PubMed] [Google Scholar]
- Galbreath K.E., Hoberg E.P. Return to Beringia: parasites reveal cryptic biogeographic history of North American pikas. Proc. R. Soc. B. 2012;279:371–378. doi: 10.1098/rspb.2011.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs H.C. Hypobiosis in parasitic nematodes – an update. Adv. Parasitol. 1986;25:129–174. [PubMed] [Google Scholar]
- Gillooly J.F., Brown J.H., West G.B., Savage V.M., Charnov E.L. Effects of size and temperature on metabolic rate. Science. 2001;293:2248–2251. doi: 10.1126/science.1061967. [DOI] [PubMed] [Google Scholar]
- Godkin G.F. The reindeer industry in Canada. Can. Vet. J. 1986;27:488–490. [PMC free article] [PubMed] [Google Scholar]
- Goebel T., Waters M.R., O’Rourke D.H. The late Pleistocene dispersal of modern humans in the Americas. Science. 2008;319:1497–1502. doi: 10.1126/science.1153569. [DOI] [PubMed] [Google Scholar]
- Guðmundsdóttir B. Faculty of Medicine, University of Iceland; Reykjavik: 2006. Parasites of reindeer (Rangifer tarandus) in Iceland. p. 100. [Google Scholar]
- Gunn A., Adamczewski J. Muskoxen. In: Stackpoles B., editor. Deer of the world: their evolution, behaviour and ecology. Mechanicsburg; Pennsylvania, USA: 1998. pp. 1076–1094. [Google Scholar]
- Gunn A., Irvine R.J. Subclinical parasitism and ruminant foraging strategies: a review. Wildl. Soc. Bull. 2003;31:117–126. [Google Scholar]
- Gunn A., Shank C., McLean B. The history, status, and management of muskoxen on Banks Island. Arctic. 1991;44:188–195. [Google Scholar]
- Gunn A., Russell D., White R.G., Kofinas G. Facing a future of change: wild migratory caribou and reindeer. Arctic. 2009;62:iii–vi. [Google Scholar]
- Guthrie R.D. Mammals of the Mammoth Steppe as paleoecological indicators. In: Hopkins D.M., Matthews J.V. Jr., Schweger C.E., Young S.B., editors. Paleoecology of Beringia. Academic Press; New York: 1982. pp. 307–326. [Google Scholar]
- Harvell C.D., Mitchell C.E., Ward J.R., Altizer S., Dobson A.P., Ostfeld R.S., Samuel M.D. Climate warming and disease risks for terrestrial and marine biota. Science. 2002;296:2158–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- Hechinger R.F., Lafferty K.D., Dobson A.P., Brown J.H., Kuris A.M. A common scaling rule for abundance, energetics, and production of parasitic and free-living species. Science. 2011;333:445–448. doi: 10.1126/science.1204337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt G.M. Some genetic consequences of the ice ages and their role in divergence and speciation. Biol. J. Linn. Soc. 1996;58:247–276. [Google Scholar]
- Hewitt G.M. Genetic consequences of climate oscillations in the Quaternary. Philos. Trans. R. Soc. Lond. B. 2004;359:183–195. doi: 10.1098/rstb.2003.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoar B. Faculty of Science, University of Calgary; Calgary: 2012. Ecology and transmission dynamics of Ostertagia gruehneri in barrenground caribou. p. 115. [Google Scholar]
- Hoar B.M., Ruckstuhl K., Kutz S. Development and availability of the free-living stages of Ostertagia gruehneri, an abomasal parasite of barrenground caribou (Rangifer tarandus groenlandicus), on the Canadian tundra. Parasitology. 2012;139:1093–1100. doi: 10.1017/S003118201200042X. [DOI] [PubMed] [Google Scholar]
- Hoberg E.P. Coevolution and biogeography among Nematodirinae (Nematoda: Trichostrongylina), Lagomorpha and Artiodactyla (Mammalia): exploring determinants of history and structure for the northern fauna across the Holarctic. J. Parasitol. 2005;91:358–369. doi: 10.1645/GE-3466. [DOI] [PubMed] [Google Scholar]
