Abstract
Purpose
Patients with immunoglobulin light chain amyloidosis (AL amyloidosis) generally present with advanced organ dysfunction and have a high risk of early death. We sought to characterize monoclonal immunoglobulin (M-Ig) light chains before clinical presentation of AL amyloidosis.
Patients and Methods
We obtained prediagnostic sera from 20 cases with AL amyloidosis and 20 healthy controls matched for age, sex, race, and age of serum sample from the Department of Defense Serum Repository. Serum protein electrophoresis with immunofixation and serum free light chain (FLC) analysis were performed on all samples.
Results
An M-Ig was detected in 100% of cases and 0% of controls (P < .001). The M-Ig was present in 100%, 80%, and 42% of cases at less than 4 years, 4 to 11 years, and more than 11 years before diagnosis, respectively. The median FLC differential (FLC-diff) was higher in cases compared with controls at all time periods, less than 4 years (174.8 v 0.3 mg/L; P < .001), 4 to 11 years (65.1 v 2.2 mg/L; P < .001), and more than 11 years (4.5 v 0.4 mg/L; P = .03) before diagnosis. The FLC-diff was greater than 23 mg/L in 85% of cases and 0% of controls (P < .001). The FLC-diff level increased more than 10% per year in 84% of cases compared with 16% of controls (P < .001).
Conclusion
Increase of FLCs, including within the accepted normal range, precedes the development of AL amyloidosis for many years.
INTRODUCTION
Immunoglobulin light chain amyloidosis (AL amyloidosis) results from the tissue deposition of amyloid fibrils derived from monoclonal immunoglobulin (M-Ig) light chains (LCs) produced by an indolent clonal plasma cell disorder.1 Diagnosis is based on detection of LCs and histologic evidence of tissue amyloid deposition. Early diagnosis and prompt treatment are essential to improve survival in AL amyloidosis. Unfortunately for most patients, the disease goes unrecognized until severe organ dysfunction develops, and nearly half die within the first year of diagnosis, generally as a result of heart failure or sudden death as a result of cardiac involvement.2
Serum free light chain (sFLC) analysis has revolutionized the management of monoclonal gammopathies and AL in particular, because this sensitive assay can detect and quantify the pathogenic LCs.3 An M-Ig can be detected in 99% of patients with AL amyloidosis by using the combination of sFLC analysis, serum protein electrophoresis (SPEP), and immunofixation electrophoresis (IFE).4 We and others have shown that an M-Ig is present in nearly all patients years before the development of multiple myeloma (MM).5,6 LC-only M-Igs represent approximately 20% of monoclonal gammopathies, and we have shown that it is the precursor to LC MM.5,7 However, the presence and characteristics of the pathogenic monoclonal LC before the clinical presentation of AL amyloidosis are unknown. We hypothesized that production of the pathogenic LC is ongoing for many years before the development of organ dysfunction and clinical presentation of AL amyloidosis. Answering this question will resolve a fundamental aspect of the pathogenesis of AL amyloidosis and be a first step toward earlier diagnosis and improved outcome. To test this hypothesis, we retrieved sera before diagnosis from patients with AL and healthy controls from the US Department of Defense Serum Repository (DoDSR) and performed SPEP with immunofixation and sFLC analysis to detect the pathogenic LCs before clinical presentation.
PATIENTS AND METHODS
Study Population
We performed a retrospective case-control study of AL amyloidosis cases seen at all U.S. Military Treatment Facilities between 2000 and 2010. Inclusion criteria were active or former active-duty service members with a diagnosis of AL based on International Classification of Diseases, Ninth Revision code 277.3. We identified a total of 109 patients coded for AL amyloidosis. Following an extensive review of each patient's individual electronic medical record (DoD global electronic health record), 25 patients were found to have biopsy-proven AL amyloidosis. Some cases were coded during an evaluation for suspected amyloidosis that were ultimately negative. Other cases were diagnosed outside U.S. Military Treatment Facilities, and we did not have access to confirmatory records. Five patients were excluded for lack of serum in the DoDSR, resulting in a total population of 20 confirmed cases of AL amyloidosis. Controls were matched by age, race, sex, and age of serum (indexed by time before diagnosis within 90 days).8 Up to three prediagnostic serum samples were retrieved from the DoDSR for cases and controls. The DoDSR has been administered by the Army Medical Surveillance Agency since 1985. It contains more than 50 million serum samples stored at −30°C from mandatory biennial HIV testing as well as from pre- and postdeployment screenings. All service members have serum banked without discrimination. Specimens are linked to demographic, occupational, and medical information.9 This study was approved by the Human Use Committee at Walter Reed National Military Medical Center, and the requirement for informed consent was waived.
