Graphical abstract
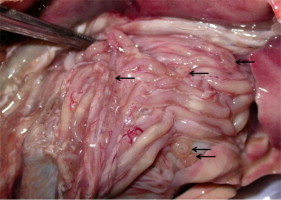
Larval stages of anisakid nematodes (arrows) encysted on the surface of the internal organs in snapper.
Keywords: Anisakidae, Australia, Anisakidosis, Taxonomy
Highlights
-
•
A list of anisakids reported in Australia and their taxonomic status were provided.
-
•
Still most species have not been adequately described in Australia.
-
•
It is possible that prevalence of anisakidosis in Australia is under estimated.
Abstract
Anisakidosis is an emerging infection associated with a wide range of clinical syndromes in humans caused by members of the family Anisakidae. Anisakid nematodes have a cosmopolitan distribution and infect a wide range of invertebrates and vertebrates during their life cycles. Since the first report of these parasites in humans during the early 60s, anisakid nematodes have attracted considerable attention as emerging zoonotic parasites. Along with rapid development of various molecular techniques during last several decades, this has caused a significant change in the taxonomy and systematics of these parasites. However, there are still huge gaps in our knowledge on various aspects of the biology and ecology of anisakid nematodes in Australia. Although the use of advanced morphological and molecular techniques to study anisakids had a late start in Australia, great biodiversity was found and unique species were discovered. Here an updated list of members within the family and the current state of knowledge on Australian anisakids will be provided. Given that the employment of advanced techniques to study these important emerging zoonotic parasites in Australia is recent, further research is needed to understand the ecology and biology of these socio economically important parasites. After a recent human case of anisakidosis in Australia, such understanding is crucial if control and preventive strategies are to be established in this country.
1. Introduction
Members of the family Anisakidae infect animals of almost all phyla, and are particularly prevalent in fish and aquatic associated animals. In the northern hemisphere, interest in these organisms grew tremendously following the discovery that larval stage of Anisakis from the North Sea herring, Clupea harengus, are able to infect humans (Van Thiel et al., 1960). Since then there have been many studies carried out that increased awareness, improved diagnostic techniques and broadened our knowledge about various aspects of their biology and pathogenicity. Anisakid nematodes are regarded as economically important parasites that are recognised as emerging seafood borne parasites with unique characteristics. It is known that even one single dead anisakid larva in properly cooked seafood may cause serious disease in humans (Audicana et al., 2002); anisakid larvae do not die immediately after the host’s death, migrating instead from internal organs to the flesh of the host, where they are more likely to be transferred to the definitive host(s). Unlike many other common parasitic diseases of humans, anisakidosis is not a problem of developing countries only (Gorokhov et al., 1999; Smith, 1999; Lopez-Serrano et al., 2000; Chai et al., 2005; Kapral et al., 2009; Shamsi and Butcher, 2011) (Fig. 1). Hence, it is not surprising that a review of the literature found a sharp increase in the number of publications since the 1960s (Fig. 2). However, to date only 2% of these publications are about Australian anisakids. In the Northern Atlantic and Northern Pacific, the economic importance of the fishing industry has prompted research on the parasites of marine fauna and as a result, many aspects of the biology and ecology of these parasites have been studied, but the parasites of Australian marine animals are poorly known.
Fig. 1.
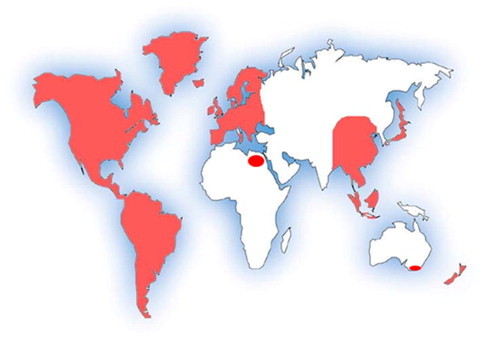
Countries where human cases of anisakidosis have been reported (Gorokhov et al., 1999; Smith, 1999; Lopez-Serrano et al., 2000; Chai et al., 2005; Kapral et al., 2009; Shamsi and Butcher, 2011).
Fig. 2.
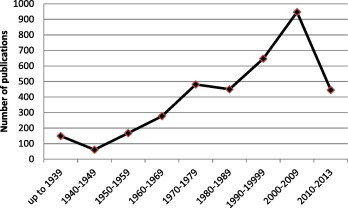
Number of published articles on anisakid nematodes (key word: Anisakidae) until 2013; based on the search on Web of Science and CABI (Gorokhov et al., 1999; Smith, 1999; Lopez-Serrano et al., 2000; Chai et al., 2005; Kapral et al., 2009; Shamsi and Butcher, 2011).
The history of studies of anisakids in Australia shows a controversial taxonomy. Australian anisakids are known mostly by the works of Johnston and Mawson in the 1940’s but since then there has been no review on importance of those findings and the current status of taxa reported by them. In some recent publications on Australian anisakids wrong taxonomy, old nomenclature and assignment of species to wrong families occur, possibly due to the lack of compact information on changes to the taxonomy and nomenclature of Australian species of anisakids. For example, a common confusion relates to the genus Hysterothylacium which was resurrected in 1981 to include those species previously considered as Thynnascaris and Contracaecum (Deardorff and Overstreet, 1981) and is now classified under family Raphidascarididae (Fagerholm, 1991; Nadler and Hudspeth, 2000) instead of Anisakidae. However, literature review shows there are still 47 publications worldwide, some from Australia, in which Thynnascaris has been reported from various hosts or studied, without referring to its significant morphological features in details. Therefore, one cannot draw conclusions about the host, geographical distribution and pathogenic aspects of the specimens reported in these articles. More importantly, Hysterothylacium is still reported as an anisakid nematode instead of raphidascarids in some publications. For decision makers in the seafood industry, health sectors and fisheries in several parts of the world, it makes a significant difference in how they deal with anisakids versus raphidascarids. Hysterothylacium larvae are very abundant in fish (Shamsi et al., 2013) but they are not as harmful as members of anisakid nematodes for human health. An article that states fish are infected with Hysterothylacium spp. and places them under anisakids can potentially lead to raising a false alert among decision makers who are not necessarily taxonomists at potentially tremendous cost to the Australian fishing industry.
This review presents the history of research on Australian anisakid nematodes, a list of taxa reported in Australia followed by their current taxonomic status, the current state of knowledge on life cycle and health impacts of Australian anisakids, and the questions these findings raise.
2. History of study of anisakid nematodes in Australia
There is a paucity of information on anisakid nematodes in Australia. Australian anisakids are known principally from early works by Johnston and Mawson (Johnston, 1910, 1913, 1937, 1938; Johnston and Mawson, 1939, 1940a,b, 1941a, 1942b,c,d, 1943a,b, 1944, 1945a,b, 1947a, 1949, 1951a, 1952, 1953; Mawson, 1953, 1957, 1969). These authors reported and described 32 species of anisakid nematodes, from a broad range of Australian animals. Although their contribution to our knowledge on anisakid nematodes in Australia is significant, most of these early reports of the species were poorly described, so that the differentiation among closely related species is not possible.
Many of these species are unidentifiable by current standards, with brief and sometimes unillustrated descriptions. Sometimes new species have been described based on a single specimen, such as is the case for Contracaecum gypsophocae (Johnston and Mawson, 1941c,e) or based on immature parasites such as Phocascaris hydrurgae (Johnston and Mawson, 1941c). Some of the nomenclature has not been used by other authors for 50 years, such as the case of Stomachus which had been used rather than Anisakis (Johnston and Mawson, 1953). For many of these species, such as Contracaecum bancrofti, C. clelandi, C. sinulabiatum and C. magnicollare, there was no information on the location of the type material in the original paper (Johnston and Mawson, 1941d). Later, a list of type specimens of animal parasites held in Australian institutions was published (Spratt, 1983). Subsequent re-examination of some of these materials by other authors showed that in some cases, hosts or parasites were misidentified. For example, specimens from the fishes, Stegostoma fasciatum (Hermann) and Orectolobus maculatus (Bonnaterre), recorded as Terranova galeocerdonis (Johnston and Mawson, 1951a) were later identified as Terranova ginglymostomae (Bruce and Cannon, 1990). Some of these species have only been reported from Australia, such as Contracaecum bancrofti, C. clelandi, C. eudyptes, C. eudyptulae, C. heardi, C. magnicollare, C. nycticoracis, C. podicipitis and C. sinulabiatum, and doubts have been raised concerning their validity (Hartwich, 1963, 1964b).
Some of the species, e.g., Anisakis simplex, Contracaecum osculatum and C. spiculigerum have been also reported from other parts of the world and their taxonomic status have been changed based on evidence provided by molecular studies. It is important to investigate the relationship between Australian taxa and con-species parasites from elsewhere and to know where Australian species stand based on new evidences. Therefore, since 2009, Shamsi et al. have employed a combined morphological (light and scanning electron microscopy) and molecular (based on the first and second ribosomal internal transcribed spacers (ITS-1 and ITS-2)) approach and have evaluated the taxonomic status of some anisakid species in Australia (Shamsi et al., 2008; Shamsi et al., 2009a,b; Shamsi et al., 2012). This was the first step toward clarifying the taxonomic status of Australian anisakids using advanced techniques.
