Abstract
Therapeutic approaches for prevention or reduction of amyloidosis are currently a main objective in basic and clinical research on Alzheimer‘s disease. Among the agents explored in clinical trials are anti-Aβ peptide antibodies and secretase inhibitors. Most anti-Aβ antibodies are considered to act via inhibition of amyloidosis and enhanced clearance of existing amyloid, although secretase inhibitors reduce the de novo production of Aβ. Limited information is currently available on the efficacy and potential advantages of combinatorial antiamyloid treatment. We performed a chronic study in APPLondon transgenic mice that received treatment with anti-Aβ antibody gantenerumab and BACE inhibitor RO5508887, either as mono- or combination treatment. Treatment aimed to evaluate efficacy on amyloid progression, similar to preexisting amyloidosis as present in Alzheimer's disease patients. Mono-treatments with either compound caused a dose-dependent reduction of total brain Aβ and amyloid burden. Combination treatment with both compounds significantly enhanced the antiamyloid effect. The observed combination effect was most pronounced for lowering of amyloid plaque load and plaque number, which suggests effective inhibition of de novo plaque formation. Moreover, significantly enhanced clearance of pre-existing amyloid plaques was observed when gantenerumab was coadministered with RO5508887. BACE inhibition led to a significant time- and dose-dependent decrease in CSF Aβ, which was not observed for gantenerumab treatment. Our results demonstrate that combining these two antiamyloid agents enhances overall efficacy and suggests that combination treatments may be of clinical relevance.
Keywords: Aβ-antibody, Alzheimer's disease, amyloidosis, BACE, tg-APP mouse
Introduction
Dementia of the Alzheimer's disease (AD) type is defined by two characteristic CNS pathologies, extracellular amyloid and intracellular neurofibrillary tangles (Ballard et al., 2011). Extracellular amyloidosis is the earlier pathology and occurs well before the clinical symptoms of AD (Aisen et al., 2010). The amyloid cascade hypothesis of AD states that accumulation of Aβ is the ultimate cause of the disease (Selkoe, 2000; Hardy and Selkoe, 2002). Aβ is derived from β-amyloid precursor protein (APP) via successive proteolytic cleavage. The extracellular domain is shedded through the activity of BACE1 (β-site β-amyloid APP cleaving enzyme 1), a membrane-bound aspartyl protease (Vassar and Citron, 2000; Cole and Vassar, 2007; Dislich and Lichtenthaler, 2012). The resulting C-terminal, membrane-bound stub becomes a substrate for γ-secretase, which liberates various Aβ peptides through progressive cleavage steps (Wolfe, 2006; Xu, 2009). The predominant Aβ species is a peptide of 40 aa length (Aβ40) but Aβ42 is recognized as the more pathogenic species (Bitan et al., 2003a,b; Walsh and Selkoe, 2004).
Prevention of amyloid formation or clearance of existing amyloid at an early in disease is currently considered a promising disease-modifying therapeutic strategy in AD (Brody and Holtzman, 2008; Jakob-Roetne and Jacobsen, 2009; De Strooper et al., 2010). Lowering BACE1 activity reduces the de novo formation of Aβ, thus preventing its subsequent aggregation into toxic aggregates (Cai et al., 2001; Vassar, 2001; Citron, 2002; McConlogue et al., 2007). Potent inhibitors of BACE1 have been described and several clinical trials are ongoing (May et al., 2011; Hamada and Kiso, 2013; Hilpert et al., 2013).
Inhibition of amyloid formation and clearance of existing amyloid have also been achieved with anti-Aβ antibodies. Phase 3 clinical trials with bapineuzumab and solanezumab have been completed recently (Doody et al., 2014; Salloway et al., 2014). Although the studies failed to demonstrate an effect on the primary endpoints, some encouraging signs on cognitive, functional, and biomarker measures have been noted. Anti-Aβ antibodies that bind directly to amyloid can act through enhanced amyloid degradation by microglial cells (Bard et al., 2000; Ostrowitzki et al., 2012), whereas antibodies like solanezumab, which bind soluble Aβ, likely interfere at the level of the aggregation process (Demattos et al., 2012).
Antibodies which target existing Aβ species act downstream of BACE1 inhibitors. We therefore evaluated whether combined pharmacological intervention with a BACE1 inhibitor and a plaque specific antibody would lead to an enhanced amyloid-lowering effect. We performed a chronic study in APPLondon transgenic mice with BACE inhibitor RO5508887 and the anti-Aβ antibody gantenerumab. Gantenerumab, a fully human monoclonal antibody preferentially binds aggregated Aβ and has demonstrated amyloid-lowering activity in transgenic mice and also in AD patients (Bohrmann et al., 2012; Ostrowitzki et al., 2012). APPLondon mice (Tanghe et al., 2010) with an established amyloidosis were treated for 4 months with either agent alone or in combination. Total brain Aβ40 and Aβ42, plaque burden, and plaque size and number were measured. We show that combined treatment with the BACE inhibitor RO5508887 and gantenerumab reduced amyloidosis significantly more than mono-treatments. Our data support the use of combination treatment as an attractive option for future clinical trials to augment the expected therapeutic benefit of antiamyloid treatment.
Materials and Methods
Transgenic mice
Female transgenic mice in mixed FVB/N × C57BL/6J background expressing heterozygously hAPP.V717I (APPLon) under control of the neuron-specific murine thy1 gene promoter have been used in this study. The construction of the FVB/N background strain and some to its properties were described earlier (Moechars et al., 1999; Tanghe et al., 2010). Genotyping by two independent PCR assays at the age of 3 weeks and at the onset of the experiments on DNA extracted from tail biopsies were affirmative of the genotype. Mice were randomly allocated to the different treatment arms. Transgenic mice overexpressing human APPSw were previously described (Richards et al., 2003).
Animal care and handling
All treatments were approved by the Local Committee for Animal Use and were performed in accordance to state and federal regulations. Mice had access to prefiltered sterile water and standard mouse chow (Ssniff Ms-H, Ssniff Spezialdiäten GmbH) ad libitum and were housed under a reversed day–night rhythm in individual ventilated macrolon T2 cages equipped with solid floors and a layer of bedding, in accordance to local legislation on animal welfare.
Treatment
In this study, BACE inhibitor RO5508887 and anti-Aβ monoclonal antibody gantenerumab were tested separately and as combination treatment. The BACE inhibitor was administered daily per os (gavage) and gantenerumab weekly intravenously (in tail vein) for a period of 4 months, between the age of 13.5 and 17.5 months. Vehicle for the BACE inhibitor was 5% ethanol (VWR Prolabo), 10% solutol (BASF Chemtrade GmbH) dissolved in sterile water (Baxter).The antibody was dissolved in 0.9% NaCl. The study comprised six treatment arms receiving one of the following: vehicle (7 ml/kg per os), BACE inhibitor (30 or 90 mg/kg, 7 ml/kg per os), anti-Aβ mAb (20 mg/kg, 5 ml/kg i.v.), or a combination of anti-Aβ mAb plus the low- or the high-dose of BACE inhibitor. Additionally, a nontreated baseline group of age 13.5 months was killed at study start. All mice in the study, except the baseline group, received one dose of an anti-CD4 mAb (hybridome clone GK 1.5, ATCC, Catalog # tib-207 RRID: AB_2260139, 0.5 mg i.v. in the tail vein, slow bolus) to deplete the CD4-positive T cells. Anti-CD4 treatment was done 1 d before start of treatment with RO5508887 and gantenerumab. Previous studies have demonstrated that the anti-CD4 treatment prevented the formation of immune response against the human gantenerumab (Bohrmann et al., 2012).