Laboratory Assays
SPEP (Capillarys 2; Sebia, Norcross, GA) and IFE (Hydrasys Focusing Unit; Sebia) with antisera to IgG, IgA, IgM, κ, and λ were performed on all samples. sFLC analysis was performed by automated immunoturbidometric assay (Advia 1650; Siemens, Tarrytown, NY) for free κ (normal range, 3.30 to 19.40 mg/L) and free λ (normal range, 5.70 to 26.30 mg/L) by using commercial reagents (Freelite; The Binding Site, Birmingham, United Kingdom). The κ:λ ratio (0.26:1.65) was calculated. An M-Ig was defined as the presence of an M-Ig on SPEP or IFE or an abnormal sFLC ratio along with an increased κ or λ LC. An LC M-Ig was defined as an increased involved LC with an abnormal sFLC ratio in the absence of heavy chain (IgG, IgA, IgM) expression on IFE.10 The FLC difference (FLC-diff) was calculated as the difference between the involved and uninvolved LCs based on the sample closest to diagnosis.
Statistical Analysis
We evaluated FLC-diff because it is the standard response criteria for AL and is not significantly affected by aging and renal function.11 Because the data were not normally distributed, continuous variables for both cases and controls are reported with median values (25% and 75%) and compared with the two-sample Kolmogorov-Smirnov test for statistical significance. Because of the matched nature of the data (to maximize statistical efficiency), categorical values were compared by using McNemar's matched χ2 using exact probabilities (because many cells had values of < 5).12 Odds ratios and CIs were omitted because the majority could not be calculated with the McNemar exact test because either all of the cases or none of the controls had levels above the specified thresholds. Specifically, we compared the percentage of cases versus matched controls with FLC-diff levels above specific thresholds, including subclinical values of 8 mg/L and 23 mg/L, respectively, and thresholds set for complete hematologic response in AL (40 mg/L) and a threshold associated with adverse prognosis (180 mg/L).13,14 Tertiles of elapsed time from date of serum draw until diagnosis (> 11.1, 4 to 11.1, and < 4 years) were used for subgroup analysis. Receiver operating characteristic (ROC) curve analysis (nonparametric) was performed at intervals of more than 11 years, 4 to 11 years, and less than 4 years before diagnosis in cases and controls. Continuous variables were explored graphically and formally for deviations from the assumption of normality. Box plots were used to display the distribution of FLC-diff levels separately in cases versus controls.
Rate of change over time for FLC-diff level was calculated by dividing the difference between the last level (LL) and the index level (IL) by the time difference in years between the last sample (LT) and the index sample (IT) as follows: (LL − IL)/(LT − IT/365). Percent change of FLC-diff level was calculated by dividing the last FLC-diff chain level (LLC2) by the first FLC-diff chain level (LLC1), subtracting 1, and then multiplying by 100 ([LLC2/LLC1] − 1) × 100). This percent change was then divided by the time in years between the two samples to obtain the percent change of FLC-diff levels over time. Stata 12.1 was used for all analyses (STATA, College Station, TX).
RESULTS
Patients
Twenty patients with AL amyloidosis were identified (Table 1). The mean age was 51 years (range, 42 to 64 years), and all patients were male. There were 14 white patients (70%) and four black patients (20%). The isotype was λ in 16 (80%) and κ in four (20%). An LC-only monoclonal Ig was present in 11 (55%) of 20 cases. An intact M-Ig was present in nine patients (45%), and of these, eight were λ LC. Heart (55%) and kidney (40%) were the most common organs affected. The median number of samples per case was three (range, one to three). The median time between the first available sample and last available sample before diagnosis was 12.2 years (range, 2.2 to 19.4 years) and 2.12 years (range, 0.2 to 13.4 years), respectively.
Table 1.