3. List of anisakid nematodes reported in Australia and their current taxonomic status
Before providing a list of Australian anisakid nematodes it is worth mentioning that the systematics and classification of these parasites and the relationships of genera and species have been the subject of extensive debate (e.g., Gibson, 1983; Sprent, 1983). Until the early 21st century, the proposed classifications were based primarily on an analysis of morphological features of high systematic significance. The lack of sufficient numbers of such characteristics and subjective interpretations of them have also led to considerable controversy over the classification of the Ascaridoidea and position and validity of the family Anisakidae within Ascaridoidea (e.g., Mozgovoi, 1953; Hartwich, 1957, 1964b, 1974; Yamaguti, 1961; Gibson, 1983; Sprent, 1983Fagerholm, 1991). Since the early 21st century, various molecular approaches have been employed to investigate taxonomic and systematic questions, such as the characterisation of distinct species and detection of genetic variation among anisakid nematodes. Nadler and Hudspeth (2000) used mitochondrial gene cytochrome oxidase subunit 2, small- and large-subunit nuclear rDNA sequences and morphological data, to produce a phylogenetic hypothesis for representatives of the superfamily Ascaridoidea. Their results were consistent with Fagerholm’s 1991 classification of anisakid nematodes. Based on these works, currently Anisakis, Contracaecum, Ophidascaris, Paranisakiopsis, Peritrachelius, Phocascaris, Pseudanisakis, Pseudoterranova, Pulchrascaris, Raillietascaris, Sulcascaris, Terranova are genera to be considered within family Anisakidae. Based on this classification, below is a list of reported species from Australia.
Anisakis brevispiculata: This is a recent report from a dwarf whale from New South Wales (Shamsi et al., 2012). A. brevispiculata was first described by Dollfus (1966) as a new species but was later synonymised with A. physeteris (Davey, 1971). However, recent molecular analysis have shown that A. brevispiculata is a valid species, genetically distinct from A. physeteris (Mattiucci et al., 2001). No further information has been published regarding its morphological characterisation and the value of different morphological characters for specific identification. In Australia, A. brevispicualata was redescribed based on a male specimen and its morphological differentiation from closely related species was discussed (Shamsi et al., 2012). Alignment of ITS-1 and ITS-2 sequences of A. brevispiculata found in Australia with those from the northern hemisphere (Nadler et al., 2005) showed that A. brevispiculata in Australia is genetically distinct (Shamsi et al., 2012).
A. diomedeae: It was first described by Johnston and Mawson (1942b) from albatrosses. The validity of this species is doubtful mainly because the identification and assignment to a new species were based on examination of immature specimens. In addition, members of the genus Anisakis do not develop into adults in birds. It was also commented later by one of the authors that these specimens might belong to a new genus and they need to be studied further (Mawson, 1983).
A. kogiae: This species currently considered invalid. A. kogiae was first described by Johnston and Mawson (1939) from a pygmy sperm whale from South Australia and Queensland waters but later was synonymised with A. simplex by Davey (1971). For further details, see below, under A. simplex.
A. marina: (= Capsularia marina; Stomachus marinus) has been reported from various hosts across Australian coasts (Johnston and Mawson, 1944, 1945b, 1949). In these reports “marina” mostly refers to larval stage of the parasite. Therefore, A. marina is not considered a valid taxon but regarded as Anisakis larval type. Both Capsularia and Stomachus have later been synonymised with Anisakis.
A. oceanicus: This species was first described from a whale, Globicephalus melas, in Australia (Johnston and Mawson, 1951b) but later synonymised with A. physeteris (Davey, 1971). Shamsi et al. (2012) raised the possibility that A. oceanicus might be a valid species, however examination of additional specimens as well as the type specimen of A. oceanicus is required before any conclusion can be drawn.
A. similis (= Stomachus similis): This species has been first reported from marine mammals in New South Wales and South Australia (Johnston and Mawson, 1939, 1941c) and then later from Queensland (Cannon, 1977a). Both have been synonymised with A. simplex (Davey, 1971). For further details, see below, under A. simplex.
A. simplex sensu lato: Currently, based on genetic analysis it is believed that A. simplex comprises three genetically distinct species, including A. pegreffii, A. simplex sensu stricto and A. simplex C (Nascetti et al., 1983; Mattiucci et al., 1997). Until recently, these species were considered indistinguishable based solely on morphological characteristics. Mattiucci et al. (2013a) used ventriculus length, tail shape, tail length/total body length ratio, plectane 1 width/plectane 3 width ratio and left and right spicule length/total body length ratios to distinguish these three sibling species and named A. simplex C as Anisakis berlandi. In Australia, recently two members of the A. simplex sensu lato have been reported, A. pegreffii and A. simplex C (= A. berlandi) from marine mammals (Shamsi et al., 2012). However, due to lack of comparable data, it is not known which species those early reports of A. simplex sensu lato (e.g., those reported as A. similis or Stomachus similis) belong to.
A. typica: In Australia this species was first reported by Cannon (1977a) from the melon-headed whale, in Queensland waters. Larval stages were later identified based on the ITS-1 and ITS-2 sequence data (Jabbar et al., 2012) from various fish species in the same region. In Davey’s revision of Anisakis spp., A. typica was considered a valid species (Davey, 1971). Later, Mattiucci and Nascetti (2006) provided molecular evidence based on the multileucos enzyme electrophoresis and supported validity of the taxonomic status of the species.
Contracaecum bancrofti: This species was first established by Johnston and Mawson (1941b) from Australian pelicans from Queensland and New South Wales in Australia but later Hartwich (1964a) synonymised it with C. micropapillatum. Therefore, C. bancrofti was treated as a synonym of C. micropapillatum in the checklist of parasites of Australian birds by Mawson et al. (1986). Later, Shamsi et al. (2009b) examined more specimens and re-established C. bancrofti as a valid species based on the morphological and genetic evidence.
C. clelandi: This species was described from Australian pelicans in Perth (Johnston and Mawson, 1941b) and since then it has not been reported elsewhere. Johnston and Mawson’s description of C. clelandi is incomplete in several respects but is sufficient to permit the recognition of C. clelandi as a valid species. However, an examination of the type specimen deposited in South Australian Museum revealed that the type specimen is female (Shamsi et al., 2008).
C. eudyptulae: This is a well established species which was first described by Johnston and Mawson (1942d) from the little penguin from Western Australia, Victoria, Tasmania and New South Wales. Later, a redescription of the species and genetic characteristaion of internal transcribed spacers were provided for this taxon (Shamsi et al., 2009b).
C. gypsophocae: Johnston and Mawson (1941c) described it as a new species in the Tasmanian fur seal (Gypsophoca tasmanica) from Derwent Heads (Tasmania, Australia) but later re-examined the specimens and synonymised it with C. osculatum (Johnston and Mawson, 1945a).
C. heardi: This species was described as a new species from penguins by Mawson (1953) from Heard Island and then later by Fonteneau et al. (2011) from Antarctic.
C. (=Thynnascaris) incurvum: First reported from swordfish in New South Wales by Johnston and Mawson (1943a) but was later synonymised as Hysterothylacium incurvum (Deardorff and Overstreet, 1981) and therefore based on the currently accepted classification, it does not belong to the family Anisakidae.
C. (Thynnascaris) legendrei: This species has been reported from various species of fish such as tiger flathead from different locations in Australia (Johnston and Mawson, 1943a, 1944, 1951a). It was later synonymised with H. incurvum (Deardorff and Overstreet, 1981) and therefore, based on the currently accepted classification, it does not belong to the family Anisakidae.
C. macquariae: This species was first described as a new species from Macquarie perch from New South Wales (Johnston and Mawson, 1940b). However, the description of excretory pore and morphology of the tail suggest that it should belong to the genus Hysterothylacium (Deardorff and Overstreet, 1981) which is no longer considered a member of the family Anisakidae.
C. magnicollare: It was described as a new species by Johnston and Mawson (1941b) from a noddy in Queensland but was later synonymised with C. magnipapillatum by Fagerholm et al. (1996).
C. magnipapillatum: Fagerholm et al. (1996) re-examined and redescribed nematode parasites obtained from ulcerated nodules in the proventriculus of the piscivorous black noddy tern, Anous minutus, by Johnston and Mawson (1941b) and considered it a senior synonym of C. magnicollare.
C. microcephalum: This widely distributed species has been reported from several birds in Australia, e.g., domestic duck and silver gull (Johnston and Mawson, 1941b, 1953). Recently a description of the species and its genetic characterisation from Australian piscivorous birds were published (Shamsi et al., 2009b).
C. multipapillatum: It was reported in Australia for the first time by Shamsi et al. (2008) and was morphologically described and genetically characterised by these authors. Until then, C. multipapillatum had only been reported from the Nearctic region (Lucker, 1941a,b; Courtney and Forrester, 1974; Navone et al., 2000; Kinsella et al., 2004).
C. murrayense: This species was described as a new species from various freshwater fish species from Tailem Bend by Johnston and Mawson (1940b). It was later synonymised with Hysterothylacium murrayense by Deardorff and Overstreet (1981).
C. (=Thynnascaris) nototheniae: First described in reported in Australia by Johnston and Mawson (1945a) from various fish species and subsequently placed under the genus Hysterothylacium (Deardorff and Overstreet, 1981).
C. nycticoracis: It was described as a new species based on the presence of a single male with broken spicule from a heron in New South Wales (Johnston and Mawson, 1941e). The description of this species is very brief and the figures provided in the article do not show sufficient detail of taxonomically important features to differentiate it from other Contracaceum spp. Therefore the validity of C. nycticoracis is questionable.