Sacrifice and biological sample collection
Blood, brain tissues, and CSF samples of untreated (baseline) mice were collected at the age of 13.5 months. Treated mice were killed at the age of 17.5 months on the last day of treatment. The following sampling schedule was used after the final treatment to obtain a composite time course: predose, 2, 5, and 8 h postdose. The mice were anesthetized with 3.5 μl per gram of body weight with a mixture of ketamine (115 mg/ml ketamine hydrochloride, Eurovet), xylazin 2% (23,32 mg/ml xylazine hydrochloride, VMD), atropine (0.50 mg/ml atropine sulfate, Sterop), and saline (4:2,5:1:2,5).
CSF collection.
CSF was collected via an incision in the neck muscles, between the skull and the first cervical vertebrae. A puncture into the cisterna magna was given with a 26-gauge needle and 10–20 μl of CSF was collected with a fine glass pipette. Samples were put into Eppendorf tubes containing 100 μl of assay buffer (50 mm Tris, pH 7.4, 60 mm NaCl, 0.1% Tween, 0.5% BSA). Tubes containing CSF and assay buffer were weighted, snap-frozen and stored at −80°C.
Brain collection and processing.
After trans-cardiac perfusion of the mice with ice-cold saline, the brain was removed from the cranium, and hindbrain and forebrain were separated with a cut in the coronal/frontal plane. The forebrain was divided evenly into left and right hemisphere by using a midline sagittal cut. Two-thirds of the right hemispheres were prepared for immunohistochemistry. Brains were frozen on a cork pad wetted with a film of saline and were kept frozen at with cork pad at 80°C wrapped in aluminum foil until sectioning in a cryostat. The other one-third of the right hemispheres was fixed in 10% neutral formalin for 24 h, after which the formalin was exchanged for PBS. All samples collected were labeled with the ID number of the mouse, without any reference to the treatment so that further processing of the samples could be done in a “blind” way. Gross inspection: different tissues (kidneys, spleen, liver, stomach, gut, lungs, and heart) were examined and checked for gross abnormalities. None was observed in any of the treatment groups.
The formalin-fixed hemisphere was embedded in paraffin blocks and slices of 4 μm were prepared. These were stained with standard hematoxylin-eosin stain and Prussian blue stain for the detection of hemorrhages. For the detection of amyloid laden vessels, cerebral amyloid angiopathy (CAA), slides were immunohistochemically stained with a rabbit polyclonal antibody directed against Aβ40 (EMD Millipore, Catalog # AB5074P RRID:AB_91672) using the Ventana DAB Map TM kit with the avidin biotin peroxidase complex method involving a biotinylated secondary antibody of donkey-anti-mouse on a Ventana Discovery XT immunostainer (Ventana Medical Systems).
Aβ assays
Preparations of brain lysates.
Frozen left brain-halves (cerebellum and brainstem removed) were homogenized in four volumes of 9 m urea/50 mm Tris (vol/wet weight) using a MagNa Lyzer (Roche; RRID:nlx_152451) for 20 min at 4000 rpm in Green Bead Tubes (Roche Applied Science). The homogenates were incubated on ice for 2 h, and then centrifuged for 20 min at 20,000 × g at 4°C. The supernatants were diluted from 1:200 to 1:100,000 in AlphaLISA assay buffer depending on Aβ content and assay sensitivity. Aβ was determined by AlphaLISA assays developed in-house.
Preparation of CSF.
CSF was diluted 1:10 in assay buffer (50 mm Tris, pH 7.4, 60 mm NaCl, 0.1% Tween, 0.5% BSA) and stored frozen at −80°C. Aβ was determined by AlphaLISA assay.
AlphaLISA.
Brain extracts were diluted in AlphaLISA assay buffer (25 mm HEPES, pH 7.4, 0.5% Triton X-100, 0.1% casein, 1 mg/ml dextran 500, pH 7.0). In a 96-well half-area plate (PerkinElmer), 20 μl of these dilutions were mixed with 5 μl of biotinylated anti-Aβ antibody 6E10 at 0.09 μg/ml final concentration (Covance, Catalog # SIG-39340-500 RRID:AB_10173400) and 5 μl of 10 μg/ml Aβ C-terminal-specific antibody BAP-24 or BAP-15-conjugated to AlphaLISA Acceptor Beads (PerkinElmer). The specificities of BAP-24 and BAP-15 were described previously (Brockhaus et al., 1998). The mix was incubated for 1 h at RT, then 20 μl AlphaLISA Donor Beads (PerkinElmer) were added and the complex was allowed to form for another 30 min before the plate was read on an EnVision MultiLabel Reader (PerkinElmer). For the quantification of Aβ40 or Aβ42 from brain extracts, the corresponding peptide standards (AnaSpec, RRID:nlx_152270; Catalog #24236 and 24224) were diluted in AlphaLISA buffer containing 45 mm urea.
Statistical analysis was done by one-way ANOVA followed by Bonferroni's multiple-comparison test using GraphPad Prism 5. P values < 0.05 were considered significant: ***p < 0.001, **p = 0.001 to 0.01, *p = 0.01 to 0.05, ns = nonsignificant (>0.05).
Immunohistochemistry and quantitative morphometry
Fresh frozen brains from APPLondon mice were sagittally sectioned and processed for further analysis by immunofluorescence and quantitative morphometry as described previously (Bohrmann et al., 2012). In this study, five sections per animal were used for quantification. Plaque-positive area and numbers was obtained from the hippocampal cortical and subiculum region using every 10th section cut at a nominal thickness of 10 μm. Sections were stained with gantenerumab at 2 μg/ml for 1 h and detected by affinity-purified goat anti-human IgG (H + L)-conjugated to AlexaFluor 555 (no. A21433, Invitrogen, RRID:nlx_152414) at 20 μg/ml for 1 h at room temperature. Fluorescence images were obtained from entire brain sections scanned with a Metafer4 slide scanner (MetaSystems). Quantitative image analysis was done by a customized rule set for automated detection of stained amyloid plaques after selection of the neocortical and hippocampal region using the Definiens XD 2.0 software package (Definiens). Calculations were made with common spreadsheet software (Microsoft Excel). Statistical evaluation was done using a two-tailed Student's t test p values <0.05 were considered significant: ***p < 0.001, **p = 0.001 to 0.01, *p = 0.01 to 0.05, ns = nonsignificant (>0.05).
Results
Characterization of BACE inhibitor RO5508887
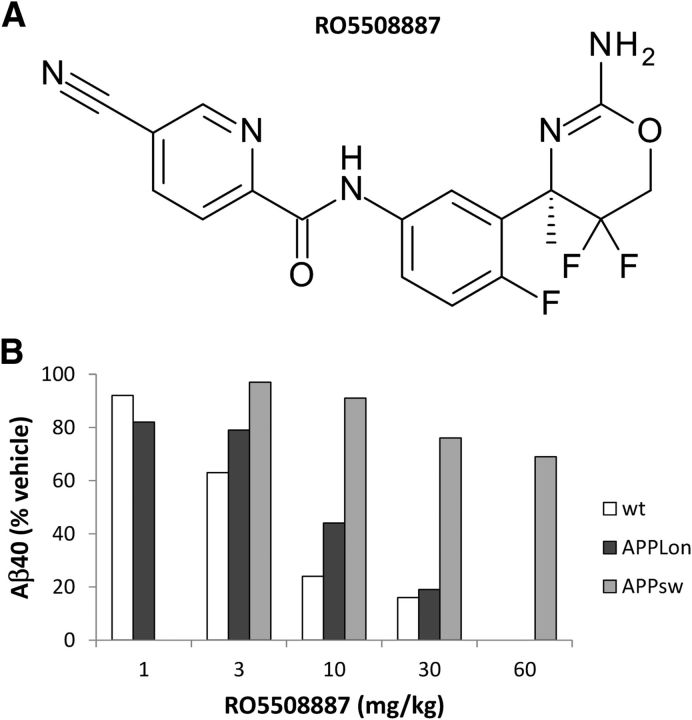
RO5508887 is a potent inhibitor of BACE (Fig. 1A). Its 50% inhibitory concentration in an enzymatic assay with human recombinant BACE1 and an APP-based substrate peptide is 30 nm. It is equally potent against BACE2 (IC50 40 nm) but >1000-fold less against cathepsin D, cathepsin E, pepsin, and renin. The IC50 in HEK293APP cells and in neuronal cultures derived from human neuronal stem cells was 18 nm, whereas an IC50 of 55 nm was measured in primary cultures of rat cortical neurons. The in vivo potency was tested in young wild-type C57BL/6 mice, which were treated once with different doses of compound and killed 4 h post-treatment. Total brain Aβ40 was measured and a 50% effective dose of 3–5 mg/kg was determined. A similar ED50 was measured in the transgenic APPLondon (V717I) mouse, whereas a significantly higher ED50 (>60 mg/kg) was observed in an APPSwedish-based transgenic line (Fig. 1B). A reduced potency of BACE inhibitors in cells carrying an APP with the Swedish mutation was previously described, and is possibly due to an aberrant subcellular localization of the mutant APP (Yamakawa et al., 2010). Thus, for the purpose of our study the APPLondon mouse was the preferred model.