Characteristics of Patients With AL Amyloidosis
| Characteristic | Cases (patients with AL) |
Controls |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Mean | 51 | 51 | ||
| Range | 42-64 | 42-64 | ||
| Male sex | 20 | 100 | 20 | 100 |
| Race | ||||
| White | 14 | 70 | 14 | 70 |
| Black | 4 | 20 | 4 | 20 |
| Other | 2 | 10 | 2 | 10 |
| Immunoglobulin light chain isotype | ||||
| Kappa | 16 | 80 | NA | |
| Lambda | 4 | 20 | ||
| Immunoglobulin heavy chain | ||||
| Overall | 9 | 45 | NA | |
| IgG | 8 | 40 | ||
| IgA | 1 | 5 | ||
| Organ involvement | ||||
| Heart | 11 | 55 | 0 | 0 |
| Kidney | 8 | 40 | 0 | 0 |
| CNS | 1 | 20 | 0 | 0 |
| Liver | 4 | 20 | 0 | 0 |
| Arthropathy | 2 | 10 | 0 | 0 |
Abbreviations: AL, amyloid light chain; IgG, immunoglobulin G; NA, not applicable.
M-Ig Abnormalities
An M-Ig was detected at some point before the diagnosis of AL amyloidosis in 100% of cases and 0% of controls (P < .001). The proportion of cases with an M-Ig was 100%, 80%, and 42% at less than 4 years, 4 to 11 years, and greater than 11 years, respectively, before diagnosis. The method of detection for the initial M-Ig was by sFLC only in eight (40%) of 20, by SPEP and IFE in three (15%) of 20, by IFE and sFLC in three (15%) of 20, and by SPEP, IFE, and SFLC in six (30%) of 20. AL amyloidosis cases had statistically significantly higher median FLC-diff levels than did matching controls at all times before diagnosis, as well as at each time period before diagnosis (Table 2 and Appendix Fig A1, online only). A greater proportion of AL amyloidosis cases versus controls had a single FLC-diff level more than 180 mg/L before diagnosis (50% v 0%; P = .002), less than 4 years (46% v 0%; P < .03), and 4 to 11 years (46% v 0%; P < .03), but not more than 11 years prior to diagnosis (17% v 0%; P = .50; Table 3). By using thresholds of 23 mg/L and 40 mg/L, there was also a statistically significant difference between cases and controls at all times, less than 4 years, and 4 to 11 years, but not more than 11 years before diagnosis (Table 3).
Table 2.
Comparison of Median FLC-Diff Levels in Patients With AL Amyloidosis Compared With Controls Prior to Diagnosis
| Years Prior to Diagnosis | Cases (patients with AL) |
Controls |
P | ||
|---|---|---|---|---|---|
| FLC-Diff (mg/L) | 25%, 75% | FLC-Diff (mg/L) | 25%, 75% | ||
| < 4 | 174.8 | 78.5, 457.6 | 0.3 | −3.1, 3.8 | < .001 |
| 4-11 | 65.1 | 16.1, 251.6 | 2.2 | −4.2, 0.7 | < .001 |
| > 11 | 4.5 | −1.1, 73.4 | −0.4 | −4.6, 0.6 | .03 |
| All time periods | 78.5 | 11.0, 297.0 | −0.65 | −3.8, 2.1 | < .001 |
Abbreviations: AL, amyloid light chain; FLC-Diff, free light chain differential.
Table 3.
A Comparison of FLC-Diff Levels in Patients With AL Amyloidosis Compared With Controls
| FLC-Diff (mg/L)/No. of Years Before Diagnosis | Cases (patients with AL) |
Controls |
McNemar Exact P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| > 8 | 20/20 | 100 | 0/20 | 0 | < .001 |
| All years | |||||
| < 4 years | 13/13 | 100 | 0/13 | 0 | < .001 |
| 4-11 years | 13/15 | 87 | 0/15 | 0 | < .001 |
| > 11 years | 6/12 | 50 | 0/12 | 0 | < .03 |
| > 23 | 17/20 | 85 | 0/20 | 0 | < .001 |
| All years | |||||
| < 4 years | 12/13 | 92 | 0/13 | 0 | < .001 |
| 4-11 years | 10/15 | 67 | 0/15 | 0 | .002 |
| > 11 years | 4/12 | 33 | 0/12 | 0 | .13 |
| > 40 | 16/20 | 80 | 0/20 | 0 | < .001 |
| All years | |||||
| < 4 years | 11/13 | 85 | 0/13 | 0 | .001 |
| 4-11 years | 9/15 | 60 | 0/15 | 0 | .004 |
| > 11 years | 4/12 | 33 | 0/12 | 0 | .13 |
| > 180 | |||||
| All years | 10/20 | 50 | 0/20 | 0 | .002 |
| < 4 years | 6/13 | 46 | 0/13 | 0 | .03 |
| 4-11 years | 6/15 | 40 | 0/15 | 0 | .03 |
| > 11 years | 2/12 | 17 | 0/12 | 0 | .50 |
Abbreviations: AL, amyloid light chain; FLC-Diff, free light chain differential.