C. ogmorhini sensu lato: A few years after the original description of C. ogmorhini, by Johnston and Mawson (1941c) from Australia, they synonymised it with C. osculatum (Johnston and Mawson, 1945a). It was also considered a synonym of C. osculatum by Hartwich (1975). Campana-Rouget and Paulian (1960) considered C. ogmorhini a valid species, Fagerholm and Gibson (1987) provided a redescription of the species and recent molecular studies based on allozyme and mtDNA data, have shown that C. ogmorhini is a species complex comprised at least two distinct genotypes which cannot be differentiated morphologically; C. ogmorhini sensu stricto and C. margolisi (Mattiucci et al., 2003) with distinct geographical distribution and host species. C. ogmorhini sensu stricto occurs in southern hemisphere while C. margolisi occurs in the northern hemisphere.
C. osculatum sensu lato: This species was reported from Australian seals (e.g., Johnston, 1937). Later it was shown C. osculatum comprises a number of sibling species that cannot be differentiated from one another morphologically, hence assigned as A to E and C. osculatum baicalensis (Nascetti et al., 1993; Orecchia et al., 1994; D’Amelio et al., 1995). Only C. osculatum D and E are found in southern hemisphere. Due to lack of comparable data it is not known earlier reports of the species in Australia belong to C. osculatum D or E.
C. pelagicum: First described as a new species from albatross in New South Wales (Johnston and Mawson, 1942b) and then it was reported from other countries (e.g., Silva et al., 2005; Garbin et al., 2007).
C. podicipitis: Described as a new species by Johnston and Mawson (1949) from crested grebe in South Australia and its validity has not been criticized since then.
C. pyripapillatum: This species was described morphologically and characterised genetically by Shamsi et al. (2008) for the first time from Australian pelican. It is morphologically most similar to C. multipapillatum, occurring in the same host species/individual (Shamsi et al., 2008) but can be differentiated based on the morphology of pre-anal papillae.
C. radiatum: This anisakid nematode has been commonly reported from the Antarctic Ocean, together with C. osculatum from seals (e.g., Johnston and Mawson, 1945a).
C. rudolphii: In Australia, anisakids from various cormorants comply morphologically with the description of C. rudolphii sensu lato (Hartwich, 1964a,b). However, morphological examination of Australian specimens followed by characterisation of internal transcribed spacers showed that they could be divided into two groups; C. rudolphii D and C. rudolphii E. These observations suggested that C. rudolphii in Australia consists of two distinct species (Shamsi et al., 2009a). In the northern hemisphere, C. rudolphii, has been reported from the stomach of a number of species of pelicans and cormorants (Hartwich, 1964a) where existence of four sibling species within C. rudolphii sensu lato named C. rudolphii A, C. rudolphii B and C. rudolphii C and C. rudolphii F was revealed (Mattiucci et al., 2002; Li et al., 2005; Zhang et al., 2009; D’Amelio et al., 2012). C. rudolphii D and C. rudolphii E from Australia are different from C. rudolphii A, B, C and F reported from northern hemisphere.
C. sinulabiatum: First described as a new species by Johnston and Mawson (1941b) and reported from various birds in Queensland and South Australia. Since then there has been no further report of this parasite. The location of the type specimen was not known, therefore later Hartwich (1964a) expressed doubt over the validity of the species. Spratt (1983) published the list of type species and it was considered a valid species in the Mawson et al. (1986) checklist.
C. spiculigerum: This parasite was reported from various birds across Australia (e.g., Johnston and Mawson, 1941b). However, Hartwich (1964a) subsequently examined the types of C. spiculigerum and found that the type material of C. spiculigerum was C. microcephalum. Therefore, he referred to this taxon as C. spiculigerum sensu auctorum and created the new name C. rudolphii.
C. (Thynnascaris) tasmaniense: First described and reported in Australia by Johnston and Mawson (1945a) from various fish species and subsequently placed under the genus Hysterothylacium (Deardorff and Overstreet, 1981).
C. variegatum: In Australia, C. variegatum was first reported in Australian Pelican and darter (Shamsi et al., 2009b). The taxonomy of this species has seen several major changes mainly due to the type specimen being a female (Rudolphi, 1809) but Hartwich (1964a) redescribed the species. Specimens reported and characterised from Australia show slight difference to previous descriptions of the species (Hartwich, 1964a; Nagasawa et al., 1999). Similar differences were observed between C. variegatum from Scottish waters and the type specimen (Fagerholm et al., 1996) suggesting it is likely that C. variegatum with a worldwide distribution, does not represent a single species.
Mawsonascaris australis: see Paranisakis australis
Ophidascaris varani: see Raillietascaris varani.
O. pyrrhus: First described by Johnston and Mawson (1942a) from a black snake and later reported from various species of snakes from several locations in Australia (Jones, 1980).
O. filarial: It was reported from carpet snake in Queensland (Johnston and Mawson, 1942a).
Paranisakiopsis sp.: The only report of this marine fish parasite in Australia is from albatross with a comment by the author that it should have been ingested with food (Mawson, 1953).
Paranisakis australis: It was described as a new species from a ray in Sydney by Johnston and Mawson (1943b) but it was assigned to the new genus Mawsonascaris (Sprent, 1990).
P. hydrurgae: The main criticism about P. hydrurgae is that it was established based on immature specimens by Johnston and Mawson (1941c). The taxonomic status of genera Contracaecum and Phocascaris, both of which have aquatic life cycles and homeothermic final hosts has been the subject of several debates. Hartwich (1974) distinguished them based on morphological characteristics, whereas, Berland (1963) considered species of Contracaecum occurring in seals as Phocascaris and those species maturing in birds remained in the genus Contracaecum which later was supported by (Mattiucci et al., 2008). Since there is no strong evidence for a close relationship between these morphological features and the evolutionary lineages for Contracaecum and Phocascaris, the taxonomic status of these genera remains unclear (D’Amelio, 2003; Nadler et al., 2000).
Porrocaecum kogiae: It was described as a new species pygmy sperm whale in South Australia and Queensland (Johnston and Mawson, 1939). Members of Porrocaecum were later placed under genus Terranova.
Pseudoterranova piscicum: Mawson (1953) reported this species from various seals in Macquarie Island as Terranova piscicum. Later, those members of the genus Terranova that developed into adults in marine mammals were placed into genus Pseudoterranova (Gibson and Colin, 1982). Since then there have been several studies on Pseudoterranova spp. using advanced molecular approaches and some newly established species, such as P. decipiens D and E were reported from Antarctic waters. However the relationship with and/or differentiation from P. piscicum has not been discussed.
Raillietascaris varani: It was first described as Ophidascaris varani by Johnston and Mawson (1947b) based on a single male from Varanus varius in Queensland and subsequently was placed into the new genus, Raillietascaris by Sprent (1985).
Stomachus similis: This currently invalid taxon, has been reported from various seals in Australia (Mawson, 1953). See A. simplex for more details.
Terranova crocodyli: It was reported from Crocodylus johnsoni in Australia by Baylis (1931) and was redescribed by Sprent (1979).
T. galeocerdonis: This species was reported from various species of sharks from Queensland and South Australia (Johnston and Mawson, 1951a; Bruce and Cannon, 1990). Gibson and Colin (1982) considered T. galeocerdonis as synonym of T. pristis. However, T. galeocerdonis was later considered a valid species and distinct from T. pristis by various authors (Tanzola and Sardella, 2006).
T. ginglymostomae: It was reported and redescribed from various sharks in Queensland (Bruce and Cannon, 1990).
T. piscicum: see Pseudoterranova piscicum.
T. pristis: Reported from elasmobranches in Queensland water by Bruce and Cannon (1990) who redescribed the species.
A summary of the current taxonomic status of the above mentioned taxa is provided in Table 1.
Table 1.
Summary of the current taxonomic status of the anisakid nematodes reported in Australia. For full report see Section 3. Those taxa previously placed in the family Anisakidae and now in other families are not listed.