Figure 1.
Chemical structure of BACE inhibitor RO5508887 (A) and its in vivo potency in wild-type, APPLon and APPsw mice (B). Reduction of total brain Aβ40 was determined in an acute study were the animals were killed 4 h post-treatment. Each bar represents the mean of three to four animals, Young, preamyloid mice were used for this comparative study to determine the changes of soluble brain Aβ following acute BACE inhibition with RO5508887. wt, wildtype C57BL/6 mice; APPLon, hAPPV717I; APPsw, hAPPKM670/671NL.
Characterization of transgenic mouse APPLondon
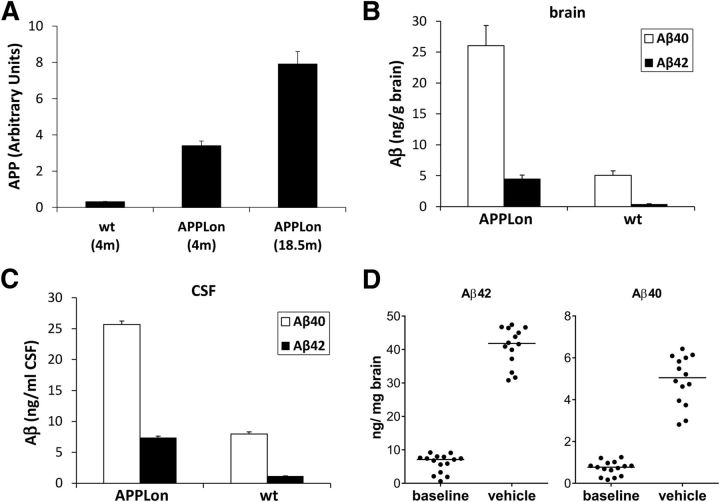
The APP-transgenic mouse line which was used in this study expresses the human APP cDNA with the FAD London mutation (V717I) under the control of the mouse thy1-gene promoter. We characterized the expression of the transgene and the resulting level of Aβ40 and Aβ42 in young, preamyloid mice. At 4 months of age, the level of total brain APP as assessed by Western blot is ∼10-fold higher than in wild-type mice of the same age and a further twofold increase of APP level is seen in mice aged 18 months (Fig. 2A). The level of Aβ40 and Aβ42 in both total brain lysates and in the CSF are likewise 5- to 10-fold higher in transgenic mice of 4 months than in aged-matched nontransgenic littermates (Fig. 2 B,C). Consistent with the presence of the FAD London mutation in the APP transgene, which shifts the γ-secretase cleavage toward the Aβ42 cleavage site, the ratio of brain Aβ40 to Aβ42 in the transgenic mouse is only approximately 6, whereas a ratio of 10–12 is known for wild-type mice. Notably, these Aβ levels reflect the steady-state Aβ-concentrations in young animals in the absence of amyloidosis.
Figure 2.
Characterization of transgenic mouse line APPLon. A, Overexpression of the transgenic APP relative to its endogenous expression (wt) was determined by Western-blot analysis. Each bar represents the mean of three to four animals (+SD). B, Levels of Aβ40 and Aβ42 were measured in DEA-brain extracts of young, preamyloid animals. C, CSF was collected from the same animals and Aβ levels determined. D, The increase in total, urea-extracted Aβ40 and Aβ42 between 13.5 months (baseline group) and 17.5 months (vehicle group) is shown. Each dot represents an individual animal; the horizontal line represents the group median. wt, wildtype C57BL/6 mice; APPLon, hAPPV717I.
Amyloidosis with typical dense-cored and diffuse plaques starts in this transgenic line ∼9 months and progresses with age. During the 4 month treatment duration, the total urea-extractable Aβ from brain lysates increased 5- to 8-fold in the vehicle-treated group. Some interindividual variability was observed for total brain Aβ40/42 levels (Fig. 2 D); however, variability is a common finding in other APP-transgenic mice, especially in the earlier phases of amyloidosis. The age-dependent increase in total Aβ40/42 showed a clear separation between baseline at study start and end of treatment period, thus providing appropriate context to evaluate the effect of study drugs. Notably, in contrast to steady-state soluble Aβ, the insoluble deposits contain predominantly Aβ42, which exceeds the Aβ40 content by ∼8-fold.
In addition to the treatment effects on brain amyloidosis and CSF Aβ level (described below) we also evaluated CAA and microhemorrhage. Only two of five animals at the age of 13.5 months showed amyloid deposits in meningeal vessels, although such deposits were present in most animals at the age of 17.5 months. Amyloid deposits in parenchymal vessels were present in individual animals at 17.5 months only. In one animal that received mono-treatment with gantenerumab, Prussian blue-positive iron consistent with former microhemorrhage was present in a meningeal vessel. However, an association with amyloid deposits within the vessel wall could not be made.
Reduction of total brain Aβ by mono-treatments with BACE inhibitor and anti-Aβ antibody
APPLon mice 13–14 months of age, which already carry a high amyloid burden, were used in this study. Thus, treatment was not aimed at preventing onset of amyloidosis but rather at halting progression or even reducing a pre-existing amyloidosis. This study design resembles more closely the situation as it is found in patients with Alzheimer's disease.
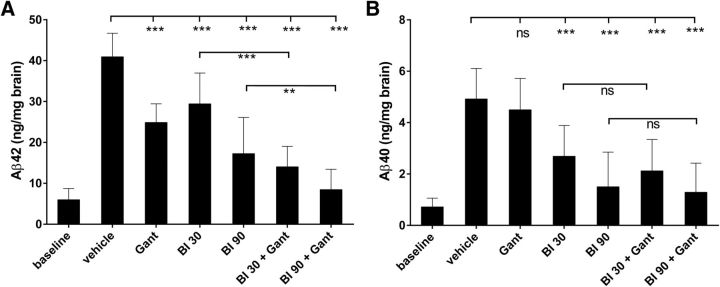
Animals were treated daily with a medium- and high-dose of BACE inhibitor or with a weekly intravenous dose of gantenerumab. In a previous study with gantenerumab in a PS2APP-double transgenic mouse model, dosed similarly over 5 months, a significant reduction of amyloid plaques was shown (Bohrmann et al., 2012). Both doses of BACE inhibitor significantly reduced the amounts of total brain Aβ40 and Aβ42 compared with the vehicle group, with a clear dose-dependency (Fig. 3). For Aβ42, the observed decrease was 28 and 58%, whereas Aβ40 was decreased by 45 and 69%, respectively. However, for both Aβ species the absolute amounts at treatment end were above those measured at baseline; i.e., amyloid buildup was slowed but not halted. Gantenerumab alone also significantly reduced the amount of predominant Aβ42 species by 39%, whereas no significant decrease was observed for Aβ40.
Figure 3.