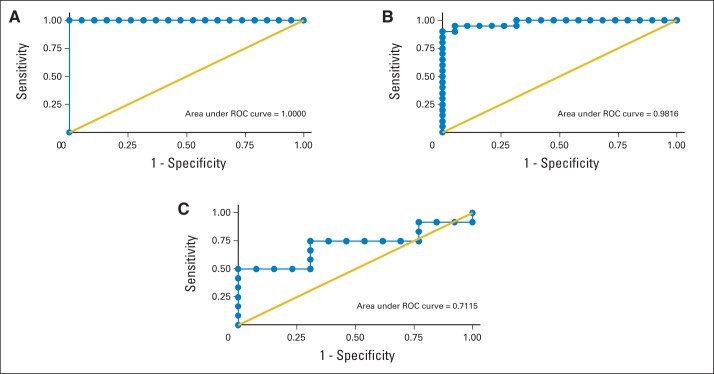
The FLC-diff ROC area under the curve was greater than 0.9 at less than 4 years and at 4 to 11 years, but not at more than 11 years before diagnosis (Fig 1). For less than 4 years before diagnosis, the area under the ROC curve was 1.00. In addition, the maximum correct classification was 100% at the FLC-diff cutoff of 11.0 with an associated 100% sensitivity and specificity. For 4 to 11 years before AL diagnosis, the area under the ROC curve was 0.98. The maximum correct classification was 95% at the FLC-diff cutoff of 8.7 with an associated sensitivity of 90% and specificity of 100%. For more than 11 years before AL diagnosis, the area under the ROC curve was 0.71. The maximum correct classification was 76% at the FLC-diff cutoff of 11.5 with an associated sensitivity of 50% and specificity of 100%.
Fig 1.
Receiver operator characteristic (ROC) curves for free light chain differential in years before diagnosis of immunoglobulin light chain amyloidosis. Free light chain differential for (A) < 4 years, (B) 4 to 11 years, and (C) > 11 years before diagnosis.
Temporal Changes of M-Ig LCs
We characterized the longitudinal changes in FLC-diff over time. Increases in FLC-diff per year of more than 10% per year occurred in 84% of cases and 16% of controls (P < .001), and increases in FLC-diff of more than 50% per year occurred in 58% of cases and 0% of controls (P < .001). We demonstrated two distinct patterns in the evolution of FLC-diff before diagnosis: (1) a slowly increasing FLC-diff over time to less than 200 mg/L near diagnosis and a more rapidly increasing FLC-diff over time to greater than 200 mg/L near the time of diagnosis (Fig 1).
DISCUSSION
In this study, we demonstrated that all patients with AL amyloidosis have an M-Ig before clinical presentation. The proportion of patients demonstrating an M-Ig increased to 100% within 4 years preceding the diagnosis and in nearly half the patients more than 11 years before diagnosis. We demonstrated that increased FLC-diff at thresholds of more than 23 mg/L, more than 40 mg/L, and more than 180 mg/L are present up to 11 years before clinical AL amyloidosis. To the best of our knowledge, there are no prior reports characterizing the pathogenic LC levels in the years before clinical diagnosis of AL amyloidosis, and thus we have defined the precursor state to AL amyloidosis.