- Hoberg E.P. Invasive processes, mosaics and the structure of helminth parasite faunas. Rev. Sci. Tech. OIE. 2010;29:255–272. [PubMed] [Google Scholar]
- Hoberg E.P., Brooks D.R. A macroevolutionary mosaic: episodic host-switching, geographical colonization and diversification in complex host–parasite systems. J. Biogeogr. 2008;35:1533–1550. [Google Scholar]
- Hoberg E.P., Brooks D.R. Beyond vicariance: integrating taxon pulses, ecological fitting and oscillation in historical biogeography and evolution. In: Morand S., Krasnow B., editors. The Geography of Host–Parasite Interactions. Oxford University Press; 2010. pp. 7–20. [Google Scholar]
- Hoberg E.P., Brooks D.R. Episodic processes, invasion and faunal mosaics in evolutionary and ecological time. In: Rodhde K., editor. The Balance of Nature and Human Impact. Cambridge University Press; 2013. pp. 199–213. [Google Scholar]
- Hoberg E.P., Polley L., Gunn A., Nishi J.S. Umingmakstrongylus pallikuukensis gen. nov. et sp. nov. (Nematoda: Protostrongylidae) from muskoxen, Ovibos moschatus, in the central Canadian Arctic, with comments on biology and biogeography. Can. J. Zool. 1995;73:2266–2282. [Google Scholar]
- Hoberg E.P., Monsen K.J., Kutz S.J., Blouin M.S. Structure, biodiversity, and historical biogeography of nematode faunas in Holarctic ruminants: morphological and molecular diagnoses for Teladorsagia boreoarcticus n. sp. (Nematoda: Ostertagiinae), a dimorphic cryptic species in muskoxen (Ovibos moschatus) J. Parasitol. 1999;85:910–934. [PubMed] [Google Scholar]
- Hoberg E.P., Kutz S., Nagy J., Jenkins E.J., Elkin B., Branigan M., Cooley D. Protostrongylus stilesi (Nematoda: Protostrongylidae): ecological isolation and putative host-switching between Dall’s sheep and muskoxen in a contact zone. Comp. Parasitol. 2002;69:1–9. [Google Scholar]
- Hoberg E.P., Polley L., Jenkins E.J., Kutz S., Veitch A., Elkin B. Integrated approaches and empirical models for investigation of parasitic diseases in northern wildlife. Emerg. Infect. Dis. 2008;14:10–17. doi: 10.3201/eid1401.071119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoberg E.P., Galbreath K.E., Cook J.A., Kutz S.J., Polley L. Northern host–parasite assemblages: history and biogeography on the borderlands of episodic climate and environmental transition. Adv. Parasitol. 2012;79:1–97. doi: 10.1016/B978-0-12-398457-9.00001-9. [DOI] [PubMed] [Google Scholar]
- Hoberg E.P., Kutz S.J., Cook J.A., Galaktionov K., Haukisalmi V., Henttonen H., Laaksonen S., Makarikov A., Marcogliese D.J. Parasites in terrestrial, freshwater and marine systems. In: Meltofte H., editor. Arctic Biodiversity Assessment – Status and Trends in Arctic Biodiversity. Conservation of Arctic Fauna and Flora, Arctic Council; Akureyri, Iceland: 2013. pp. 420–449. [Google Scholar]
- Hughes J.R. University of Aberdeen; 2006. The influence of forage and parasites on the migration of the Dolphin-Union caribou herd. [Google Scholar]
- Hughes J., Albon S.D., Irvine R.J., Woodin S. Is there a cost of parasites to caribou? Parasitology. 2009;136:253–265. doi: 10.1017/S0031182008005246. [DOI] [PubMed] [Google Scholar]
- IPCC, 2013. Summary for Policymakers. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In: Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M.e. (Eds.).