Efficacy of mono-treatments and combination treatments on total brain Aβ. Animals were treated with BACE inhibitor RO5508887 at either 30 or 90 mg/kg daily (BI30, BI90), with anti-Aβ antibody gantenerumab (Gant) at 20 mg/kg weekly or with both compounds (BI30+Gant, BI90+Gant). Total brain Aβ was urea-extracted and Aβ42 (A) and Aβ40 (B) levels determined (A, B). Each bar represents the mean of 10–14 animals (+SD). BACE inhibition alone causes a dose-dependent decrease of brain Aβ40 and Aβ42, whereas mono-treatment with gantenerumab only decreases brain Aβ42. Combination treatment is significantly more efficacious on Aβ42 but only marginally enhances the reductions of Aβ40. The Aβ levels at baseline are included for comparison.
Reduction of total brain Aβ by combination treatment
In parallel to the mono-treatments, additional groups of mice were treated for the same period with a combination of BACE inhibitor plus gantenerumab. Doses and treatment schedules were the same as those used for the mono-treatments, and total brain Aβ42 and Aβ40 were determined at study end. Notably, combination treatment caused a much stronger reduction of Aβ42 than any of the BACE inhibitor mono-treatments or antibody mono-treatment (Fig. 3), and this effect was highly significant for both combination-treatment arms. In the 30 mg/kg group the decrease of Aβ42 in the combination treatment was 66% compared with 28% with BACE inhibitor alone, a clear doubling of efficacy. The effect is additive, because the reduction with antibody alone was 39%. For the 90 mg/kg group the corresponding numbers are 79% reduction of Aβ42 by combination treatment versus 58% for mono-treatment with BACE inhibitor. Here the additive effect of the combination is less, probably as a result of the already strong effect of BACE inhibition alone. Nevertheless, in both combination groups there is a clear and significant increase in efficacy compared with mono-treatments. The combination outcome is much less pronounced for Aβ40. Although there appears to be a trend toward lower levels in the combination arms compared with treatment for BACE inhibitor alone, the difference is not statistically significant. In the 30 mg/kg group the decrease of Aβ40 in the combination treatment was 57% compared with 45% for BACE inhibitor alone. For the 90 mg/kg group the corresponding numbers are 74% reduction of Aβ40 by combination treatment versus 70% for mono-treatment with BACE inhibitor. The absence of a significant combination effect is consistent with the minor trend for efficacy on Aβ40 in the antibody mono-treatment group (9%). Thus, the reduction of Aβ40 seen in the combination treatment is predominantly driven by BACE inhibition. We also compared the Aβ level at baseline for high-dose BACE inhibitor alone or plus gantenerumab. For Aβ42 there is still a significant increase after BACE inhibitor mono-treatment. Thus, despite substantial reduction of de novo Aβ42 the remaining production is still sufficient to increase amyloidosis. In contrast, in the combination arm, we found at study end, levels of total Aβ comparable to levels at baseline, which are insignificantly different both for Aβ40 and Aβ42. In summary, amyloidosis as measured as total brain Aβ is effectively stopped at pretreatment level by combination treatment with the high BACE inhibitor dose.
Effects on amyloid burden and plaque number
In addition to total brain Aβ measured by specific immune assays, we also measured by quantitative microscopy the effect of different treatments on amyloid load, i.e., the area covered by amyloid, as well as plaque number and plaque size distribution. We considered these measurements as important because gantenerumab binds preferentially to aggregated Aβ and enhances microglia-mediated clearance of plaques (Bohrmann et al., 2012). We measured amyloid load and plaque number in brain areas that exhibit the most prominent amyloidosis, cortex, and hippocampus. For detection of Aβ on sections, we used immune-labeling with gantenerumab, which detects with high-specificity and -sensitivity all amyloid deposits, independent of their C-terminal sequence. It had been established before that treatment of animals with gantenerumab does not affect the subsequent use of the same antibody on brain sections when followed by secondary human IgG-specific detection antibody. We also attempted to measure plaque load by staining with the C-terminal antibodies specific for Aβ42 or Aβ40 (BAP15 and BAP24, respectively). These were, however, less efficient in labeling amyloid deposits compared with gantenerumab, possibly because the C-terminus of Aβ is less accessible in amyloid deposits (data not shown).
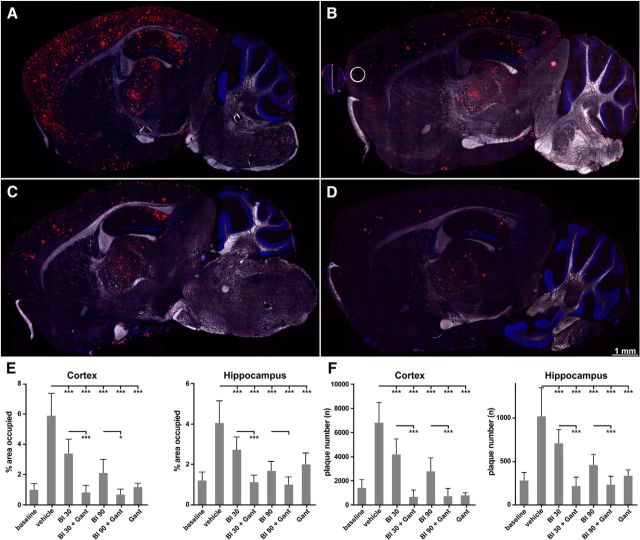
There are revealing differences between treatment effects on total brain Aβ40/Aβ42 as measured by quantitative immunotype assays and amyloidosis determined by immunohistochemistry and quantitative microscopy (Fig. 4). The extent of plaque reduction can be appreciated in representative brain sections by comparing the substantial plaque deposition present in vehicle-treated animals (Fig. 4A) and the considerable plaque reduction after mono-treatments of BACE inhibitor at high-dose, gantenerumab, and combination treatment with gantenerumab and BACE inhibitor at high-dose (Fig. 4B–D). The quantification of plaques by image processing revealed in neocortices and the hippocampal formation that the surface area of immunostained amyloid plaques decreased with BACE inhibitor in a dose-dependent manner (Fig. 4E); cortex: mean vehicle 5.9%, BI30 3.4%, BI90 2.1%). However, for both doses of BACE inhibitor, the amyloid load at study end clearly exceeded that measured at baseline (mean 0.96%). This is in agreement with the finding that total brain Aβ also increased over the study period. Gantenerumab treatment alone significantly reduced plaque surface area in the cortical and hippocampal regions to a larger extent than treatment with the high-dose of BACE inhibitor (mean mAb 1.1%), approaching the level at baseline. The plaque lowering was more pronounced in the cortical region than in the hippocampus. A plausible explanation is the later onset of amyloidosis in the cortex leading to more pronounced efficacy of treatment.
Figure 4.
Efficacy of mono- and combination treatments on brain amyloid burden and plaque number. Animals were treated with BACE inhibitor RO5508887 at either 30 or 90 mg/kg daily (BI30, BI90), with anti-Aβ antibody gantenerumab (Gant) at 20 mg/kg weekly or with both compounds (BI30+Gant, BI90+Gant). A–D, Fluorescence images of entire sagittal brain sections after staining of amyloid plaques at study end. Representative images are shown after vehicle-treatment (A), treatment with BI90 (B), gantenerumab (C), and gantenerumab combined with BI90 (D). Notably, plaque reduction is most substantial in the animals that received both drugs. E, The quantification and statistical evaluation of the area surface occupied by plaques across all treatment groups for cortex and hippocampus. Decrease of plaque surface is significant while variable in treatment groups compared with vehicle-treated mice. The combination treatment significantly enhances the amyloid-plaque lowering for both the low- and the high-dose of RO5508887 in the cortex. F, Effect of mono- and combination treatments on total plaque number. Significantly enhanced efficacy by combination treatment is seen in both brain regions examined. Bars represent the mean of 10–4 animals (+SD).
