Abstract
Mutations in CSF3R (colony-stimulating factor 3 receptor) are frequent oncogenic drivers in chronic neutrophilic leukemia (CNL) and atypical chronic myeloid leukemia (aCML). Here we describe a 75 year old man who was diagnosed with CSF3R-T618I-positive atypical CML. He presented with leukocytosis, anemia, and thrombocytopenia and developed massive splenomegaly and severe constitutional symptoms. Hydroxyurea was given over a 6 month period but failed to provide any measureable clinical benefit. Eventually, he was treated with ruxolitinib, an FDA-approved JAK1/2 inhibitor, which resulted in dramatic improvement of his blood counts. He also had significant reduction of spleen volume and constitutional symptoms. This case highlights the need for a clinical trial to interrogate JAK1/2 as a potential molecular target in CNL and aCML in patients with or without CSF3R mutation. A clinical trial evaluating the safety and efficacy of ruxolitinib for this patient population is registered at ClinicalTrials.gov (NCT02092324).
Keywords: Colony-stimulating factor 3 receptor, Chronic neutrophilic leukemia, Atypical chronic myeloid leukemia, Ruxolitinib
Highlights
-
•
Mutations in CSF3R are frequent in CNL and aCML.
-
•
Here we describe a 75 year old man with CSF3R-T618I-positive atypical CML.
-
•
Treatment with ruxolitinib (JAK1/2 inhibitor) provided significant clinical benefit.
-
•
A clinical trial evaluating safety and efficacy of ruxolitinib is underway.
1. Introduction
Clinically, CNL and aCML are rare leukemias characterized by varying degrees of leukocytosis, anemia, thrombocytopenia, splenomegaly, and constitutional symptoms. Key distinguishing pathologic features between CNL and aCML are summarized in Table 1 [1]. No standard of care is established for CNL and aCML and the reported median overall survival is approximately two years [2]. Recently, Maxson, et al. reported CSF3R mutations in ~90% of patients with CNL and in ~40% of patients with aCML [3]. Subsequent studies confirmed this high frequency of CSF3R mutation in CNL while observing a lower frequency in aCML [4]. Mutations in CSF3R generally occur in the extracellular membrane proximal domain or result in premature truncation of the cytoplasmic tail. Of note, membrane proximal mutations are far more common [3]. These membrane proximal mutations cause significant activation of JAK/STAT signaling. Therefore, targeting of the JAK/STAT pathway may inhibit granulocytic proliferation and provide clinical benefit to patients with CNL or aCML. Ruxolitinib (Incyte Corporation) is the first FDA-approved JAK1/JAK2 inhibitor with a reported IC50 of 3.3 nM and 2.8 nM, respectively [5]. In preclinical studies, targeting JAK1/2 with ruxolitinib significantly suppressed CSF3R-T618I-induced malignant colony growth compared to no drug treatment controls [6]. Transplantation of T618I-CSF3R-expressing mouse bone marrow cells was sufficient to produce a highly penetrant, lethal neutrophilic leukemia in a mouse model. Treatment of experimental mice with ruxolitinib provided disease control and improved survival compared to untreated controls [6]. These studies suggest that targeting JAK1/2 may provide clinical benefit to patients with these rare types of leukemia.
Table 1.
Key distinguishing pathologic differences between CNL and aCML according to the 2008 WHO Classification.[1].
| Chronic neutrophilic leukemia | Atypical chronic myeloid leukemia | |
|---|---|---|
| Blood | ||
| Immature granulocytesa | <10% | ≥10% |
| Myeloblasts | <1% | <20% |
| Marrow | ||
| Granulocytic hyperplasia | Present | Present |
| Myeloblasts | <5% | <20% |
| Granulocytic dysplasia | Minimal/absent | Present |
| Megakaryocytic dysplasia | Absent | Present |
Promyelocytes, myelocytes, and metamyelocytes.