- Irlich U.M., Terblanche J.S., Blackburn T.M., Chown S.L. Insect rate-temperature relationships: environmental variation and the metabolic theory of ecology. Am. Nat. 2009;174:819–835. doi: 10.1086/647904. [DOI] [PubMed] [Google Scholar]
- Jenkins E.J., Kutz S.J., Hoberg E.P., Polley L. Bionomics of larvae of Parelaphostrongylus odocoilei (Nematoda: Protostrongylidae) in experimentally infected gastropod intermediate hosts. J. Parasitol. 2006;92:298–305. doi: 10.1645/GE-629R.1. [DOI] [PubMed] [Google Scholar]
- Jenkins E.J., Veitch A.M., Kutz S.J., Hoberg E.P., Polley L. Climate change and the epidemiology of protostrongylid nematodes in northern ecosystems: Parelaphostrongylus odocoilei and Protostrongylus stilesi in Dall’s sheep (Ovis d. dalli) Parasitology. 2006;132:387–401. doi: 10.1017/S0031182005009145. [DOI] [PubMed] [Google Scholar]
- Jepsen B.I., Siegismund H.R., Fredholm M. Population genetics of the native caribou (Rangifer tarandus groenlandicus) and the semi-domestic reindeer (Rangifer tarandus tarandus) in Southwestern Greenland: evidence of introgression. Conserv. Genet. 2002;3:401–409. [Google Scholar]
- Johnson C.J., Boyce M.S., Case R.L., Cluff H.D., Gau R.J., Gunn A., Mulders R. Cumulative effects of human developments on arctic wildlife. Wildl. Monogr. 2005;160:1–36. [Google Scholar]
- Kashivakura C.K. Faculty of Veterinary Medicine, University of Calgary; Calgary: 2013. Detecting Dermacentor albipictus (Packard, 1869), the winter tick, at the northern extent of its distribution range: Hunter-based monitoring and serological assay development, Ecosystem and Public Health. [Google Scholar]
- Klütsch C.F.C., Manseau M., Wilson P.J. Phylogeographical analysis of mtDNA data indicates postglacial expansion from multiple glacial refugia in Woodland Caribou (Rangifer tarandus caribou) PLoS One. 2012;7:e52661. doi: 10.1371/journal.pone.0052661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsholm H., Olesen C.R. Preliminary investigations on the parasite burden and distribution of endoparasite species of muskox (Ovibos moschatus) and caribou (Rangifer tarandus groenlandicus) in West Greenland. Rangifer. 1993;13:185–189. [Google Scholar]
- Kutz, S.J., 2007. An Evaluation of the Role of Climate Change in the Emergence of Pathogens and Diseases in Arctic and Subarctic Caribou Populations. Climate Change Action Fund, Project A760. In: Prepared for the Climate Change Action Fund, G.o.C. (Ed.), Climate Change Action Fund, Project A760. Research Group for Arctic Parasitology (RGAP), Faculty of Veterinary Medicine, University of Calgary, Calgary, Alberta, Canada.
- Kutz S.J., Hoberg E.P., Polley L. Emergence of third-stage larvae of Umingmakstrongylus pallikuukensis from three gastropod intermediate host species. J. Parasitol. 2000;86:743–749. doi: 10.1645/0022-3395(2000)086[0743:EOTSLO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kutz S.J., Hoberg E.P., Polley L. A new lungworm in muskoxen: an exploration in Arctic parasitology. Trends Parasitol. 2001;17:276–280. doi: 10.1016/s1471-4922(01)01882-7. [DOI] [PubMed] [Google Scholar]
- Kutz S.J., Hoberg E.P., Nishi J., Polley L. Development of the muskox lungworm, Umingmakstrongylus pallikuukensis (Protostrongylidae), in gastropods in the Arctic. Can. J. Zool. 2002;80:1977–1985. [Google Scholar]
- Kutz S.J., Hoberg E.P., Polley L., Jenkins E.J. Global warming is changing the dynamics of Arctic host–parasite systems. Proc. R. Soc. B. 2005;272:2571–2576. doi: 10.1098/rspb.2005.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutz S.J., Asmundsson I., Hoberg E.P., Appleyard G.D., Jenkins E.J., Beckmen K., Branigan M., Butler L., Chilton N.B., Cooley D., Elkin B., Huby-Chilton F., Johnson D., Kuchboev A., Nagy J., Oakley M., Polley L., Popko R., Scheer A., Simard M., Veitch A. Serendipitous discovery of a novel protostrongylid (Nematoda: Metastrongyloidea) in caribou, muskoxen, and moose from high latitudes of North America based on DNA sequence comparisons. Can. J. Zool. 2007;85:1143–1156. [Google Scholar]