Combination treatments reduced the plaque load below baseline levels (0.8 and 0.6%) for low- and high-doses of BACE inhibitor, respectively, but the differences to baseline did not reach significance. However, plaque surface area was significantly (p > 0.001) reduced in combination groups compared with gantenerumab alone.
To get more insight into the process of amyloid plaque lowering, we analyzed the total plaque number in the same brain regions where plaque surface area was assessed (Fig. 4F). Total plaque number in the vehicle control group increased 4- to 5-fold over the study period. In the treatment groups the increase of plaque number was significant for the low-dose BACE inhibitor group but not for high-dose and antibody mono-treatments. In the combination arms, the decrease in plaque number appears dependent on gantenerumab. This is evident by similar levels of plaque numbers measured with gantenerumab alone or in combination with BACE-inhibitor. In contrast, the number of plaques with high dose BACE inhibitor was significantly larger than for antibody mono-treatment. When both high- and low-doses of BACE inhibitor are compared with combination with gantenerumab, a statistically significant (p < 0.001) decrease of plaque numbers are seen in cortex and hippocampus. Notably, the total plaque number in both combination doses with BACE inhibitor and gantenerumab are slightly reduced below levels measured at baseline, which suggests that new plaque formation was efficiently inhibited.
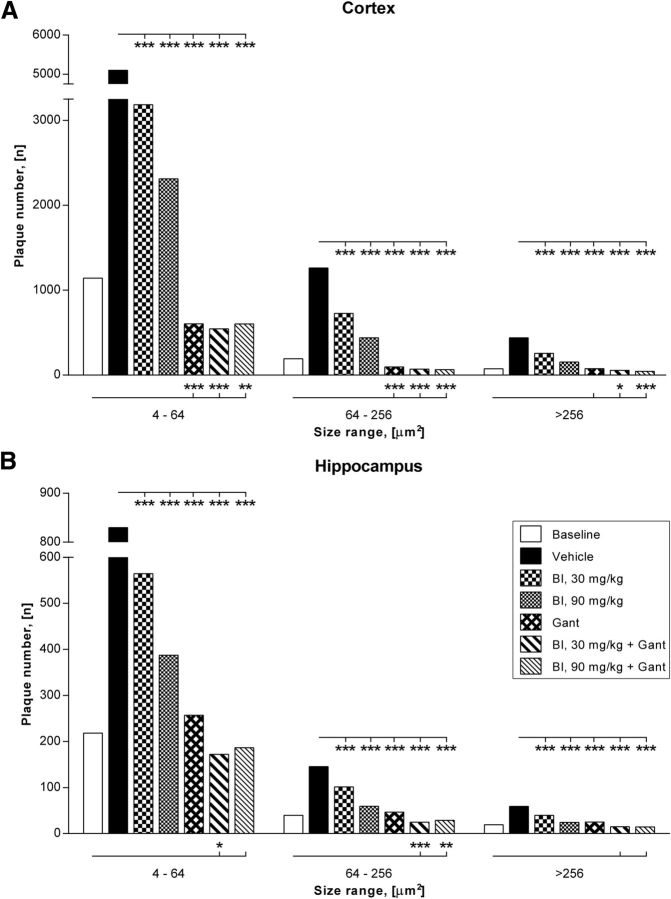
A more detailed morphometrical analysis of the plaque number in relation to their size was performed to investigate whether in addition to reduced plaque formation clearance of pre-existing amyloid plaques occurred (Fig. 5). Mono-treatment by BACE inhibition resulted in a clear dose-dependent reduction of all plaque sizes compared with the vehicle group as shown quantitatively for the cortex and hippocampus. The reduction in number of plaques compared with vehicle is most evident in the treatment arms with gantenerumab (monotherapy or in combination with BACE inhibitor). Reduction of plaque number was significant (p < 0.001) in all treatment groups. Nearly complete prevention of de novo plaque formation was observed with gantenerumab alone and in combination with BACE inhibitor.
Figure 5.
A morphometrical analysis of the treatment effect in relationship to plaque sizes is shown for cortex and hippocampus. Decrease of plaque numbers is significant in all treatment groups compared with vehicle treated mice. Reduction of plaque number is dose dependent for BACE inhibitor (BI) monotherapy and most pronounced for gantenerumab (Gant) treatment groups. Notably, a more pronounced and statistically significant decrease of plaque numbers below baseline levels is observed in the combination groups. The enhanced clearance is more consistent in the cortical region (A) and observed in all plaque sizes measured, including very large plaques with diameters >256 μm. Compared with cortex, a similar but slightly attenuated plaque reduction is seen in the hippocampus (B).
The decrease of plaque numbers was most apparent and statistical significant for all plaque sizes in the cortical region when baseline levels are compared with combination groups (Fig. 5A), although less pronounced in the hippocampus (Fig. 5B). In the neocortices, which carry highest levels of plaque burden, the number of smaller, 4–64 μm2 plaques was found to be significantly reduced compared with baseline. Plaque numbers were also significantly (p < 0.001 to p < 0.05) reduced for larger plaques of diameters ranging from 64–256 μm2 and also for very large plaques >256 μm2 when compared with the baseline group of APPLondon mice.
Overall, combination treatment effectively and nearly completely arrested further increase in amyloid plaque burden even for low-dose BACE inhibition. In addition, clearance of preexisting amyloid plaques that involves microglia engagement (Bohrmann et al., 2012) was significantly enhanced by combination treatment. Thus, the capacity of amyloid removal by microglia engagement that is seen with gantenerumab treatment alone can be further enhanced by cotreatment with a BACE inhibitor. A likely explanation is that plaques grow at reduced rate and microglia phagocytosis is facilitated under conditions that lower the soluble Aβ pool as achieved with BACE inhibition.
Effect of treatments on CSF Aβ
CSF Aβ is widely used as a specific biomarker for diagnosis of AD, as the ratio of CSF Aβ42 to Aβ40 was reported to decrease in the presence of amyloidosis in the brain parenchyma (Lewczuk et al., 2004; Hansson et al., 2007). Rapid, dose-dependent changes in CSF Aβ have been observed after treatment with γ-secretase inhibitors and BACE-inhibitors. Because CSF Aβ is largely brain-derived it has become a valuable biomarker for monitoring the Aβ-lowering activities of secretase inhibitors (Siemers et al., 2007; May et al., 2011).
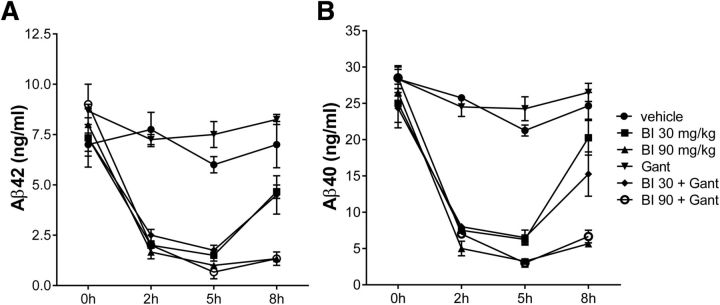
On the final day of the 4 month treatment, the animals received a last dose of BACE inhibitor (where applicable) and were randomized into groups of two to three animals and subsequently killed at 0, 2, 5, and 8 h post-treatment to obtain a composite time course. CSF was sampled for determination of Aβ40 and Aβ42 (Fig. 6). In the vehicle and antibody-only treatment arms Aβ concentrations remained constant over the observation period, as expected. All groups receiving BACE inhibitor exhibited a time- and dose-dependent decrease of Aβ40 and 42. In the low-dose group Aβ levels increased again after 8 h, whereas sustained inhibition was observed in the high-dose group, as expected from the pharmacokinetic properties of the compound. The presence of the antibody in the combo-treatment groups had no effect above that seen in the BACE inhibitor mono-treatment groups; thus, the reduction is entirely driven by BACE inhibition. The absolute predose amounts in all groups were similar and thus independent of the vastly different amounts of total brain Aβ and amyloidosis between vehicle group and the different treatment groups. The concentrations of Aβ40 to Aβ42 and their ratio are similar to what was observed in young preamyloid mice and therefore appear not to be modified by brain amyloidosis. It should be noted that at peak inhibition (∼5 h postdose) the residual Aβ concentrations were comparable with the baseline level in untreated wild-type mice. Considering that inhibition wears off and full transgenic overproduction resumes 8 h after dosing with BACE inhibitor, the total Aβ produced over a 24 h period is still sufficient to further drive amyloid deposition.