2. Case study
A 75 year old man with Parkinson׳s disease was diagnosed with aCML in April 2013 based on pathologic review of his bone marrow biopsy and on the finding of the CSF3R-T618I mutation. According to old records, leukocytosis (not otherwise specified) was present as far back as 2005. His CBC before he began taking hydroxyurea showed WBC 71.3×10e3/microliter, ANC 42.1×10e3/microliter, Hgb 9.8 g/dL, MCV 98.3 fL, and platelet 97×10e3/microliter. His peripheral blood showed 12% immature granulocytes and hypogranular neutrophils and rare pseudo-Pelger–Huet neutrophils. His bone marrow showed granulocytic hyperplasia (myeloid:erythroid ratio >15:1) and hypolobated megakaryocytes in 30% of megakaryocytes. Peripheral and marrow blasts were less than 1%. In addition to the CSF3R-T618I mutation (50% allele frequency), his disease also harbored CBL-I383T (70% allele frequency) and KDM6A-S114C (100% allele frequency on one X chromosome) mutations evaluated by massively parallel sequencing. SETBP1 was wildtype. His disease was characterized by progressive leukocytosis, anemia, thrombocytopenia, splenomegaly, and constitutional symptoms. Performance status was ECOG of 3. Physical exam revealed a chronically ill-appearing, cachectic male with massive splenomegaly. He began taking hydroxyurea in April 2013 at 1000 mg three times per week and 500 mg four times per week (total 5000 mg per week).
Despite taking hydroxyurea for 6 months, his leukocytosis, splenomegaly, and constitutional symptoms were poorly controlled and his performance status and overall function had declined even further. There was little clinical benefit to gain by increasing the hydroxyurea dose further especially in the setting of severe thrombocytopenia. Interferon therapy was considered contraindicated due to the potential for exacerbating his mood disturbances associated with Parkinson׳s disease. The patient was eventually prescribed ruxolitinib through his commercial insurance. Shortly before starting ruxolitinib but while still on hydroxyurea, his CBC showed WBC 56.0×10e3/microliter, ANC 48.7×10e3/microliter, Hgb 8.6 g/dL, MCV 121 fL, and platelet 28×10e3/microliter. His spleen volume was 4342 cm3 [3] and his total symptom score was 164 (total max of 270, MPN-symptom assessment form [MPN-SAF]) [7].
On December 2013 the patient was started on ruxolitinib 10 mg twice a day (day 0). His dose was increased to 15 mg twice a day on day +57 and to 20 mg twice a day on day +84. He was tapered off hydroxyurea and was completely off on day +14. As shown in Fig. 1A, the WBC and MCV were reduced gradually over 3 months while his Hgb and platelets steadily increased, indicating improved marrow function by targeting JAK1/JAK2 signaling and going off hydroxyurea. His peripheral blood showed reduced immature granulocytes while the neutrophils displayed increased cytoplasmic granularity/toxic granulation. His bone marrow had reduced granulocytic hyperplasia (myeloid:erythroid ratio 10:1) and fewer hypolobated megakaryocytes compared with the pre-ruxolitinib bone marrow evaluation. Peripheral and marrow blasts were less than 1%. In addition, his spleen volume was reduced by approximately 75% after approximately 3 months of ruxolitinib therapy as shown in Fig. 1B. Furthermore, his quality of life and total symptom score improved dramatically as shown in Fig. 1C. As a result of his excellent tolerance and response to ruxolitinib, his dose was increased further to the target dose of 20 mg twice a day. After this adjustment, his platelets increased further as shown in Fig. 1A. He has gained weight and his performance status improved to ECOG of 1. As of this report, he continues to do well and remains on ruxolitnib 20 mg twice a day. Interestingly, his dramatic clinical response was not associated with a reduction in CSF3R-T618I allele frequency based on peripheral blood studies after approximately 4 months of ruxolitinib treatment. Single colony assays confirmed that the allele frequency was not significantly reduced.
Fig. 1.