- Kutz S.J., Jenkins E.J., Veitch A.M., Ducrocq J., Polley L., Elkin B., Lair S. The Arctic as a model for anticipating, preventing, and mitigating climate change impacts on host–parasite interactions. Vet. Parasitol. 2009;163:217–228. doi: 10.1016/j.vetpar.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Kutz S.J., Ducrocq J., Verocai G.G., Hoar B.M., Colwell D.D., Beckmen K., Polley L., Elkin B.T., Hoberg E.P. Parasites of ungulates of arctic North America and Greenland: a view of contemporary diversity, ecology, and impact in a world under change. Adv. Parasitol. 2012;79:99–252. doi: 10.1016/B978-0-12-398457-9.00002-0. [DOI] [PubMed] [Google Scholar]
- Kutz S.J., Checkley S., Verocai G.G., Dumond M., Hoberg E., Peacock R., Wu J., Orsel K., Seegers K., Warren A., Abrams A. Invasion, establishment, and range expansion of two protostrongylid nematodes in the Canadian Arctic. Glob. Change Biol. 2013;19:3254–3262. doi: 10.1111/gcb.12315. [DOI] [PubMed] [Google Scholar]
- Lafferty K.D. The ecology of climate change and infectious diseases. Ecol. Lett. 2009;90:888–900. doi: 10.1890/08-0079.1. [DOI] [PubMed] [Google Scholar]
- Lankester M.W., Fong D. Distribution of elaphostrongyline nematodes (Metastrongyloidea, Protostrongylidae) in Cervidae and possible effects of moving Rangifer spp. into and within North America. Alces. 1989;25:133–145. [Google Scholar]
- Leader-Williams N. Observations on the internal parasites of reindeer introduced into South Georgia. Vet. Rec. 1980;107:393–395. doi: 10.1136/vr.107.17.393. [DOI] [PubMed] [Google Scholar]
- Lent P.C. University of Oklahoma Press; Norman: 1999. Muskoxen and their Hunters: A History. [Google Scholar]
- Madubata C., Dunams-Morel D.B., Elkin B., Oksanen A., Rosenthal B.M. Evidence for a recent population bottleneck in an Apicomplexan parasite of caribou and reindeer, Besnoitia tarandi. Infect. Genet. Evol. 2012;12:1605–1613. doi: 10.1016/j.meegid.2012.06.007. [DOI] [PubMed] [Google Scholar]
- McDevitt A.D., Mariani S., Hebblewhite M., Decesare N.J., Morgantini L., Seip D., Weckworth B.V., Musiani M. Survival in the Rockies of an endangered hybrid swarm from diverged caribou (Rangifer tarandus) lineages. Mol. Ecol. 2009;18:665–679. doi: 10.1111/j.1365-294X.2008.04050.x. [DOI] [PubMed] [Google Scholar]
- Meakin, S., Kurvits, T., 2009. Assessing the impacts of climate change on food security in the Canadian Arctic, in: Affairs, I.a.N. (Ed.). GRID-Arendal, Ottawa, Canada, p. 46.
- Meltofte H., Barry T., Berteaux D., Bultman H., Christianson J.S., Cook J.A. Arctic biodiversity assessment, status and trends in arctic biodiversity: synthesis. In: Meltofte H., Josefson A.P., Payer D., editors. Arctic Biodiversity Assessment, Status and Trends in Arctic Biodiversity Arctic Council. Conservation of Arctic Fauna and Flora (CAFF); 2013. pp. 1–120. [Google Scholar]
- Molnár P.K., Dobson A.P., Kutz S.J. Gimme Shelter – the relative sensitivity of parasitic nematodes with direct and indirect life cycles to climate change. Glob. Change Biol. 2013;19:3291–3305. doi: 10.1111/gcb.12303. [DOI] [PubMed] [Google Scholar]
- Molnár P.K., Kutz S.J., Hoar B.M., Dobson A.P. Metabolic approaches to understanding climate change impacts on seasonal host–macroparasite dynamics. Ecol. Lett. 2013;16:9–21. doi: 10.1111/ele.12022. [DOI] [PubMed] [Google Scholar]
- Nesbitt, L., Adamczewski, J., 2013. In: Environment and Natural Resources, G.o.t.N.T. (Ed.). Government of the Northwest Territories, Yellowknife, p. 56.