Figure 6.
Changes in CSF Aβ42 (A) and Aβ40 (B). On the last day of the study period following the last dosing with BACE inhibitor the different treatment groups were subdivided onto groups of two to three animals which were then killed at 0, 2, 5, and 8 h after receiving RO5508887. BACE inhibition causes a time- and dose-dependent reduction in CSF Aβ. Extent and kinetics of Aβ changes are not affected by gantenerumab. Last dosing with gantenerumab was done 1 week before sacrifice. Labeling of treatment arms is as described in Figure 3.
Discussion
Here, we demonstrate that combined treatment with two compounds targeting different steps in the amyloid cascade significantly enhances the amyloid-lowering activity. This is to our knowledge the first pharmacological evaluation of such effect in a relevant model with already widespread amyloidosis. Furthermore, the two antiamyloid drugs used have potential clinical relevance for therapy in AD. An APP-transgenic mouse model was used with expression of the transgenic human APP exceeding by ∼10-fold that of the endogenous protein. The excess APP production is reflected in a correspondingly increased Aβ production in the brain as measured in young, preamyloid mice. Furthermore, the APPLon mutation shifts the Aβ40 to Aβ42 ratio toward Aβ42, the more amyloidogenic species. The increases in Aβ production and Aβ42 level cause a rapid onset and progression of amyloidosis. Nevertheless, combining the Aβ lowering compounds largely prevented further increase of amyloidosis over the 4 month study duration. In contrast, total brain Aβ in the vehicle group increased 5- to 8-fold over the same period. This clearly demonstrates the potential of the proposed combination therapy.
Combination treatments effectively reduced plaque load and total plaque number below baseline levels. However, increments in total brain Aβ40 and Aβ42 as measured by biochemical assays could not be prevented. This was most obvious for the combination treatment with the lower dose of BACE inhibitor. This raises the questions, where and in what state the de novo produced Aβ accumulates under those treatment conditions. There are several potential reservoirs of Aβ independent from plaques, such as small, soluble aggregates (Haass and Selkoe, 2007), membrane-associated oligomerized Aβ (Zhang et al., 2012), and intracellular Aβ (Christensen et al., 2010). These may not have been detected by the staining procedure used in this study. It is interesting to note that also in brain tissue from nondemented elderly, high levels of Aβ are found that may not be detected with PIB PET (Roher et al., 2009).
Attempts to separate total brain Aβ in a defined soluble and insoluble fraction were inconclusive. The “soluble fraction” was always proportionally related to the insoluble, urea-extractable pool, thus likely representing extraction-caused spillover from the insoluble deposits. This could be a feature of this mouse model because soluble Aβ, as a distinct fraction of total Aβ, was reported for other models (Kawarabayashi et al., 2001; Westerman et al., 2002).
The lack of efficacy of mono-treatment with gantenerumab on total brain Aβ40 is likely explained by its differential binding to aggregated versus monomeric Aβ. Gantenerumab recognizes a conformational epitope comprising N-terminal and central parts of Aβ that is present in aggregated Aβ. It has a markedly higher affinity for aggregated Aβ and is less stably bound to monomeric Aβ (Bohrmann et al., 2012). In the APPLon mouse model, Aβ42 is the predominant Aβ peptide which forms the deposits. This is evidenced in the ratio of Aβ42 to Aβ40 in the insoluble, urea-extractable amyloid compared with the ratio of soluble Aβ in the young, preamyloid mice. It thus appears that after gantenerumab-dependent clearance of aggregated, predominantly Aβ42, the residual Aβ40 likely remains in a nonaggregated state. In an earlier study, in a different transgenic mouse model with a very aggressive amyloidosis, it was shown that treatment for 5 months with the same dose of gantenerumab caused significant reduction of amyloid plaque formation and clearance of small plaques. On the other hand, very large plaques were less affected and total Aβ40/42 was not significantly lowered (Bohrmann et al., 2012). However, the phagocytic clearance capacity involving Fc functionality of human IgG1 is less efficient for murine Fcγ receptors. Thus, for a fully human IgG1 like gantenerumab, phagocytosis by murine microglia is likely not optimally supported and predictive for therapeutic use in AD (Ostrowitzki et al., 2012).
CSF Aβ is an important biomarker for the diagnosis of AD and is also used to monitor the central inhibition of Aβ production by e.g., secretase inhibitors (Siemers et al., 2005; May et al., 2011). We measured CSF Aβ40 and Aβ42 at the end of the 4 month study period after the last administration. Their time- and dose-dependent changes seem to be exclusively mediated by inhibition of BACE1. As expected, treatment with gantenerumab alone does not lower Aβ CSF levels and for a given BACE inhibitor dose, inhibition is comparable between treatment arms with or without gantenerumab. This is entirely in agreement with the different mechanisms of action, i.e., reduction of de novo production by the BACE inhibitor complementary to high affinity binding of gantenerumab to aggregated Aβ. The extent of Aβ reduction by BACE1 inhibition is similar to what was observed before in acute studies in young, preamyloid mice (data not shown). This suggests that even in the presence of substantial brain amyloid burden CSF Aβ is mostly derived from de novo production and to no or very small extent reflects soluble Aβ originating from amyloid deposits. Thus, CSF Aβ may be considered a valuable pharmacodynamic biomarker of BACE1 inhibition also in patients with high CNS amyloidosis. The ratio of Aβ40 to Aβ42 was shown to change in the early and prodromal phase of AD, presumably due to the preferential deposition of Aβ42 in the insoluble fraction. We did not observe a significant change in this ratio when comparing young mice in their preamyloid phase to old mice. This is likely due to the substantial overproduction of total Aβ and the increased Aβ42 fraction caused by the APPLondon mutation, which provides sufficient Aβ42 to drive amyloidosis without significantly affecting the ratio of soluble Aβ42 to Aβ40 in CSF.
A previous study with γ-secretase inhibitor in the J20 transgenic APP mouse model reported a similar reduction of interstitial Aβ40 by γ-secretase inhibition in young mice (Hong et al., 2011). However, the reduction of Aβ42 was considerably less in aged mice with existing brain amyloidosis, presumably because a significant fraction of soluble Aβ42 was plaque-derived. This seems to differ from our findings and it may either reflect differences in the mouse models or suggest that Aβ changes in CSF and interstitial fluid are not entirely correlated in extent and kinetics.
Despite the significant amyloid-lowering activity the investigated mono- and combination treatments did not reduce total brain Aβ or amyloid load below the level measured at study start. However, this may be a particularity of the highly aggressive course of amyloidosis in this mouse model that is considerably different from the physiological situation in human, due to the aforementioned overproduction of Aβ and its shift toward Aβ42, The extent and kinetics of Aβ changes in CSF also show that even at peak inhibition the residual concentration is comparable with wild-type mice steady-state levels. Due to the relatively short half-life of the BACE inhibitor in rodents, there remains a significant Aβ overproduction over the 24 h dosing intervals.