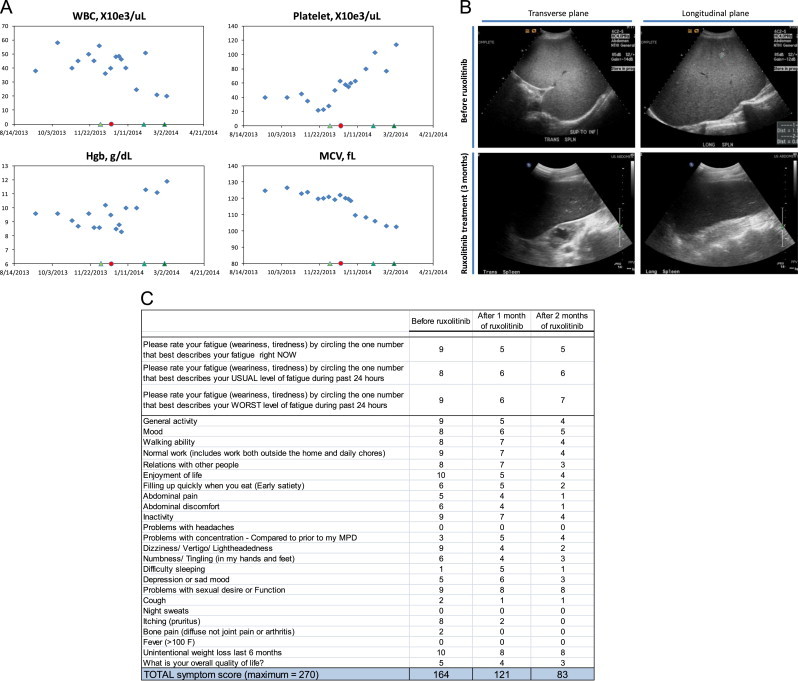
Clinical response in a patient with CSF3R-T618I-positive aCML treated with ruxolitinib. (A) WBC, platelet, Hgb, and MCV laboratory values during ruxolitinib treatment. Refer to case study description for details. Hydroxyurea was stopped at the indicated red circle and ruxolitinib was started at 10 mg twice daily at the indicated green triangle, both on the x-axis. Subsequent green triangles indicate when ruxolitinib was increased to 15 mg twice daily, then to 20 mg twice daily. (B) Spleen volume was determined by conventional prolate ellipsoid method (0.524×W×T×ML; width, thickness, and max length). Two different ultrasound views are shown, the transverse and longitudinal views. Before ruxolitinib treatment, the spleen was diffusely echogenic with hyperechoic nodules. The spleen measured 25.6×19.5×16.6 cm; volume=4342 cm3. After 3 months of ruxolitinib treatment, the spleen was normal in echogenicity and the hyperechoic nodules resolved. The spleen measured 18.5×14.3×7.6 cm; volume=1053 cm3. (C) Shown is the MPN-SAF symptom questionnaire as filled out by the patient. The total symptom score (the sum of each line) is quantitative and a useful tool to monitor symptoms and treatment responses [7]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3. Discussion
Here we report a case of CSF3R-T618I-positive aCML with a robust clinical response to JAK1/2 inhibition with ruxolitinib. The clinical benefit in this case was not merely leukoreduction but also improved marrow function as evident by near normalization of Hgb, MCV, and platelet counts. In addition, the patient had a significant reduction in spleen volume and constitutional symptoms, which were refractory to hydroxyurea. In the report by Maxson et al. [3] a clinical case of CSF3R-T618I-positive CNL was described where the patient also had platelet and Hgb improvement with ruxolitinib. These clinical cases suggest interesting differences between myelofibrosis (primary, post-polycythemia vera, and post-essential thrombocytosis) and CNL/aCML. As noted in the Comfort I study, a randomized placebo-controlled phase III clinical trial with ruxolitinib in patients with myelofibrosis, (1) thrombocytopenia is a dose-limiting toxicity, (2) WBC is not substantially reduced in myelofibrosis patients, and (3) marrow function is not measureably improved with ruxolitinib despite clinical responses (spleen and symptom reduction). One similarity is that the allele burden of the oncogenic driver is not significantly reduced with ruxolitinib [8].