- Paterson A.M., Palma R.L., Gray R.D. How frequently do avian lice miss the boat? Implications for coevolutionary studies. Syst. Biol. 1999;48:214–223. [Google Scholar]
- Paterson A.M., Palma R.L., Gray R.D. Drowning on arrival, missing the boat and X events: how likely are sorting events? In: Page R.D., editor. Tangled Trees: Phylogeny, Cospeciation and Coevolution. University of Chicago Press; 2003. pp. 287–309. [Google Scholar]
- Pitulko V.V., Nikolsky P.A., Girya E.Y., Basilyan A.E., Tumskoy V.E., Koulokov S.A., Astakhov S.N., Yu P.E., Anisimov N.A. The Yana RHS site: humans in the Arctic before the last glacial maximum. Science. 2004;303:52–56. doi: 10.1126/science.1085219. [DOI] [PubMed] [Google Scholar]
- Poole K., Gunn A., Patterson B.R., Dumond M. Sea ice and migration of the Dolphin and Union caribou herd in the Canadian Arctic: an uncertain future. Arctic. 2010;63:414–428. [Google Scholar]
- Post E., Forchhammer M.C. Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Philos. Trans. R. Soc. Lond. B. 2008;363:2367–2373. doi: 10.1098/rstb.2007.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post E., Forchhammer M.C., Bret-Harte M.S., Callaghan T.V., Christensen T.R., Elberling B., Fox A.D., Gilg O., Hik D.S., Hoye T.T., Ims R.A., Jeppesen E., Klein D.R., Madsen J., McGuire A.D., Rysgaard S., Schindler D.E., Stirling I., Tamstorf M.P., Tyler N.J.C., van der Wal R., Welker J., Wookey P.A., Schmidt N.M., Aastrup P. Ecological dynamics across the Arctic associated with recent climate change. Science. 2009;325:1355–1358. doi: 10.1126/science.1173113. [DOI] [PubMed] [Google Scholar]
- Post E., Bhatt U., Bitz C., Brodie J., Fulton T.L., Hebblewhite M., Kerby J., Kutz S.J., Stirling I., Walker D.A. Ecological consequences of sea-ice decline. Science. 2013;341:519–524. doi: 10.1126/science.1235225. [DOI] [PubMed] [Google Scholar]
- Poulin R., Morand S. Smithsonian Institution Books; Washington, DC: 2004. Parasite Biodiversity. [Google Scholar]
- Reynolds P.E. Dynamics and range expansion of a re-established muskox population. J. Wildl. Manage. 1998;62:734–744. [Google Scholar]
- Rohr J.R., Dobson A.P., Johnson P.T.J., Kilpatrick A.M., Paull S.H., Raffel T.R., Ruiz-Moreno D., Thomas M.B. Frontiers in climate change-disease research. Trends Ecol. Evol. 2011;26:270–277. doi: 10.1016/j.tree.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotter G.W. Reindeer ranching in Canada. J. Range Manage. 1972;25:167–174. [Google Scholar]
- Shafer A.B.A., Cullingham C.I., CÔTÉ S.D., Coltman D.W. Of glaciers and refugia: a decade of study sheds new light on the phylogeography of northwestern North America. Mol. Ecol. 2010;19:4589–4621. doi: 10.1111/j.1365-294X.2010.04828.x. [DOI] [PubMed] [Google Scholar]
- Stanford D.J., Bradley B.A. University of California Press; Berkeley: 2012. Across Atlantic Ice: The Origin of America’s Clovis Culture. [Google Scholar]
- Steele J. University of Calgary; Calgary: 2013. The Devil’s in the Diversity: Divergent Parasite Faunas and their Impacts on Body Condition in Two Greenland Caribou Populations, Veterinary Medical Sciences. p. 139. [Google Scholar]
- Steele J., Orsel K., Cuyler C., Hoberg E., Schmidt N.M., Kutz S.J. Divergent parasite faunas in adjacent populations of west Greenland caribou: natural and anthropogenic influences on diversity. Int. J. Parasitol. 2013;2:197–202. doi: 10.1016/j.ijppaw.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stien A., Irvine R.J., Ropstad E., Halvorsen O., Langvatn R., Albon S.D. The impact of gastrointestinal nematodes on wild reindeer: experimental and cross-sectional studies. J. Anim. Ecol. 2002;71:937–945. [Google Scholar]
- Tessaro S.V., Forbes L.B. Brucella suis biotype 4: a case of granulomatous nephritis in a barren ground caribou (Rangifer tarandus groenlandicus) with a review of the distribution of rangiferine brucellosis in Canada. J. Wildl. Dis. 1986;22:479–483. doi: 10.7589/0090-3558-22.4.479. [DOI] [PubMed] [Google Scholar]
- Torchin M.E., Lafferty K.D., Dobson A.P., McKenzie V.J., Kuris A.M. Introduced species and their missing parasites. Nature. 2003;421:628–630. doi: 10.1038/nature01346. [DOI] [PubMed] [Google Scholar]
- van Beest F.M., Milner J.M. Behavioural responses to thermal conditions affect seasonal mass chane in a heat-senisitive northern ungulate. PLoS One. 2013;8:e65972. doi: 10.1371/journal.pone.0065972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verocai, G.G., Kutz, S.J., Simard, M., Hoberg, E.P., 2011. A newly discovered Varestrongylus species (Nematoda: Protostrongylidae) in ungulates at high latitudes of North America: Taxonomy and Phylogeny. In: Proceedings of the 23rd International Conference of the World Association for the Advancement of Veterinary Parasitology, Buenos Aires, Argentina.