Although this appears to be the first report on antiamyloid treatment combining two pharmacological agents, there have been previous reports that simulated combination treatment by using genetic tools. Chow et al. (2010) constructed transgenic mice with a partial reduction of both BACE1 and γ-secretase activity, and demonstrated that the combination exceeded the effect of each single deletion. Even though this previous study indicated the potential benefit of combined treatment with BACE and γ-secretase inhibitors, it clearly differs from our study because these genetic reductions are in place from early development and thus resemble a preventive treatment. Furthermore, both genetic manipulations targeted the Aβ-production process and their effect on existing amyloidosis remains unclear. Of direct importance to our approach is a recent study by Wang et al. (2011) which combined treatment with an anti-Aβ antibody and genetic reduction of APP expression using a regulatable human APP transgene. Suppression of APP expression reduced Αβ production and thus mimicked the effect of secretase inhibitors, but by itself only delayed amyloidosis and did not reduce existing amyloid burden. Suppressed production of Aβ plus treatment with the antibody reduced amyloid burden below pretreatment levels, a finding which is largely in agreement with the outcome of the present study. Recently a combination study in 5xFAD mice was reported which compared treatment with scyllo-inositol alone to combination treatment with R-flurbiprofen, a γ-secretase modulator. Here, combination treatment did not facilitate amyloid lowering beyond that seen with scyllo-inositol alone (Aytan et al., 2013).
Although combination therapy for symptomatic treatment with small molecule drugs, such as acetylcholine esterase inhibitors and NMDA antagonists, may improve their efficacy, they are not expected to modify the underlying disease progression in AD. Therefore, a combination therapy that targets the amyloid cascade with different mechanisms of action may offer an improved benefit to risk profile. Notably, recent data obtained with anti-Aβ antibodies in Phase 3 trials indicate at least partial efficacy in some biomarker and cognition measures in mild to moderate AD patients (Doody et al., 2014; Salloway et al., 2014). Thus, earlier intervention and combination of drugs exhibiting additive or synergistic interaction represent a promising approach for clinical studies in AD. It will be interesting to explore such combination modalities not only for coadministration but also for alternating mono- and combination treatment paradigms. Our findings support further investigation of combinatorial therapeutic concepts in preclinical models to support future clinical trials.
Footnotes
We thank D. Reinhardt for Aβ analysis, Jürg Messer for expert assistance in the morphometrical analysis and preparation of figures, Françoise Gerber for performing immunohistochemical stainings, and Marleen Lox, Annelies Termont, Sarah Mertens, and Leen Wolfs for performing the in-life phase of the study.
B. Bohrmann, A. Caruso, H. Hilpert, B. Jacobsen, H. Jacobsen, R. Narquizian, and L. Ozmen are employees of Hoffmann-La Roche AG, Basel, Switzerland; D. Terwel and A. Tanghe are employees of reMYND, Leuven, Belgium.
References
- Aisen PS, Petersen RC, Donohue MC, Gamst A, Raman R, Thomas RG, Walter S, Trojanowski JQ, Shaw LM, Beckett LA, Jack CR, Jr, Jagust W, Toga AW, Saykin AJ, Morris JC, Green RC, Weiner MW. Clinical core of the Alzheimer's disease neuroimaging initiative: progress and plans. Alzheimers Dement. 2010;6:239–246. doi: 10.1016/j.jalz.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aytan N, Choi JK, Carreras I, Kowall NW, Jenkins BG, Dedeoglu A. Combination therapy in a transgenic model of Alzheimer's disease. Exp Neurol. 2013;250:228–238. doi: 10.1016/j.expneurol.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. 2011;377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Bitan G, Kirkitadze MD, Lomakin A, Vollers SS, Benedek GB, Teplow DB. Amyloid beta-protein (Aβ) assembly: Aβ40 and Aβ42 oligomerize through distinct pathways. Proc Natl Acad Sci U S A. 2003a;100:330–335. doi: 10.1073/pnas.222681699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan G, Vollers SS, Teplow DB. Elucidation of primary structure elements controlling early amyloid beta-protein oligomerization. J Biol Chem. 2003b;278:34882–34889. doi: 10.1074/jbc.M300825200. [DOI] [PubMed] [Google Scholar]
- Bohrmann B, Baumann K, Benz J, Gerber F, Huber W, Knoflach F, Messer J, Oroszlan K, Rauchenberger R, Richter WF, Rothe C, Urban M, Bardroff M, Winter M, Nordstedt C, Loetscher H. Gantenerumab: a novel human anti-Aβ antibody demonstrates sustained cerebral amyloid-β binding and elicits cell-mediated removal of human amyloid-beta. J Alzheimers Dis. 2012;28:49–69. doi: 10.3233/JAD-2011-110977. [DOI] [PubMed] [Google Scholar]
- Brockhaus M, Grünberg J, Röhrig S, Loetscher H, Wittenburg N, Baumeister R, Jacobsen H, Haass C. Caspase-mediated cleavage is not required for the activity of presenilins in amyloidogenesis and NOTCH signaling. Neuroreport. 1998;9:1481–1486. doi: 10.1097/00001756-199805110-00043. [DOI] [PubMed] [Google Scholar]
- Brody DL, Holtzman DM. Active and passive immunotherapy for neurodegenerative disorders. Annu Rev Neurosci. 2008;31:175–193. doi: 10.1146/annurev.neuro.31.060407.125529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. BACE1 is the major beta-secretase for generation of Aβ peptides by neurons. Nat Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- Chow VW, Savonenko AV, Melnikova T, Kim H, Price DL, Li T, Wong PC. Modeling an anti-amyloid combination therapy for Alzheimer's disease. Sci Transl Med. 2010;2:13ra1. doi: 10.1126/scitranslmed.3000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DZ, Bayer TA, Wirths O. Intracellular Aβ triggers neuron loss in the cholinergic system of the APP/PS1KI mouse model of Alzheimer's disease. Neurobiol Aging. 2010;31:1153–1163. doi: 10.1016/j.neurobiolaging.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Citron M. Emerging Alzheimer's disease therapies: inhibition of beta-secretase. Neurobiol Aging. 2002;23:1017–1022. doi: 10.1016/S0197-4580(02)00122-7. [DOI] [PubMed] [Google Scholar]
- Cole SL, Vassar R. The Alzheimer's disease β-secretase enzyme, BACE1. Mol Neurodegener. 2007;2:22. doi: 10.1186/1750-1326-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demattos RB, Lu J, Tang Y, Racke MM, Delong CA, Tzaferis JA, Hole JT, Forster BM, McDonnell PC, Liu F, Kinley RD, Jordan WH, Hutton ML. A plaque-specific antibody clears existing beta-amyloid plaques in Alzheimer's disease mice. Neuron. 2012;76:908–920. doi: 10.1016/j.neuron.2012.10.029. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Vassar R, Golde T. The secretases: enzymes with therapeutic potential in Alzheimer disease. Nat Rev Neurol. 2010;6:99–107. doi: 10.1038/nrneurol.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dislich B, Lichtenthaler SF. The membrane-bound aspartyl protease BACE1: molecular and functional properties in Alzheimer's disease and beyond. Front Physiol. 2012;3:8. doi: 10.3389/fphys.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, Raman R, Sun X, Aisen PS, Siemers E, Liu-Seifert H, Mohs R. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Kiso Y. Advances in the identification of beta-secretase inhibitors. Expert Opin Drug Discov. 2013;8:709–731. doi: 10.1517/17460441.2013.784267. [DOI] [PubMed] [Google Scholar]