Notably, we found statistically significant differences between the percentage of cases and controls above thresholds in the clinically normal range. The accepted normal range for sFLCs was established in patients with active plasma cell disorders and healthy controls.11 Our findings suggest that in the precursor state to AL amyloidosis, abnormal FLC-diff levels occur within the accepted normal range. This is analogous to our work in autoimmune kidney diseases, in which we have demonstrated preclinical autoantibodies above thresholds within the accepted normal range compared with matched controls.9,15,16 In AL amyloidosis, the concentration of the M-Ig is generally modest when compared with that in MM.17 The pathogenesis of AL amyloidosis is driven by misfolded LCs, which form an insoluble β-pleated conformation that deposits in tissues resulting in organ dysfunction.18 Thus, it is the specific quality of the propensity of LCs toward amyloid formation over the quantity. It is known that LCs derived from specific Ig genes have a greater propensity toward amyloid formation.19–21 Our findings demonstrate that prolonged and sustained production of relatively low concentrations of amyloidogenic LCs, even within the accepted normal range, results in the development of AL amyloidosis.
We also found that modest increases in LCs over time are associated with the future development of AL amyloidosis. In 84% of cases, there was at least a 10% annual increase in the FLC-diff. We observed two patterns of FLC-diff evolution before presentation: (1) a slowly increasing level and (2) a rapidly increasing level with a threshold of about 200 mg/L near the time of diagnosis separating these groups. Taken together, these findings suggest that there is heterogeneity among patients with AL amyloidosis in the degree of proliferation in the underlying plasma cell clone.3,14 Recently, an FLC-diff level ≥ 180 mg/L has been shown to confer an adverse prognosis in newly diagnosed AL amyloidosis, which provides further support for the biologic and clinical significance of our findings.13 Our findings are also consistent with our prior work and the findings of Blade et al22 and Kyle et al23 that describe the evolution of M-Ig before development of MM.5
Our study has several strengths. Besides skewing younger and male, the clinical and laboratory features of our cases are consistent with the typical AL amyloidosis population.24 We used highly sensitive and widely available assays to detect M-Ig.4 Serial samples were available for up to 19 years before diagnosis. This allowed us to describe in detail the longitudinal changes in the pathogenic LCs before diagnosis. Because of mandatory scheduled military health screenings, we were able to limit verification, observer, recall, and Berkson's biases.
We acknowledge the following limitations of our study. The small sample size may have been underpowered to detect significant differences between cases and controls more than 11 years before diagnosis or to confirm that no such difference exists in this time interval. Because of the retrospective nature of the study and the arbitrary availability of banked serum, not all patients had assays for M-Ig at all time points (Fig 2). In addition, because of limited sample volume, we could not perform cardiac troponin, N-terminal pro-brain natriuretic peptide (NT-proBNP), or creatinine analyses to describe the temporal relationship between the increase of sFLCs and onset of organ dysfunction. Changes in FLC-diff level over time assume a linear increase before diagnosis, but more than the three permissible samples would be required to prove this assumption. DoDSR de-identification requirements prevented the linkage of study results and background characteristics for specific cases and controls, which precluded secondary analyses. Finally, there were no statistical adjustments made for the multiple comparisons required to evaluate the various FLC-diff thresholds.
Fig 2.
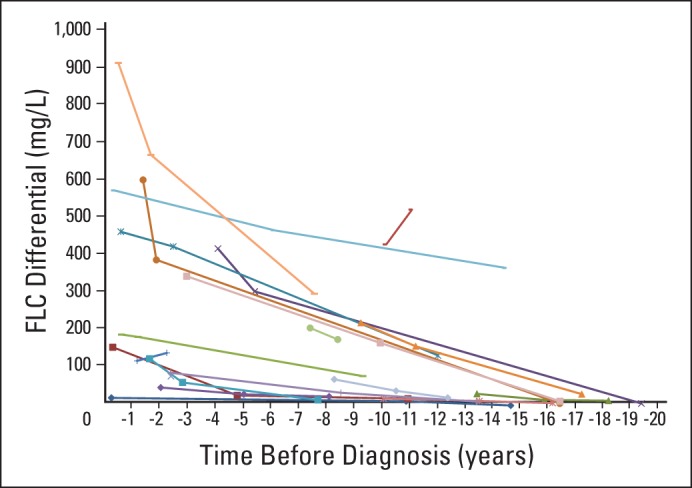
Serial free light chain (FLC) differential levels before clinical presentation in patients with immunoglubulin light chain amyloidosis.