| Taxa | Current status | Note |
|---|---|---|
| Anisakis brevispiculata | Valid | A. brevispiculata in Australia is genetically distinct from those reported previously (Shamsi et al., 2012) |
| A. berlandi | Valid | Previously known as A. simplex C |
| A. diomedeae | Invalid | |
| A. kogiae | Invalid | |
| A. marina | Invalid | |
| A. oceanicus | Inquirenda | For further information see Shamsi et al. (2012) |
| A. pegreffii | Valid | Previously known as A. simplex A |
| A. similis | Invalid | |
| A. typica | Valid | A. typica in Australia seems to be genetically different from those reported in other countries (Shamsi, 2007) |
| Contracaecum bancrofti | Valid | |
| C. clelandi | Inquirenda | Type specimen is female however the description by Johnson and Mawson (1941b) is sufficient to recognize a distinct species |
| C. eudyptulae | Valid | |
| C. gypsophocae | Invalid | |
| C. heardi | Valid | |
| C. magnicollare | Invalid | |
| C. magnipapillatum | Valid | |
| C. microcephalum | Valid | |
| C. multipapillatum | Valid | |
| C. nycticoracis | Inquirenda | The validity of C. nycticoracis is questionable due to insufficient description as new species based on a single male with broken spicule |
| C. ogmorhini sensu stricto | Valid | |
| C. osculatum sensu lato | Valid | Only C. osculatum D and E are found in southern hemisphere. Due to lack of comparable data it is not known earlier reports of the species in Australia belong to C. osculatum D or E |
| C. pelagicum | Valid | |
| C. podicipitis | Valid | |
| C. pyripapillatum | Valid | |
| C. radiatum | Valid | |
| C. rudolphii sensu lato | Valid | C. rudolphii D and C. rudolphii E were reported from Australia. They are different from C. rudolphii A, B, C and F reported from northern hemisphere |
| C. sinulabiatum | Valid | |
| C. spiculigerum | Invalid | |
| C. variegatum | Valid | |
| Capsularia marina | Invalid | |
| Mawsonascaris australis | Valid | |
| Ophidascaris filarial | Valid | |
| O. pyrrhus | Valid | |
| O. varani | Invalid | |
| Paranisakis australis | Invalid | |
| Phocascaris hydrurgae | Inquirenda | |
| Porrocaecum kogiae | Invalid | |
| Pseudoterranova piscicum | Inquirenda | |
| Raillietascaris varani | Valid | |
| Stomachus marinus | Invalid | |
| S. similis | Invalid | |
| Terranova crocodyli | Valid | |
| T. galeocerdonis | Valid | |
| T. ginglymostomae | Valid | |
| T. piscicum | Invalid | |
| T. pristis | Valid |
4. Life cycle of Australian anisakid nematodes
The common life cycle pattern for all genera and species within the family Anisakidae according to (Anderson, 2000) is as follows: eggs pass out in the faeces of the definitive host and enter the water where they embryonate into first stage larvae within the egg (L1). They then develop further and moult to the second stage (L2). Eggs or larvae can be ingested by first intermediate hosts (crustaceans, usually copepods) and then grow in their haemocoel. When infected copepods are eaten by second intermediate hosts, larvae reach the third larval stage (L3). A great variety of teleost fishes can play a role as paratenic hosts. Piscivorous fishes may accumulate enormous numbers of larvae due to preying upon infected small fishes. Larvae in small fishes can infect piscivorous fish without moulting. This general life history pattern is variable and there are differences in the type of intermediate/definitive hosts among different genera and species of anisakids. Adults of Anisakis spp., also known as “whaleworms”, are parasites of the stomach of pinnipeds, such as elephant seals, fur seals, grey seals, leopard seals, monk seals, ringed seals, sea lions and walrus as well as cetaceans such as dolphins, narwhals, porpoises and whales (Baker, 1992; Ugland et al., 2004). Marine crustaceans, such as euphausiids, are their first invertebrate intermediate hosts. For Contracaecum spp. various species of piscivorous birds and mammals associated with freshwater, brackish and marine environments (such as cormorants, pelicans and seals) are definitive hosts (Kloser et al., 1992) and a broad range of invertebrates, including coelenterates, ctenophores, gastropods, cephalopods, polychaetes, copepods, mysids, amphipods, euphasiids, decapods, echinoderms and chaetognaths can act as first intermediate hosts (Semenova, 1979), although their roles in the natural transmission of the larvae to fish intermediate hosts is not completely clear. Life cycles of the other genera within the family Anisakidae have not been studied as extensively. Species of reptiles and elasmobranches could act as the definitive hosts for other genera, such as Terranova and Ophidascaris.
In Australia, our knowledge on the life cycle of various anisakid nematodes is poor mainly because larval stage of anisakids cannot be identified to species based solely on morphological characteristics. Several taxonomically important features, such as spicules, papillae and lips are absent in larval stages. Hence, many aspects of their biology and ecology remain unknown.
Larval stages of anisakid nematodes in Australia were mainly studied by (Cannon, 1977c) who reported, described and illustrated several distinct larval types from marine fishes in south-eastern Queensland including Anisakis Type I, Terranova Types I and II, and Contracaecum Types I and II . He also described Hysterothylacium (=Thynnascaris) Types I, II, III and IV larvae which now are placed under Raphidascarididae. He also reported and described fourth stage larval ascaridoids found in bivalves, Pinna menkei, from Moreton Bay, in Spondylus ducalis and Amusium balloti from the Bundaberg region as well as a gastropod, Cypraea tigris, in Queensland (Cannon, 1978) which he found to be consistent with description of larvae found in pearl oysters, reported previously (Shipley and Hornell, 1906). In his study on the parasites of Queensland marine fishes in Australia, Cannon (1977b) suggested that Anisakis is essentially pelagic and has been found in the adult stage in the predators of nektons.
Since, Cannon, there have been few reports of larval anisakids from fishes in Australian waters. Some authors followed Cannon’s description to differentiate anisakid larvae occurring in Australian waters at least to different types (Hooper, 1983; Lymbery et al., 2002), whereas others simply placed them into different genera (Doupe et al., 2003).
One of the outcomes of employing molecular techniques to study Australian anisakids was the specific identification of anisakid larvae from a range of hosts, therefore clarifying part of the life cycle. Since larval stages of anisakid nematodes cannot be identified specifically using morphological characters, ITS-1 and ITS-2 sequences of larval stages of anisakid nematodes were compared with ITS-1 and ITS-2 sequences from well described adult stages, which allowed unequivocal identification of anisakid larvae to species (Shamsi et al., 2011b, 2012). Therefore, it was shown that Anisakis larval type I in Australia comprises at least three known species, including A. pegreffii, A. simplex C (= A. berlandi) and A. typica (Shamsi, 2007). It was also shown that Contracaecum larval type I, found in mullet, Mugil cephalus, and hardyheads (Atherinidae), were distinct from one another and comprised at least two distinct genotypes. Contracaecum larval type I from mullet was matched with adult C. multipapillatum and Contracaecum larval type I from hardyheads (Atherinidae) was matched with adult C. pyripapillatum (Shamsi et al., 2011b). By contrast, Contracaecum larval type II, found in two different host species including mackerel, Scomber australasicus, and king fish, Seriola lalandi, had identical ITS-1 and ITS-2 sequences to adult C. ogmorhini sensu stricto (Shamsi et al., 2011b). Finally, Contracaecum larval type III found in flathead, Platycephalus laevigatus, had identical ITS-1 and ITS-2 sequences as adults of C. rudolphii D (Shamsi et al., 2011b). This partly clarified the life cycle of these species. However, larval characteristics, intermediate hosts and life cycle patterns of other anisakids in Australia remain unclear. Twelve different morphotypes of Hysterothylacium larvae were described and genetically characterised from Australian waters (Shamsi et al., 2013) however they are now placed in the family Raphidascarididae and their details will not be discussed here.
5. Public health significance of anisakid nematodes in Australia
Despite the popularity of consuming seafood in Australia as well as the multicultural environment of the country in which different cuisines use raw or undercooked sea foods, there has been (to date) only one report of human anisakidosis acquired in Australia (Shamsi and Butcher, 2011) and two cases were diagnosed in Australia but acquired abroad (unpublished data). The Australian case of anisakidosis was due to Contracaecum larval type. The patient’s symptoms, vomiting, diarrhoea, abdominal pain, sore throat, rhinorrhoea, nasal congestion and cough lasted about 3 weeks until a larva was passed in a bowel motion. As human infestations occur after eating infested seafood, and the patient had history of eating South Australian mackerel, it is implied that the mackerel eaten by the patient was infected.
The Australian case of Anisakidosis was unusual for two reasons. Firstly, infection of humans with Contracaecum type larva, as occurred in Australia is very rare. Most cases of anisakidosis around the world are due to Anisakis (Fumarola et al., 2009; Mattiucci et al., 2013b) or Pseudoterranova larval types (Arizono et al., 2011). Secondly, in most cases the anisakid larvae penetrate patient’s gastro-intestinal wall. The transient form of the anisakidosis in which larvae pass the alimentary tract and leave it alive, as it happened in Australia, have rarely been reported (Smith, 1999).
In Australia, larval and adult stages of various species of anisakids infest a broad variety of fish, including mackerels (Shamsi et al., 2011a). However, there has been only one human case of anisakidosis in the country. Given that the popularity of consuming raw or undercooked fish (e.g., sushi and sashimi) is increasing, it is possible that anisakidosis is under diagnosed in Australia due to the vague symptoms and limited diagnostic tests, which raises the question as to the extent of hidden cases of anisakidosis in this country. It is important to design and implement strategies to prevent anisakidosis in Australia. Educational campaigns would also be useful to inform the public and medical professionals/practitioners.
6. Significance of anisakid nematodes on Australian wildlife health
It has been shown that anisakid nematodes are relatively prevalent in Australian marine fish (Cannon, 1977c; Shamsi et al., 2011a; Doupe et al., 2003). There is no report on the impact of anisakids on the health of Australian fishes. It is noteworthy that infection of fish with anisakid nematodes is of importance not only because of anisakidosis in humans, but also because of the effect they have on the infected fish. Anisakid larvae, particularly when located in the musculature, can affect the commercial value of fish and thus result in significant economic losses to the fishing industry (Smith and Wootten, 1975; Angot and Brasseur, 1995). Moreover, anisakids can cause disease in fish. The symptoms and severity of disease can vary considerably depending on factors such as the species of fish, species and intensity of infecting parasite in the fish and the particular organ invaded (Woo, 1995). In brief, disease is most severe when the anisakid larvae infect the liver. Fibrosis of the liver can lead to atrophy of this organ and a significant loss in body weight. Other symptoms can also be granulomata, inflammation and necrosis of the muscularis externa of the pyloric caeca, gallbladder, intestine and body cavity, which can cause substantial mortality in fish (Woo, 1995).