- Hansson O, Zetterberg H, Buchhave P, Andreasson U, Londos E, Minthon L, Blennow K. Prediction of Alzheimer's disease using the CSF Aβ42/Aβ40 ratio in patients with mild cognitive impairment. Dement Geriatr Cogn Disord. 2007;23:316–320. doi: 10.1159/000100926. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hilpert H, Guba W, Woltering TJ, Wostl W, Pinard E, Mauser H, Mayweg AV, Rogers-Evans M, Humm R, Krummenacher D, Muser T, Schnider C, Jacobsen H, Ozmen L, Bergadano A, Banner DW, Hochstrasser R, Kuglstatter A, David-Pierson P, Fischer H, Polara A, Narquizian R. β-Secretase (BACE1) inhibitors with high in vivo efficacy suitable for clinical evaluation in Alzheimer's disease. J Med Chem. 2013;56:3980–3995. doi: 10.1021/jm400225m. [DOI] [PubMed] [Google Scholar]
- Hong S, Quintero-Monzon O, Ostaszewski BL, Podlisny DR, Cavanaugh WT, Yang T, Holtzman DM, Cirrito JR, Selkoe DJ. Dynamic analysis of amyloid β-protein in behaving mice reveals opposing changes in ISF versus parenchymal Aβ during age-related plaque formation. J Neurosci. 2011;31:15861–15869. doi: 10.1523/JNEUROSCI.3272-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob-Roetne R, Jacobsen H. Alzheimer's disease: from pathology to therapeutic approaches. Angewandte Chemie. 2009;48:3030–3059. doi: 10.1002/anie.200802808. [DOI] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid β protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewczuk P, Esselmann H, Otto M, Maler JM, Henkel AW, Henkel MK, Eikenberg O, Antz C, Krause WR, Reulbach U, Kornhuber J, Wiltfang J. Neurochemical diagnosis of Alzheimer's dementia by CSF Aβ42, Aβ42/Aβ40 ratio and total tau. Neurobiol Aging. 2004;25:273–281. doi: 10.1016/S0197-4580(03)00086-1. [DOI] [PubMed] [Google Scholar]
- May PC, Dean RA, Lowe SL, Martenyi F, Sheehan SM, Boggs LN, Monk SA, Mathes BM, Mergott DJ, Watson BM, Stout SL, Timm DE, Smith Labell E, Gonzales CR, Nakano M, Jhee SS, Yen M, Ereshefsky L, Lindstrom TD, Calligaro DO, Cocke PJ, Greg Hall D, Friedrich S, Citron M, Audia JE. Robust central reduction of amyloid-β in humans with an orally available, non-peptidic β-secretase inhibitor. J Neurosci. 2011;31:16507–16516. doi: 10.1523/JNEUROSCI.3647-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConlogue L, Buttini M, Anderson JP, Brigham EF, Chen KS, Freedman SB, Games D, Johnson-Wood K, Lee M, Zeller M, Liu W, Motter R, Sinha S. Partial reduction of BACE1 has dramatic effects on Alzheimer plaque and synaptic pathology in APP transgenic mice. J Biol Chem. 2007;282:26326–26334. doi: 10.1074/jbc.M611687200. [DOI] [PubMed] [Google Scholar]
- Moechars D, Dewachter I, Lorent K, Reversé D, Baekelandt V, Naidu A, Tesseur I, Spittaels K, Haute CV, Checler F, Godaux E, Cordell B, Van Leuven F. Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J Biol Chem. 1999;274:6483–6492. doi: 10.1074/jbc.274.10.6483. [DOI] [PubMed] [Google Scholar]
- Ostrowitzki S, Deptula D, Thurfjell L, Barkhof F, Bohrmann B, Brooks DJ, Klunk WE, Ashford E, Yoo K, Xu ZX, Loetscher H, Santarelli L. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol. 2012;69:198–207. doi: 10.1001/archneurol.2011.1538. [DOI] [PubMed] [Google Scholar]
- Richards JG, Higgins GA, Ouagazzal AM, Ozmen L, Kew JN, Bohrmann B, Malherbe P, Brockhaus M, Loetscher H, Czech C, Huber G, Bluethmann H, Jacobsen H, Kemp JA. PS2APP transgenic mice, coexpressing hPS2mut and hAPPswe, show age-related cognitive deficits associated with discrete brain amyloid deposition and inflammation. J Neurosci. 2003;23:8989–9003. doi: 10.1523/JNEUROSCI.23-26-08989.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roher AE, Esh CL, Kokjohn TA, Castaño EM, Van Vickle GD, Kalback WM, Patton RL, Luehrs DC, Daugs ID, Kuo YM, Emmerling MR, Soares H, Quinn JF, Kaye J, Connor DJ, Silverberg NB, Adler CH, Seward JD, Beach TG, Sabbagh MN. Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer's disease. Alzheimers Dement. 2009;5:18–29. doi: 10.1016/j.jalz.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, Reichert M, Ketter N, Nejadnik B, Guenzler V, Miloslavsky M, Wang D, Lu Y, Lull J, Tudor IC, Liu E, Grundman M, Yuen E, Black R, Brashear HR. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Toward a comprehensive theory for Alzheimer's disease. Hypothesis: Alzheimer's disease is caused by the cerebral accumulation and cytotoxicity of amyloid β-protein. Ann N Y Acad Sci. 2000;924:17–25. doi: 10.1111/j.1749-6632.2000.tb05554.x. [DOI] [PubMed] [Google Scholar]
- Siemers ER, Dean RA, Friedrich S, Ferguson-Sells L, Gonzales C, Farlow MR, May PC. Safety, tolerability, and effects on plasma and cerebrospinal fluid amyloid-beta after inhibition of gamma-secretase. Clin Neuropharmacol. 2007;30:317–325. doi: 10.1097/WNF.0b013e31805b7660. [DOI] [PubMed] [Google Scholar]
- Siemers E, Skinner M, Dean RA, Gonzales C, Satterwhite J, Farlow M, Ness D, May PC. Safety, tolerability, and changes in amyloid beta concentrations after administration of a gamma-secretase inhibitor in volunteers. Clin Neuropharmacol. 2005;28:126–132. doi: 10.1097/01.wnf.0000167360.27670.29. [DOI] [PubMed] [Google Scholar]
- Tanghe A, Termont A, Merchiers P, Schilling S, Demuth HU, Scrocchi L, Van Leuven F, Griffioen G, Van Dooren T. Pathological hallmarks, clinical parallels, and value for drug testing in Alzheimer's disease of the APP[V717I] London transgenic mouse model. Int J Alzheimers Dis. 2010;2010:417314. doi: 10.4061/2010/417314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R. The beta-secretase, BACE: a prime drug target for Alzheimer's disease. J Mol Neurosci. 2001;17:157–170. doi: 10.1385/JMN:17:2:157. [DOI] [PubMed] [Google Scholar]
- Vassar R, Citron M. Aβ-generating enzymes: recent advances in β- and γ-secretase research. Neuron. 2000;27:419–422. doi: 10.1016/S0896-6273(00)00051-9. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Wang A, Das P, Switzer RC, 3rd, Golde TE, Jankowsky JL. Robust amyloid clearance in a mouse model of Alzheimer's disease provides novel insights into the mechanism of amyloid-β immunotherapy. J Neurosci. 2011;31:4124–4136. doi: 10.1523/JNEUROSCI.5077-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. The relationship between Aβ and memory in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe MS. The γ-secretase complex: membrane-embedded proteolytic ensemble. Biochemistry. 2006;45:7931–7939. doi: 10.1021/bi060799c. [DOI] [PubMed] [Google Scholar]
- Xu X. γ-Secretase catalyzes sequential cleavages of the AβPP transmembrane domain. J Alzheimers Dis. 2009;16:211–224. doi: 10.3233/JAD-2009-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa H, Yagishita S, Futai E, Ishiura S. β-Secretase inhibitor potency is decreased by aberrant β-cleavage location of the “Swedish mutant” amyloid precursor protein. J Biol Chem. 2010;285:1634–1642. doi: 10.1074/jbc.M109.066753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, Shi JM, Bai CJ, Wang H, Li HY, Wu Y, Ji SR. Intra-membrane oligomerization and extra-membrane oligomerization of amyloid-beta peptide are competing processes as a result of distinct patterns of motif interplay. J Biol Chem. 2012;287:748–756. doi: 10.1074/jbc.M111.281295. [DOI] [PMC free article] [PubMed] [Google Scholar]