Our findings have important implications. Early diagnosis and treatment of AL amyloidosis before the development of severe organ dysfunction is essential to improve outcomes, but until now, the window of opportunity for early diagnosis was unknown.10 We have described a prolonged precursor state to AL amyloidosis. AL amyloidosis cases have higher FLC-diff levels, even within the accepted normal range, that steadily increase over time for years before diagnosis. Our data suggest that patients with an FLC-diff greater than 11 mg/L that increases annually may benefit from an evaluation for organ dysfunction with cardiac biomarkers (troponins, NT-proBNP), echocardiography, and a fat pad aspirate with Congo Red staining for detection of tissue amyloid deposition.25,26 Recently, Merlini and Palladini27 suggested that patients with monoclonal gammopathies should have periodic assessments for cardiac biomarkers to detect organ dysfunction, which our data indirectly support. Our findings need to be confirmed in larger studies with longitudinal follow-up. Future studies are needed to better understand our findings in the context of the myeloma precursor states, monoclonal gammopathy of undetermined significance and smoldering MM. There are no immunophenotypic or genomic features that can clearly distinguish plasma cells in AL amyloidosis from those in other plasma cell disorders.28–31 These studies should incorporate additional biomarkers for assessment of potential amyloidogenicity and/or organ dysfunction, such as clusterin, cardiac troponins, NT-proBNP, midregional proadrenomedulin, or other novel biomarkers.25,32,33 Comparing M-Ig in cohorts of patients with stable monoclonal gammopathy of undetermined significance, those in evolution to AL amyloidosis, and those in evolution to MM would be clinically useful. Comparing this study to our previous work, in which we investigated M-Ig before development of MM, an M-Ig is present before AL amyloidosis in 42%, 80%, and 100% compared with 80%, 77%, and 95% in MM in the time periods more than 11, 4 to 11, and less than 4 years, respectively. This analysis is limited because of the imbalance between LC-only disorders in MM (approximately 20%) compared with AL amyloidosis (50%), and thus larger studies are needed to elucidate differences among these subsets of monoclonal gammopathies.
In conclusion, we detected an M-Ig in all patients with AL amyloidosis many years before the patients presented with symptomatic disease. We have definitively demonstrated that AL amyloidosis results from prolonged and sustained production of amyloidogenic LCs. Novel biomarkers sensitive for the amyloidogenicity of sFLCs or the presence of tissue amyloid deposition are urgently needed to facilitate early diagnosis and improve outcomes in this devastating disease. Until that time, clinical acumen remains the only route to early diagnosis of AL amyloidosis.
Acknowledgment
We thank Angelia Eick (Armed Forces Medical Surveillance Agency, Silver Spring, MD) for support and assistance with the Department of Defense Serum Repository, and Dan T. Vogl, Elizabeth O. Hexner, and Adam Cohen (University of Pennsylvania) for critical reviews of earlier versions of this article.
Appendix
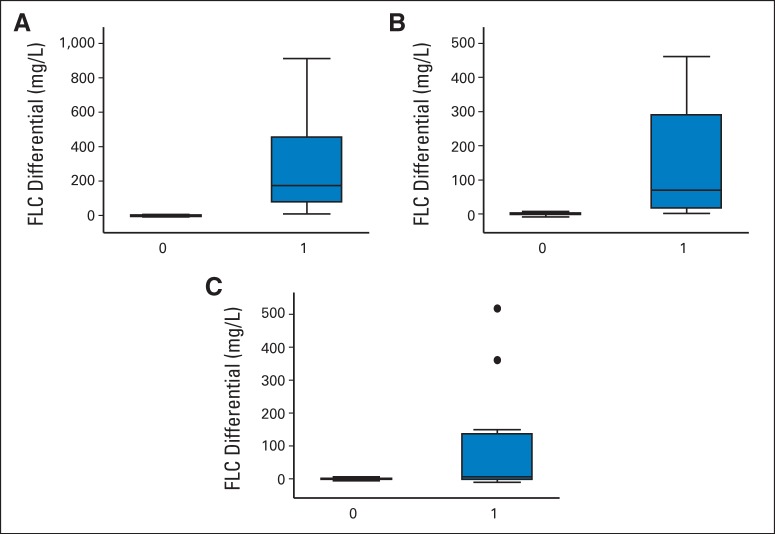
Fig A1.