It seems that these parasites may not be host specific at the larval stages which means a wide range of fish species can act as their intermediate or paratenic host. It has been shown that larval anisakids can pass through several fish species via predation and can be accumulated in larger fishes (Jensen, 1997). Hence, fishes of different species can play an important role in the distribution of anisakids in the environment. Different species of fish can be the source of infestation in humans as well as a wide range of marine mammals and piscivorous birds (Shamsi et al., 2011b).
There is controversy about the effect and pathogenicity of anisakid nematodes on marine mammals and piscivorous birds. Whilst some authors believe that infections with anisakid nematodes are not serious in marine mammal hosts (Geraci and St Aubin, 1987), others have stated that anisakids can be quite harmful in the alimentary tract of marine mammals (Stroud and Roffe, 1979; McColl and Obendorf, 1982; Jefferies et al., 1990; Abollo et al., 1998). Anisakids have also been reported from terrestrial mammals, such as dogs and pigs, which are fed fish harbouring anisakid larvae (Usui et al., 1973) with pathological changes resembling those found in marine mammal final hosts but differing in some aspects, for example in fewer macroscopic granulomata in pigs.
In other countries, there are numerous reports of infection of piscivorous birds with anisakid nematodes (e.g., Torres et al., 2005), describing their pathogenicity in different species of birds. Some authors considered large numbers of nematodes in the stomachs of pelecaniform birds as a benefit for the host, assisting in the digestion of food (Owre, 1962). By contrast, other authors reported immature Contracaecum nematodes attached tightly to ulcers in the proventriculus of white pelicans, causing an extensive inflammatory reaction with necrotic debris (Liu and Edward, 1971). In Australia the only study to investigate the impact of anisakids on marine mammals and piscivorous birds is by Norman (2005) who did not find a direct impact on animal health due to infection with anisakids.
In Australia anisakid nematodes have also been reported in reptiles (Glazebrook and Campbell, 1990). Anisakis spp. type I in pen-reared green turtles, Chelonia mydas caused haemorrhagic ulcers in the lower stomach and upper intestine (Glazebrook and Campbell, 1990). Ulceration of the gastric and intestinal mucosa, as well as visceral adhesions, have also been reported in farmed turtles in northern Australia (due to the migration of larval Anisakis from the lumen of the gut to the pleuro-peritoneal cavity) (Glazebrook and Campbell, 1990).
7. Conclusion
Despite considerable past achievements, there are still huge gaps in our knowledge about many aspects of the biology and ecology of these important parasites and huge potential remains for future work. In Australia, it is evident that there is a broad range of anisakid nematodes, infecting a broad variety of invertebrate and vertebrate hosts, however, most species have not yet been adequately described. There is a need to employ tools such as electron microscopy and molecular approaches for a reliable and accurate specific identification and differentiation of species, particularly those that have only been reported from Australia. In spite of a significant increase in the body of the literature on the biology and life cycle of anisakids, many aspects of their developmental stages and life cycle still remain unknown. For example, the part of the life cycle which occurs in crustaceans is not known. In addition, the specific identity of the larval stages of most species has not been determined. Therefore, the host-specificity of these parasites in different developmental stages, their life history patterns and geographical distributions remain unclear.
Acknowledgements
The author is especially grateful to her PhD supervisor Professor Ian Beveridge form the University of Melbourne for his continuous support and advice through her research on Australian anisakids. The author is a co-author in some publications cited in this article. The author has no conflict of interest regarding materials in this article.
References
- Abollo E., Lopez A., Gestal C., Benavente P., Pascual S. Long-term recording of gastric ulcers in cetaceans stranded on the Galician (NW Spain) coast. Dis. Aquat. Organ. 1998;32:71–73. doi: 10.3354/dao032071. [DOI] [PubMed] [Google Scholar]
- Anderson R.C. CABI Publishing; Wallingford, UK: 2000. Nematode Parasites of Vertebrates: Their Development and Transmission. [Google Scholar]
- Angot V., Brasseur P. Les larves d’anisakides et leur incidence sur la qualite des poissons et produits de poisson. Rev. Med. Vet. 1995;146:791–804. [Google Scholar]
- Arizono N., Miura T., Yamada M., Tegoshi T., Onishi K. Human infection with Pseudoterranova azarasi roundworm. Emerg. Infect. Dis. 2011;17:555–556. doi: 10.3201/eid1703.101350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audicana M.T., Ansotegui I.J., Fernandez de Corres L., Kennedy M.W. Anisakis simplex: dangerous – dead and alive? Trends Parasitol. 2002;18:20–25. doi: 10.1016/s1471-4922(01)02152-3. [DOI] [PubMed] [Google Scholar]
- Baker J.A. Causes of mortality and parasites and incidental lesions in dolphins and whales from British waters. Vet. Rec. 1992;130:569–572. doi: 10.1136/vr.130.26.569. [DOI] [PubMed] [Google Scholar]
- Baylis H.A. Some ascaridae from Queenzland. Ann. Mag. Nat. Hist. 1931;8:95–102. [Google Scholar]
- Bruce N.L., Cannon L.R.G. Ascaridoid nematodes from sharks from Australia and the Solomon Islands, Southwestern Pacific Ocean. Invert. Taxon. 1990;4:763–783. [Google Scholar]
- Campana-Rouget Y., Paulian P. Demonstration of the synonymy of 2 species of Contracaecum, parasites of marine mammals. Ann. Parasitol. Hum. Comp. 1960;35:191–192. [PubMed] [Google Scholar]
- Cannon L.R.G. Some aspects of the biology of Peponocephala electra (Cetacea: Delphinidae). II. Parasites. Aust. J. Mar. Freshwater Res. 1977;28:717–722. [Google Scholar]
- Cannon L.R.G. Some ecological relationships of larval ascaridoids from south-eastern Queensland marine fishes. Int. J. Parasitol. 1977;7:227–232. doi: 10.1016/0020-7519(77)90052-2. [DOI] [PubMed] [Google Scholar]
- Cannon L.R.G. Some larval ascaridoids from south-eastern Queensland marine fishes. Int. J. Parasitol. 1977;7:233–243. doi: 10.1016/0020-7519(77)90053-4. [DOI] [PubMed] [Google Scholar]
- Cannon L.R.G. A larval ascaridoid nematode from Queensland scallops. Int. J. Parasitol. 1978;8:75–80. doi: 10.1016/0020-7519(78)90055-3. [DOI] [PubMed] [Google Scholar]
- Chai J., Murrell K.D., Lymbery A.J. Fish-borne parasitic zoonoses: status and issues. Int. J. Parasitol. 2005;35:1233–1254. doi: 10.1016/j.ijpara.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Courtney C.H., Forrester D.J. Helminth parasites of the brown pelican in Florida and Louisiana. Proc. Helminthol. Soc. Wash. 1974;41:89–93. [Google Scholar]
- D’Amelio S. Phylogeny of anisakid nematodes: a review. Helminthologia. 2003;40:87–91. [Google Scholar]
- D’Amelio, S., Mattiucci, S., Paggi, L., Koie, M., Podvyaznaya, I., Pugachev, O., Rusinek, O., Timoshkin, O., Nascetti, G., 1995. Taxonomic rank and origin of Contracaecum osculatum baicalensis Mozgovoy and Ryjikov, 1950, parasite of Phoca sibirica from Lake Baikal, with data on its occurrence in fish hosts. In: IVth International Symposium of Fish Parasitology Munich, Germany, 27.