- Verocai G.G., Lejeune M., Beckmen K.B., Kashivakura C.K., Veitch A.M., Popko R., Fuentealba C., Hoberg E.P., Kutz S.J. Defining parasite biodiversity at high latitudes of North America: new host and geographic records for Onchocerca cervipedis (Nematoda: Onchocercidae) in moose and caribou. Parasite Vectors. 2012;5:242. doi: 10.1186/1756-3305-5-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verocai G.G., Lejeune M., Finstad G.L., Kutz S.J. A Nearctic parasite in a Palearctic host: Parelaphostrongylus andersoni (Nematoda; Protostrongylidae) infecting semi-domesticated reindeer in Alaska. Int. J. Parasitol. 2013;2:119–123. doi: 10.1016/j.ijppaw.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vors L.S., Boyce M.S. Global declines of caribou and reindeer. Glob. Change Biol. 2009;15:2626–2633. [Google Scholar]
- Waller P.J., Rudby-Martin L., Ljungstrom B.L., Rydzik A. The epidemiology of abomasal nematodes of sheep in Sweden, with particular reference to over-winter survival strategies. Vet. Parasitol. 2004;122:207–220. doi: 10.1016/j.vetpar.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Waltari E., Hoberg E.P., Lessa E.P., Cook J.A. Eastward Ho: phylogeographical perspectives on colonization of hosts and parasites across the Beringian nexus. J. Biogeogr. 2007;34:561–574. [Google Scholar]
- Weckworth B.V., Musiani M., McDevitt A.D., Hebblewhite M., Mariani S. Reconstruction of caribou evolutionary history in Western North America and its implications for conservation. Mol. Ecol. 2012;12:3610–3624. doi: 10.1111/j.1365-294X.2012.05621.x. [DOI] [PubMed] [Google Scholar]
- Welch D.A., Samuel W.M., Wilke C.J. Dermacentor albipictus (Acari, Ixodidae) on captive reindeer and free-ranging woodland caribou. J. Wildl. Dis. 1990;26:410–411. doi: 10.7589/0090-3558-26.3.410. [DOI] [PubMed] [Google Scholar]
- Wilkinson P.R. The distribution of Dermacentor ticks in Canada in relation to bioclimatic zones. Can. J. Zool. 1967;45:517–537. doi: 10.1139/z67-066. [DOI] [PubMed] [Google Scholar]
- Yannic G., Pellissier L., Ortego J., Lecomte N., Couturier S., Cuyler C., Dussault C., Hundertmark K.J., Irvine R.J., Jenkins D.A., Kolpashikov L., Mager K., Musiani M., Parker K.L., Røed K.H., Sipko T., Þórisson S.G., Weckworth B.V., Guisan A., Bernatchez L., Côté S.D. Genetic diversity in caribou linked to past and future climate change. Nature Clim. Change. 2014;4:132–137. [Google Scholar]
- Ytrehus B., Bretten T., Bergsjo B., Isaksen K. Fatal pneumonia epizootic in muskox (Ovibos moschatus) in a period of extraordinary weather conditions. EcoHealth. 2008;5:213. doi: 10.1007/s10393-008-0166-0. [DOI] [PubMed] [Google Scholar]