Box plots representing free light chain (FLC) differential levels in cases and controls in the years before diagnosis of immunoglobulin light chain (controls = 0; cases = 1). FLC differential by case (A) < 4 years, (B) 4 to 11 years, and (C) > 11 years before diagnosis. The horizontal bar represents the median, vertical bars represent the highest and lowest values that are not outliers, and the dots are outliers (defined as outside 1.5 times the interquartile range). The lower and upper end of each box represents the first and third quartile, respectively. Interquartile range is defined as the third quartile subtracted from the first quartile.
Footnotes
See accompanying editorial on page 2679
Supported by the Walter Reed National Military Medical Center and the Armed Forces Medical Surveillance Agency.
Presented in part at the American Society of Nephrology, Philadelphia, PA, November 8-13, 2011, and the American Society of Hematology, San Diego, CA, December 10-13, 2011.
The views expressed in this paper are those of the authors and are not official views of the Department of Defense.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Brendan M. Weiss, Joseph Hebreo, Thomas P. Baker, Stephen W. Olson
Collection and assembly of data: Brendan M. Weiss, Joseph Hebreo, Daniel V. Cordaro, Stephen W. Olson
Data analysis and interpretation: Brendan M. Weiss, Joseph Hebreo, Mark J. Roschewski, Kevin C. Abbott, Stephen W. Olson
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Merlini G, Stone MJ. Dangerous small B-cell clones. Blood. 2006;108:2520–2530. doi: 10.1182/blood-2006-03-001164. [DOI] [PubMed] [Google Scholar]
- 2.Kumar SK, Gertz MA, Lacy MQ, et al. Recent improvements in survival in primary systemic amyloidosis and the importance of an early mortality risk score. Mayo Clinic Proc. 2011;86:12–18. doi: 10.4065/mcp.2010.0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23:215–224. doi: 10.1038/leu.2008.307. [DOI] [PubMed] [Google Scholar]
- 4.Katzmann JA, Abraham RS, Dispenzieri A, et al. …..: Diagnostic performance of quantitative kappa and lambda free light chain assays in clinical practice. Clin Chem. 2005;51:878–881. doi: 10.1373/clinchem.2004.046870. [DOI] [PubMed] [Google Scholar]
- 5.Weiss BM, Abadie J, Verma P, et al. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood. 2009;113:5418–5422. doi: 10.1182/blood-2008-12-195008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: A prospective study. Blood. 2009;113:5412–5417. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dispenzieri A, Katzmann JA, Kyle RA, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: A retrospective population-based cohort study. Lancet. 2010;375:1721–1728. doi: 10.1016/S0140-6736(10)60482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niven DJ, Berthiaume LR, Fick GH, et al. Matched case-control studies: A review of reported statistical methodology. Clin Epidemiol. 2012;4:99–110. doi: 10.2147/CLEP.S30816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olson SW, Arbogast CB, Baker TP, et al. Asymptomatic autoantibodies associate with future anti-glomerular basement membrane disease. J Am Soc Nephrol. 2011;22:1946–1952. doi: 10.1681/ASN.2010090928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comenzo RL. How I treat amyloidosis. Blood. 2009;114:3147–3157. doi: 10.1182/blood-2009-04-202879. [DOI] [PubMed] [Google Scholar]
- 11.Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: Relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48:1437–1444. [PubMed] [Google Scholar]
- 12.McNemar Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika. 1947;12:153–157. doi: 10.1007/BF02295996. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S, Dispenzieri A, Lacy MQ, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30:989–995. doi: 10.1200/JCO.2011.38.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar SK, Dispenzieri A, Lacy MQ, et al. Changes in serum-free light chain rather than intact monoclonal immunoglobulin levels predicts outcome following therapy in primary amyloidosis. Am J Hematol. 2011;86:251–255. doi: 10.1002/ajh.21948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olson SW, Lee JJ, Prince LK, et al. Elevated subclinical double-stranded DNA antibodies and future proliferative lupus nephritis. Clin J Am Soc Nephrol. 