- D’Amelio S., Cavallero S., Dronen N.O., Barros N.B., Paggi L. Two new species of Contracaecum Railliet & Henry, 1912 (Nematoda: Anisakidae), C. fagerholmi n. sp and C. rudolphii F from the brown pelican Pelecanus occidentalis in the northern Gulf of Mexico. Syst. Parasitol. 2012;81:1–16. doi: 10.1007/s11230-011-9323-x. [DOI] [PubMed] [Google Scholar]
- Davey J.T. A revision of the genus Anisakis Dujardin, 1845 (Nematoda: Ascaridata) J. Helminthol. 1971;45:51–72. [Google Scholar]
- Deardorff T.L., Overstreet R.M. Review of Hysterothylacium and Iheringascaris (both previously = Thynnascaris) (Nematoda: Anisakidae) from the northern Gulf of Mexico. Proc. Biol. Soc. Wash. 1981;93:1035–1079. [Google Scholar]
- Dollfus R.P. Helminthofaune de Kogia breviceps (Blainville 1838) cetacé odontocète. Annales de la Société des Sciences Naturelle, Charente-Maritime. 1966;4:3–6. [Google Scholar]
- Doupe R.G., Lymbery A.J., Wong S., Hobbs R.P. Larval anisakid infections of some tropical fish species from North–West Australia. J. Helminthol. 2003;77:363–365. doi: 10.1079/joh2003193. [DOI] [PubMed] [Google Scholar]
- Fagerholm H.P. Systematic implications of male caudal morphology in ascaridoid nematode parasites. Syst. Parasitol. 1991;19:215–228. [Google Scholar]
- Fagerholm H.P., Gibson D.I. A redescription of the pinniped parasite Contracaecum ogmorhini (Nematoda, Ascaridoidea), with an assessment of its antiboreal circumpolar distribution. Zool. Scr. 1987;16:19–24. [Google Scholar]
- Fagerholm H.P., Overstreet R.M., Humphrey-Smith I. Contracaecum magnipapillatum (Nematoda, Ascaridoidea): resurrection and pathogenic effect of a common parasite from the proventriculus of Anous minutus from the Great Barrier Reef, with a note on C. variegatum. Helminthologia. 1996;33:195–207. [Google Scholar]
- Fonteneau F., Geiger S., Marion L., Le Maho Y., Robin J.P., Kinsella J.M. Gastrointestinal helminths of King penguins (Aptenodytes patagonicus) at Crozet Archipelago. Polar Biol. 2011;34:1249–1252. [Google Scholar]
- Fumarola L., Monno R., Ierardi E., Rizzo G., Giannelli G., Lalle M., Pozio E. Anisakis pegreffi etiological agent of gastric infections in two Italian women. Foodborne Pathog. Dis. 2009;6:1157–1159. doi: 10.1089/fpd.2009.0325. [DOI] [PubMed] [Google Scholar]
- Garbin L.E., Navone G.T., Diaz J.I., Cremonte F. Further study of Contracaecum pelagicum (Nematoda: Anisakidae) in Spheniscus magellanicus (Aves: Spheniscidae) from Argentinean coasts. J. Parasitol. 2007;93:143–150. doi: 10.1645/GE-875R1.1. [DOI] [PubMed] [Google Scholar]
- Geraci J.R., St Aubin D.J. Effects of parasites on marine mammals. Int. J. Parasitol. 1987;17:407–414. doi: 10.1016/0020-7519(87)90116-0. [DOI] [PubMed] [Google Scholar]
- Gibson D.I. The systematics of ascaridoid nematodes-acurrent assessment. In: Stone A.R., Platt H.M., Khalil L.F., editors. Concepts in Nematode Systematics. Academic Press; London: 1983. pp. 321–338. [Google Scholar]
- Gibson D.I., Colin J.A. The Terranova enigma. Parasitology. 1982;85:R36–R37. [Google Scholar]
- Glazebrook J.S., Campbell R.S.F. A survey of the diseases of marine turtles in northern Australia. I. Farmed turtles. Dis. Aquat. Organ. 1990;9:83–104. [Google Scholar]
- Gorokhov V.V., Sergiev V.P., Romanenko N.A. Anisakiasis as a growing ecological and social problem. Med. Parazitol. (Mosk) 1999:50–54. [PubMed] [Google Scholar]
- Hartwich G. Zur systematik der Nematoden-superfamilie Ascaridoidea. Zool. Jb. Syst. (Jena) 1957;85:211–252. [Google Scholar]
- Hartwich, G., 1963. Revision der vogelparasitischen nematoden mitteleuropas. II. Die Gattung Contracaecum Railliet & Henry, 1912 (Ascaridoidea). Aus dem Institut fur Spezielle Zoologie und Zoologischen Museum der Humboldt-Universitat zu Berlin, 16–51.
- Hartwich G. Revision der vogelparasitischen nematoden mitteleuropas, II. De gattung Contracaecum Railliet & Henry, 1912. Mitt. Zool. Mus. Berl. 1964;40:15–53. [Google Scholar]
- Hartwich G. Uber die wirtsspezifitat bei den gattungen Porrocaecum und Contracaecum (Nematoda, Ascaridoidea) Ceskoslovenska Parasitologie XI. 1964:323–325. [Google Scholar]
- Hartwich G. Keys to genera of the Ascaridoidea. In: Anderson R.C., A.G.C., Willmott S., editors. CIH Keys to the Nematode Parasites of Vertebrates. Commonwealth Agricultural Bureaux; 1974. pp. 1–15. [Google Scholar]
- Hartwich, G., 1975. Parasitic nematodes of vertebrates. I. Rhabditida and Ascaridida. Schlauchwurmer, Nemathelminthes; Rund- oder Fadenwurmer, Nematoda; Parasitische Rundwurmer von Wirbeltieren. I. Rhabditida und Ascaridia. [Die Tierwelt Deutschlands, 62. Teil.]. pp. 256.
- Hooper J.N.A. Parasites of estuarine and oceanic flathead fishes (family Platycephalidae) from northern New South Wales. Aust. J. Zool. Suppl. Ser. 1983;(90):1–69. [Google Scholar]
- Jabbar A., Asnoussi A., Norbury L.J., Eisenbarth A., Shamsi S., Gasser R.B., Lopata A.L., Beveridge I. Larval anisakid nematodes in teleost fishes from Lizard Island, northern Great Barrier Reef, Australia. Mar. Freshw. Res. 2012;63:1283–1299. [Google Scholar]
- Jefferies D.J., Hanson H.M., Harris E.A. The prevalence of Pseudoterranova decipiens (Nematoda) and Corynosoma strumosum (Acanthocephala) in otters Lutra lutra from coastal sites in Britain. J. Zool. 1990;221:316–321. [Google Scholar]
- Jensen T. Experimental infection/transmission of sculpins (Myoxocephalus scorpius) and cod (Gadus morhua) by sealworm (Pseudoterranova decipiens) larvae. Parasitol. Res. 1997;83:380–382. doi: 10.1007/s004360050266. [DOI] [PubMed] [Google Scholar]
- Johnston T.H. Exhibit of a series of entozoa. Proc. Linn. Soc. N.S.W. 1910;35:309–310. [Google Scholar]
- Johnston T.H. Notes on some entozoa. Proc. R. Soc. Qld. 1913;24:63–91. [Google Scholar]
- Johnston T.H. Entozoa from Australian hair seal. Proc. Linn. Soc. N.S.W. 1937;62:9–16. [Google Scholar]
- Johnston, T.H., 1938. Parasitic nematoda. Scientific reports of the Australian Antarctic Expedition. Ser. C 10, 1–31.
- Johnston T.H., Mawson P.M. Internal parasites of the pigmy sperm whale. Rec. S. Aust. Mus. 1939;6:263–274. [Google Scholar]
- Johnston T.H., Mawson P.M. Some filarial parasites of Australian birds. Trans. R. Soc. S. Aust. 1940;64:355–361. [Google Scholar]
- Johnston T.H., Mawson P.M. Some nematode parasitic in Australian freshwater fish. Trans. R. Soc. S. Aust. 1940;64:340–352. [Google Scholar]
- Johnston T.H., Mawson P.M. Additional nematodes from Australian birds. Trans. R. Soc. S. Aust. 1941;65:254–262. [Google Scholar]
- Johnston T.H., Mawson P.M. Ascaroid nematodes from Australian birds. Trans. R. Soc. S. Aust. 1941;65:110–115. [Google Scholar]
- Johnston T.H., Mawson P.M. Nematodes from Australian marine mammals. Rec. S. Aust. Mus. 1941;6:429–434. [Google Scholar]
- Johnston T.H., Mawson P.M. Some nematode parasites of Australian birds. Proc. Linn. Soc. N.S.W. 1941;66:250–256. [Google Scholar]
- Johnston T.H., Mawson P.M. Some parasitic nematodes in the collection of the Australian museum. Rec. Aust. Mus. 1941;21:9–16. [Google Scholar]
- Johnston T.H., Mawson P.M. The Gallard collection of parasitic nematodes in the Australian museum. Rec. Aust. Mus. 1942;21:110–115. [Google Scholar]
- Johnston T.H., Mawson P.M. Nematodes from Australian albatrosses and petrels. Trans. R. Soc. S. Aust. 1942;66:66–70. [Google Scholar]
- Johnston T.H., Mawson P.M. Remarks on some parasitic nematodes. Rec. S. Aust. Mus. 1942;7:183–186. [Google Scholar]
- Johnston T.H., Mawson P.M. Some new and known Australian parasitic nematodes. Proc. Linn. Soc. N.S.W. 1942;67:90–94. [Google Scholar]
- Johnston T.H., Mawson P.M. Some ascarid nematodes from Australian marine fish. Trans. R. Soc. S. Aust. 1943;67:20–35. [Google Scholar]
- Johnston T.H., Mawson P.M. Some nematodes from Australian elasmobranchs. Trans. R. Soc. S. Aust. 1943;67:187–190. [Google Scholar]
- Johnston T.H., Mawson P.M. Remarks on some parasitic nematodes from Australia and New Zealand. Trans. R. Soc. S. Aust. 1944;68:60–66. [Google Scholar]
- Johnston, T.H., Mawson, P.M., 1945a. Parasitic nematodes. British, Australian and New Zealand Antarctic Research Expedition. Reports Series B 5, 73–159.