The case described here suggests that targeting JAK1/2 in CNL/aCML may provide clinical benefit beyond spleen and symptom reduction, and may suppress the malignant clone and enhance normal hematopoiesis more effectively than that seen in myelofibrosis. Interestingly, we did not detect a reduction in CSF3R-T618I allele frequency or overall bone marrow cellularity after 3 months of treatment, suggesting that JAK1/2 inhibition may preferentially affect cells not in the sanctuary of the bone marrow microenvironment. It will be important to expand upon prior work to confidently show that specific inhibition of JAK kinases rather than other kinase targets underlie these clinical responses. The safety profile and clinical benefit of ruxolitinib treatment or other JAK1/2 inhibitors in patients with CNL and aCML in a prospective clinical trial is needed to establish the (1) frequency of clinical responses, (2) durability and depth of clinical responses, (3) genetic modifiers of disease characteristics and clinical responses such as co-existing SETBP1 or TET2 mutations, and (4) impact on long-term quality of life and overall survival. As of this report, a prospective, multi-center phase II clinical trial investigating the safety and efficacy of ruxolitinib in this patient population is registered at ClinicalTrials.gov (NCT02092324) and is open for participant recruitment.
In summary, this case highlights the potential clinical benefit of JAK1/2 inhibition for patients with CNL or aCML with CSF3R mutations. It is not known yet whether patients with wildtype CSF3R will experience the same clinical benefit. Many patients with CNL and aCML present with severe anemia and thrombocytopenia thus limiting the usefulness of hydroxyurea. The recent discovery of frequent mutations in CSF3R in patients with CNL and aCML and the preclinical studies supporting JAK1/2 as a molecular target have uncovered a potential line of therapy for these rare types of leukemia associated with very poor prognosis.
Acknowledgments
KTD receives support from the OHSU Knight Cancer Institute and the National Institutes of Health, National Heart, Lung and Blood Institute (1K08HL111280). BJD is supported by the Howard Hughes Medical Institute. JWT is supported by Grants from the V Foundation for Cancer Research, the Leukemia and Lymphoma Society, the Gabrielle׳s Angel Foundation for Cancer Research, and the National Cancer Institute (5R00CA151457 and 1R01CA183974).
References
- 1.The International Agency for Research on Cancer . In: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue (IARC WHO Classification of Tumours) 4th ed. Swerdlow S., Campo E., Lee Harris N., Pileri S.A., Stein H., Thiele J., Vardiman J.W., editors. World Health Organization; 2008. p. 441. [Google Scholar]
- 2.Gotlib J., Maxson J.E., George T.I., Tyner J.W. The new genetics of chronic neutrophilic leukemia and atypical CML: implications for diagnosis and treatment. Blood. 2013;122(10):1707–1711. doi: 10.1182/blood-2013-05-500959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maxson J.E., Gotlib J., Pollyea D.A., Fleischman A.G., Agarwal A., Eide C.A. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N Engl J Med. 2013;368(19):1781–1790. doi: 10.1056/NEJMoa1214514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardanani A., Lasho T.L., Laborde R.R., Elliott M., Hanson C.A., Knudson R.A. CSF3R T618I is a highly prevalent and specific mutation in chronic neutrophilic leukemia. Leukemia. 2013;27(9):1870–1873. doi: 10.1038/leu.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mascarenhas J., Hoffman R. Ruxolitinib: the first FDA approved therapy for the treatment of myelofibrosis. Clin Cancer Res. 2012;18(11):3008–3014. doi: 10.1158/1078-0432.CCR-11-3145. [DOI] [PubMed] [Google Scholar]
- 6.Fleischman A.G., Maxson J.E., Luty S.B., Agarwal A., Royer L.R., Abel M.L. The CSF3R T618I mutation causes a lethal neutrophilic neoplasia in mice that is responsive to therapeutic JAK inhibition. Blood. 2013;122(22):3628–3631. doi: 10.1182/blood-2013-06-509976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emanuel R.M., Dueck A.C., Geyer H.L., Kiladjian J.J., Slot S., Zweegman S. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30(33):4098–4103. doi: 10.1200/JCO.2012.42.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verstovsek S., Mesa R.A., Gotlib J., Levy R.S., Gupta V., DiPersio J.F. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]