2013;8:1702–1708. doi: 10.2215/CJN.01910213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson SW, Owshalimpur D, Yuan CM, et al. Relation between asymptomatic proteinase 3 antibodies and future granulomatosis with polyangiitis. Clin J Am Soc Nephrol. 2013;8:1312–1318. doi: 10.2215/CJN.10411012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clinic Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 18.Korde N, Kristinsson SY, Landgren O. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM): Novel biological insights and development of early treatment strategies. Blood. 2011;117:5573–5581. doi: 10.1182/blood-2011-01-270140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comenzo RL, Zhang Y, Martinez C, et al. The tropism of organ involvement in primary systemic amyloidosis: Contributions of Ig V(L) germ line gene use and clonal plasma cell burden. Blood. 2001;98:714–720. doi: 10.1182/blood.v98.3.714. [DOI] [PubMed] [Google Scholar]
- 20.Perfetti V, Casarini S, Palladini G, et al. Analysis of V(lambda)-J(lambda) expression in plasma cells from primary (AL) amyloidosis and normal bone marrow identifies 3r (lambdaIII) as a new amyloid-associated germline gene segment. Blood. 2002;100:948–953. doi: 10.1182/blood-2002-01-0114. [DOI] [PubMed] [Google Scholar]
- 21.Perfetti V, Palladini G, Casarini S, et al. The repertoire of λ light chains causing predominant amyloid heart involvement and identification of a preferentially involved germline gene, IGLV1-44. Blood. 2012;119:144–150. doi: 10.1182/blood-2011-05-355784. [DOI] [PubMed] [Google Scholar]
- 22.Bladé J, Rosiñol L, Cibeira MT, et al. Pathogenesis and progression of monoclonal gammopathy of undetermined significance. Leukemia. 2008;22:1651–1657. doi: 10.1038/leu.2008.203. [DOI] [PubMed] [Google Scholar]
- 23.Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–569. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 24.Cohen AD, Comenzo RL. Systemic light-chain amyloidosis: Advances in diagnosis, prognosis, and therapy. Hematology Am Soc Hematol Educ Program. 2010;2010:287–294. doi: 10.1182/asheducation-2010.1.287. [DOI] [PubMed] [Google Scholar]
- 25.Palladini G, Campana C, Klersy C, et al. Serum N-terminal pro-brain natriuretic peptide is a sensitive marker of myocardial dysfunction in AL amyloidosis. Circulation. 2003;107:2440–2445. doi: 10.1161/01.CIR.0000068314.02595.B2. [DOI] [PubMed] [Google Scholar]
- 26.Gertz MA, Li CY, Shirahama T, et al. Utility of subcutaneous fat aspiration for the diagnosis of systemic amyloidosis (immunoglobulin light chain) Arch Intern Med. 1988;148:929–933. [PubMed] [Google Scholar]
- 27.Merlini G, Palladini G. Differential diagnosis of monoclonal gammopathy of undetermined significance. Hematology Am Soc Hematol Educ Program. 2012;2012:595–603. doi: 10.1182/asheducation-2012.1.595. [DOI] [PubMed] [Google Scholar]
- 28.Fonseca R, Bailey RJ, Ahmann GJ, et al. Genomic abnormalities in monoclonal gammopathy of undetermined significance. Blood. 2002;100:1417–1424. [PubMed] [Google Scholar]
- 29.Paiva B, Vídriales MB, Pérez JJ, et al. The clinical utility and prognostic value of multiparameter flow cytometry immunophenotyping in light-chain amyloidosis. Blood. 2011;117:3613–3616. doi: 10.1182/blood-2010-12-324665. [DOI] [PubMed] [Google Scholar]
- 30.Fonseca R, Ahmann GJ, Jalal SM, et al. Chromosomal abnormalities in systemic amyloidosis. Br J Haematol. 1998;103:704–710. doi: 10.1046/j.1365-2141.1998.01034.x. [DOI] [PubMed] [Google Scholar]
- 31.Bochtler T, Hegenbart U, Cremer FW, et al. Evaluation of the cytogenetic aberration pattern in amyloid light chain amyloidosis as compared with monoclonal gammopathy of undetermined significance reveals common pathways of karyotypic instability. Blood. 2008;111:4700–4705. doi: 10.1182/blood-2007-11-122101. [DOI] [PubMed] [Google Scholar]
- 32.Greene MJ, Sam F, Soo Hoo PT, et al. Evidence for a functional role of the molecular chaperone clusterin in amyloidotic cardiomyopathy. Am J Pathol. 2011;178:61–68. doi: 10.1016/j.ajpath.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palladini G, Barassi A, Perlini S, et al. Midregional proadrenomedullin (MR-proADM) is a powerful predictor of early death in AL amyloidosis. Amyloid. 2011;18:216–221. doi: 10.3109/13506129.2011.627069. [DOI] [PubMed] [Google Scholar]