- Johnston T.H., Mawson P.M. Some parasitic nematodes from south Australian marine fish. Trans. R. Soc. S. Aust. 1945;69:114–117. [Google Scholar]
- Johnston T.H., Mawson P.M. Some avian and fish nematodes, chiefly from Tailem Bend, South Australia. Rec. S. Aust. Mus. 1947;8:547–553. [Google Scholar]
- Johnston T.H., Mawson P.M. Some nematodes from Australian lizards. Trans. R. Soc. S. Aust. 1947;71:22–37. [Google Scholar]
- Johnston T.H., Mawson P.M. Some nematodes from Australian hosts, together with a note on Rhabditis allgeni. Trans. R. Soc. S. Aust. 1949;73:63–71. [Google Scholar]
- Johnston T.H., Mawson P.M. Additional nematodes from Australian fish. Trans. R. Soc. S. Aust. 1951;74:18–24. [Google Scholar]
- Johnston T.H., Mawson P.M. Report on some parasitic nematodes from the Australian Museum. Rec. Aust. Mus. 1951;22:289–297. [Google Scholar]
- Johnston T.H., Mawson P.M. Some nematodes from Australian birds and mammals. Trans. R. Soc. S. Aust. 1952;75:30–37. [Google Scholar]
- Johnston T.H., Mawson P.M. Parasitic nematodes and trematodes from Campbell and Auckland Islands (cape expedition) Rec. Dom. Mus. 1953;2:63–71. [Google Scholar]
- Jones H.I. Observations on nematodes from west and central Australian snakes. Aust. J. Zool. 1980;28:423–433. [Google Scholar]
- Kapral C., Haditsch M., Wewalka F., Schatzlmayr W., Lenz K., Auer H. The first case of anisakiasis acquired in Austria. Z. Gastroenterol. 2009;47:1059–1061. doi: 10.1055/s-0028-1109468. [DOI] [PubMed] [Google Scholar]
- Kinsella J.M., Spalding M.G., Forrester D.J. Parasitic helminths of the American white pelican, Pelecanus erythrorhynchos, from Florida, USA. Comp. Parasitol. 2004;71:29–36. [Google Scholar]
- Kloser H., Plotz J., Palm H., Bartsch A., Hubold G. Adjustment of anisakid nemaode life cycles to the high Antarctic food web as shown by Contracaecum radiatum and C. osculatum in the Weddell Sea. Antarct. Sci. 1992;4:171–178. [Google Scholar]
- Li A., D’Amelio S., Paggi L., He F., Gasser R.B., Lun Z., Abollo E., Turchetto M., Zhu X. Genetic evidence for the existence of sibling species within Contracaecum rudolphii (Hartwich, 1964) and the validity of Contracaecum septentrionale (Kreis, 1955) (Nematoda: Anisakidae) Parasitol. Res. 2005;96:361–366. doi: 10.1007/s00436-005-1366-y. [DOI] [PubMed] [Google Scholar]
- Liu S., Edward A. Gastric ulcers associated with Contracaecum spp. (Nematoda: Ascaroidea) in a Steller sea lion and a white pelican. J. Wildl. Dis. 1971;7:266–271. [PubMed] [Google Scholar]
- Lopez-Serrano M.C., Gomez A.A., Daschner A., Moreno-Ancillo A., De Parga J.M.S., Caballero M.T., Barranco P., Cabanas R. Gastroallergic anisakiasis: findings in 22 patients. J. Gastroenterol. Hepatol. 2000;15:503–506. doi: 10.1046/j.1440-1746.2000.02153.x. [DOI] [PubMed] [Google Scholar]
- Lucker J.T. Contracaecum quincuspis, a new species of nematode from the American waterturkey. J. Wash. Acad. Sci. 1941;31:33–37. [Google Scholar]
- Lucker J.T. A redescription of Contracaecum multipapillatum (von Drasche, 1882) J. Parasitol. 1941;27:505–512. [PubMed] [Google Scholar]
- Lymbery A.J., Doupe R.G., Munshi M.A., Wong T. Larvae of Contracaecum sp. among inshore fish species of southwestern Australia. Dis. Aquat. Organ. 2002;51:157–159. doi: 10.3354/dao051157. [DOI] [PubMed] [Google Scholar]
- Mattiucci S., Cianchi R., Nascetti G., Paggi L., Sardella N., Timi J., Webb S.C., Bastida R., Rodriguez D., Bullini L. Genetic evidence for two sibling species within Contracaecum ogmorhini Johnston & Mawson, 1941 (Nematoda: Anisakidae) from otariid seals of boreal and austral regions. Syst. Parasitol. 2003;54:13–23. doi: 10.1023/a:1022145926409. [DOI] [PubMed] [Google Scholar]
- Mattiucci S., Cipriani P., Webb Stephen C., Paoletti M., Marcer F., Bellisario B., Gibson David I., Nascetti G. Genetic and morphological approaches distinguish the three sibling species of the Anisakis simplex species complex with a species designation as Anisakis berlandi n. sp. for A. simplex sp. C (Nematoda: Anisakidae) J. Parasitol. 2013;15:12–15. doi: 10.1645/12-120.1. [DOI] [PubMed] [Google Scholar]
- Mattiucci S., Fazii P., De Rosa A., Paoletti M., Megna A.S., Glielmo A., De Angelis M., Costa A., Meucci C., Calvaruso V., Sorrentini I., Palma G., Bruschi F., Nascetti G. Anisakiasis and gastroallergic reactions associated with Anisakis pegreffii infection, Italy. Emerg. Infect. Dis. 2013;19:496–499. doi: 10.3201/eid1903.121017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiucci S., Nascetti G. Molecular systematics, phylogeny and ecology of anisakid nematodes of the genus Anisakis Dujardin, 1845: an update. Parasite. 2006;13:99–113. doi: 10.1051/parasite/2006132099. [DOI] [PubMed] [Google Scholar]
- Mattiucci S., Nascetti G., Cianchi R., Paggi L., Arduino P., Margolis L., Brattey J., Webb S., D’Amelio S., Orecchia P., Bullini L. Genetic and ecological data on the Anisakis simplex complex, with evidence for a new species (Nematoda, Ascaridoidea, Anisakidae) J. Parasitol. 1997;83:401–416. [PubMed] [Google Scholar]
- Mattiucci S., Paggi L., Nascetti G., Abollo E., Webb S.C., Pascual S., Cianchi R., Bullini L. Genetic divergence and reproductive isolation between Anisakis brevispiculata and Anisakis physeteris (Nematoda: Anisakidae) Int. J. Parasitol. 2001;31:9–14. doi: 10.1016/s0020-7519(00)00125-9. [DOI] [PubMed] [Google Scholar]
- Mattiucci S., Turchetto M., Bragantini F., Nascetti G. On the occurrence of the sibling species of Contracaecum rudolphii complex (Nematoda: Anisakidae) in cormorants (Phalacrocorax carbo sinensis) from Venice and Caorle lagoons: genetic markers and ecological studies. Parassitologia. 2002;44:105. [Google Scholar]
- Mattiucci S., Paoletti M., Webb S.C., Sardella N., Timi J.T., Berland B., Nascetti G. Genetic relationships among species of Contracaecum Railliet & Henry, 1912 and Phocascaris Host, 1932 (Nematoda: Anisakidae) from pinnipeds inferred from mitochondrial cox2 sequences, and congruence with allozyme data. Parasite. 2008;15:408–419. doi: 10.1051/parasite/2008153408. [DOI] [PubMed] [Google Scholar]
- Mawson P. Parasitic nematoda collected by the Australian national Antarctic research expedition: Heard Island and Macquarie Island, 1948–1951. Parasitology. 1953;43:291–297. doi: 10.1017/s0031182000018667. [DOI] [PubMed] [Google Scholar]
- Mawson P. Some nematodes from fish from Heron Island, Queensland. Trans. R. Soc. S. Aust. 1957;80:177–179. [Google Scholar]
- Mawson P. Some nematodes from Australian gulls and terns. J. Fish. Res. Res. Bd. Canada. 1969;26:1103–1111. [Google Scholar]
- Mawson P.M. The status of some nematode species from Australian birds. Trans. R. Soc. S. Aust. 1983;107:247–248. [Google Scholar]
- Mawson P.M., Angel M., Edmonds S.J. A checklist of helminths from Australian birds. Rec. S. Aust. Mus. 1986;19:219–325. [Google Scholar]
- McColl K.A., Obendorf D.L. Helminth parasites and associated pathology in stranded Fraser’s dolphins, Lagenodelphis hosei (Fraser, 1956) Aquat. Mamm. 1982;9:30–34. [Google Scholar]
- Mozgovoi A.A. Fundamental of Nematodology. Nauk SSSR; 1953. Ascaridata of animals and man and the diseases caused by them. pp. 8–42. [Google Scholar]
- Nadler S.A., Hudspeth D.S.S. Phylogeny of the Ascaridoidea (Nematoda: Ascaridida) based on three genes and morphology: hypotheses of structural and sequence evolution. J. Parasitol. 2000;86:380–393. doi: 10.1645/0022-3395(2000)086[0380:POTANA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Nadler S.A., D’Amelio S., Fagerholm H.P., Berland B., Paggi L. Phylogenetic relationships among species of Contracaecum Railliet & Henry, 1912 and Phocascaris Host, 1932 (Nematoda: Ascaridoidea) based on nuclear rDNA sequence data. Parasitology. 2000;121:455–463. doi: 10.1017/s0031182099006423. [DOI] [PubMed] [Google Scholar]
- Nadler S.A., D’Amelio S., Dailey M.D., Paggi L., Siu S., Sakanari J.A. Molecular phylogenetics and diagnosis of Anisakis, Pseudoterranova, and Contracaecum from Northern Pacific marine mammals. J. Parasitol. 2005;91:1413–1429. doi: 10.1645/GE-522R.1. [DOI] [PubMed] [Google Scholar]
- Nagasawa K., Barus V., Tenora F., Oka N. Contracaecum variegatum (Nematoda: Anisakidae) from the Pacific diver (Gavia pacifica) in Japan. Biogeography. 1999;1:107–110. [Google Scholar]
- Nascetti G., Paggi L., Orecchia P., Mattiucci S., Bullini L. Two sibling species within Anisakis simplex (Ascaridida: Anisakidae) Parassitologia. 1983;25:306–307. [Google Scholar]
- Nascetti G., Cianchi R., Mattiucci S., D’amelio S., Orecchia P., Paggi L., Brattey J., Berland B., Smith J.W., Bullini L. Three sibling species within Contracaecum osculatum (Nematoda, Ascaridida, Ascaridoidea) from the atlantic arctic-boreal region: reproductive isolation and host preferences. Int. J. Parasitol. 1993;23:105–120. doi: 10.1016/0020-7519(93)90103-6. [DOI] [PubMed] [Google Scholar]
- Navone G.T., Etchegoin J.A., Cremonte F. Contracaecum multipapillatum (Nematoda: Anisakidae) from Egretta alba (Aves: Ardeidae) and comments on other species of this genus in Argentina. J. Parasitol. 2000;86:807–810. doi: 10.1645/0022-3395(2000)086[0807:CMNAFE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Norman R.J.D.B. University of Melbourne, Department of Veterinary Science; 2005. Parasitic diseases of the little penguin: Eudyptula minor (Forster, 1781), with emphasis on nematodes of the genus contracaecum Railliet & Henry, 1912 (Anisakidae) PhD. [Google Scholar]
- Orecchia P., Mattiucci S., D’Amelio S., Paggi L., Plotz J., Cianchi R., Nascetti G., Arduino P., Bullini L. Two new members in the Contracaecum osculatum complex (Nematoda: Ascaridoidea) from the Antarctic. Int. J. Parasitol. 1994;24:367–377. doi: 10.1016/0020-7519(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Owre O.T. Nematodes in birds of the order Pelecaniformes. Auk. 1962;79 114-114. [Google Scholar]
- Rudolphi C.A. vol. 2. Amsteodami; 1809. (Entozoorum Sive Vermium Intestinalium Historia Naturalis). 457 p. [Google Scholar]
- Semenova, M.K., 1979. Host–parasite relationships of fish and nematode larvae of the genus Contracaecum. In: VII Vsesoyuznoe Soveshchanie po parazitam i boleznyam ryb, Leningrad, Sentyabr’, 1979 g (Tezisy dokladov).
- Shamsi S. University of Melbourne; Melbourne: 2007. Morphologic and Genetic Characterisation of Selected Ascaridoid Nematodes. [Google Scholar]
- Shamsi S., Butcher A.R. First report of human anisakidosis in Australia. Med. J. Aust. 2011;194:199–200. doi: 10.5694/j.1326-5377.2011.tb03772.x. [DOI] [PubMed] [Google Scholar]
- Shamsi S., Gasser R., Beveridge I., Shabani A.A. Contracaecum pyripapillatum n. sp. and a description of C. multipapillatum (von Drasche, 1882) from the Australian pelican, Pelecanus conspicillatus. Parasitol. Res. 2008;103:1031–1039. doi: 10.1007/s00436-008-1088-z. [DOI] [PubMed] [Google Scholar]
- Shamsi S., Norman R., Gasser R., Beveridge I. Genetic and morphological evidences for the existence of sibling species within Contracaecum rudolphii (Hartwich, 1964) (Nematoda: Anisakidae) in Australia. Parasitol. Res. 2009;105:529–538. doi: 10.1007/s00436-009-1424-y. [DOI] [PubMed] [Google Scholar]
- Shamsi S., Norman R., Gasser R., Beveridge I. Redescription and genetic characterization of selected Contracaecum spp. (Nematoda: Anisakidae) from various hosts in Australia. Parasitol. Res. 2009;104:1507–1525. doi: 10.1007/s00436-009-1357-5. [DOI] [PubMed] [Google Scholar]
- Shamsi S., Eisenbarth A., Saptarshi S., Beveridge I., Gasser R.B., Lopata A.L. Occurrence and abundance of anisakid nematode larvae in five species of fish from southern Australian waters. Parasitol. Res. 2011;108:927–934. doi: 10.1007/s00436-010-2134-1. [DOI] [PubMed] [Google Scholar]
- Shamsi S., Gasser R.B., Beveridge I. Mutation scanning-coupled sequencing of nuclear ribosomal DNA spacers (as a taxonomic tool) for the specific identification of different Contracaecum (Nematoda: Anisakidae) larval types. Mol. Cell. Probes. 2011;25:13–18. doi: 10.1016/j.mcp.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Shamsi S., Gasser R., Beveridge I. Genetic characterisation and taxonomy of species of Anisakis (Nematoda: Anisakidae) parasitic in Australian marine mammals. Invert. Syst. 2012;26:204–212. [Google Scholar]
- Shamsi S., Gasser R., Beveridge I. Description and genetic characterisation of Hysterothylacium (Nematoda: Raphidascarididae) larvae parasitic in Australian marine fishes. Parasitol. Int. 2013;62:320–328. doi: 10.1016/j.parint.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Shipley A.E., Hornell J. The parasites of the pearl oyster. In: Herdman W.A., editor. Report to the Government of Ceylon on the Pearl Oyster Fisheries of the Gulf of Manaar. Part II. The Royal Society; London: 1906. pp. 77–106+plates. [Google Scholar]
- Silva R.J., Raso T.F., Faria P.J., Campos F.P. Occurrence of Contracaecum pelagicum Johnston & Mawson 1942 (Nematoda, Anisakidae) in Sula leucogaster Boddaert 1783 (Pelecaniformes, Sulidae) Arq. Bras. Med. Vet. Zootec. 2005;57:565–567. [Google Scholar]
- Smith J.W. Ascaridoid nematodes and pathology of the alimentary tract and its associated organs in vertebrates, including man: a literature review. Helminthol. Abstr. 1999;68:49–96. [Google Scholar]
- Smith J.W., Wootten R. Experimental studies on the migration of Anisakis sp. larvae (Nematoda: Ascaridida) into the flesh of herring, Clupea harengus L. Int. J. Parasitol. 1975;5:133–136. doi: 10.1016/0020-7519(75)90019-3. [DOI] [PubMed] [Google Scholar]
- Spratt D.M. CSIRO; 1983. A List of Type Specimens of Animal Parasites Held in Australian, Institutions, Technical Memorandum, Division of Wildlife and Rangelands Research. 64 pp. [Google Scholar]
- Sprent J.F.A. Ascaridoid nematodes of amphibians and reptiles: Terranova. J. Helminthol. 1979;53:265–282. doi: 10.1017/s0022149x00005782. [DOI] [PubMed] [Google Scholar]
- Sprent J.F.A. Observations on the systematics of ascaridoid nematodes. In: Stone A.R., Platt H.M., Khalil L.F., editors. Concepts in Nematode Systematics. Academic Press; London: 1983. pp. 303–319. [Google Scholar]
- Sprent J.F.A. Ascaridoid nematodes of amphibians and reptiles – Raillietascaris N G. Ann. Parasitol. Hum. Comp. 1985;60:601–611. doi: 10.1051/parasite/1985605601. [DOI] [PubMed] [Google Scholar]
- Sprent J.F.A. Some ascaridoid nematodes of fishes: Paranisakis and Mawsonascaris n.g. Syst. Parasitol. 1990;15:41–63. [Google Scholar]
- Stroud R.K., Roffe T.J. Causes of death in marine mammals stranded along the Oregon Coast. J. Wildl. Dis. 1979;15:91–97. doi: 10.7589/0090-3558-15.1.91. [DOI] [PubMed] [Google Scholar]
- Tanzola R.D., Sardella N.H. Terranova galeocerdonis (Thwaite, 1927) (Nematoda: Anisakidae) from Carcharias taurus (Chondrichthyes: Odontaspididae) off Argentina, with comments on some related species. Syst. Parasitol. 2006;64:27–36. doi: 10.1007/s11230-005-9015-5. [DOI] [PubMed] [Google Scholar]
- Torres P., Ortega J., Schlatter R. Nematode parasites of the digestive tract in neotropic cormorant chicks (Phalacrocorax brasilianus) from the River Cruces Ramsar site in southern Chile. Parasitol. Res. 2005;97:103–107. doi: 10.1007/s00436-005-1372-0. [DOI] [PubMed] [Google Scholar]
- Ugland K.I., Stromnes E., Berland B., Aspholm P.E. Growth, fecundity and sex ratio of adult whaleworm (Anisakis simplex; Nematoda, Ascaridoidea, Anisakidae) in three whale species from the North–East Atlantic. Parasitol. Res. 2004;92:484–489. doi: 10.1007/s00436-003-1065-5. [DOI] [PubMed] [Google Scholar]
- Usui, M., Ashizawa, H., Nosaka, D., Tateyama, S., 1973. Studies on swine gastric anisakiasis. 1. Morphological findings on worms. Bulletin of the Faculty of Agriculture, Miyazaki University 20.
- Van Thiel P.H., Kuipers F.C., Roskam R.T. A nematode parasitic to herring, causing acute abdominal syndromes in man. Trop. Geogr. Med. 1960;2:97–113. [PubMed] [Google Scholar]
- Woo P.T.K. Fish diseases and disorders. Volume 1: protozoan and metazoan infections. CAB International; Wallingford. UK: 1995. [Google Scholar]
- Yamaguti S. Interscience Publishers Inc.; New York: 1961. (Systema Helminthum. Vol. 3. The Nematodes of Vertebrates. Part II). [Google Scholar]
- Zhang Y., Lin R., Zhao G., Yuan Z., Song H., Zhu X. ITS rDNA sequencing and phylogenetic analysis of the new species Contracaecum rudolphii C (Nematoda: Anisakidae) Chinese Veterinary Science/Zhongguo Shouyi Kexue. 2009;39:298–302. [Google Scholar]


