Abstract
Purpose
To investigate the prognostic value of the BRAF V600E mutation and the recently identified TERT promoter mutation chr5:1,295,228C>T (C228T), individually and in their coexistence, in papillary thyroid cancer (PTC).
Patients and Methods
We performed a retrospective study of the relationship of BRAF and TERT C228T mutations with clinicopathologic outcomes of PTC in 507 patients (365 women and 142 men) age 45.9 ± 14.0 years (mean ± SD) with a median follow-up of 24 months (interquartile range, 8 to 78 months).
Results
Coexisting BRAF V600E and TERT C228T mutations were more commonly associated with high-risk clinicopathologic characteristics of PTC than they were individually. Tumor recurrence rates were 25.8% (50 of 194;77.60 recurrences per 1,000 person-years; 95% CI, 58.81 to 102.38) versus 9.6% (30 of 313; 22.88 recurrences per 1,000 person-years; 95% CI, 16.00 to 32.72) in BRAF mutation–positive versus –negative patients (hazard ratio [HR], 3.22; 95% CI, 2.05 to 5.07) and 47.5% (29 of 61; 108.55 recurrences per 1,000 person-years; 95% CI, 75.43 to 156.20) versus 11.4% (51 of 446; 30.21 recurrences per 1,000 person-years; 95% CI, 22.96 to 39.74) in TERT mutation–positive versus –negative patients (HR, 3.46; 95% CI, 2.19 to 5.45). Recurrence rates were 68.6% (24 of 35; 211.76 recurrences per 1,000 person-years; 95% CI, 141.94 to 315.94) versus 8.7% (25 of 287; 21.60 recurrences per 1,000 person-years; 95% CI, 14.59 to 31.97) in patients harboring both mutations versus patients harboring neither mutation (HR, 8.51; 95% CI, 4.84 to 14.97), which remained significant after clinicopathologic cofactor adjustments. Disease-free patient survival curves displayed a moderate decline with BRAF V600E or TERT C228T alone but a sharp decline with two coexisting mutations.
Conclusion
Coexisting BRAF V600E and TERT C228T mutations form a novel genetic background that defines PTC with the worst clinicopathologic outcomes, providing unique prognostic and therapeutic implications.
INTRODUCTION
Papillary thyroid cancer (PTC) is a common endocrine malignancy that accounts for 80% to 85% of thyroid malignancies.1,2 It can be classified further as conventional variant (CPTC), follicular variant (FVPTC), tall-cell variant (TCPTC), or one of a few other rare variants, among which CPTC is the most common. Although PTC is highly curable in general, approximately 10% of patients are destined for a progressive disease course with aggressive tumor behaviors and high disease recurrence and mortality rates.3–5 This wide spectrum of disease behaviors often creates dilemmas in clinical risk stratification and decision making for the management of PTC. The aggressive group of PTCs poses a particularly difficult prognostic and therapeutic challenge. It has been suggested that novel molecular-based management would help tackle this challenge,6 but the molecular mechanisms, particularly the genetic backgrounds, for the aggressiveness of this special group of PTCs remain to be better defined.
Molecular-based risk stratification of PTC using BRAF V600E mutation has been proposed in recent years.6,7 This is based on the association of BRAF mutation with poor clinicopathologic outcomes of PTC.8–11 BRAF V600E is the most common oncogene in PTC, with an average prevalence of 45%,12 and it promotes PTC tumorigenesis through constitutively activating the mitogen-activated protein kinase pathway and other mechanisms.13 We recently reported for the first time common mutations in the promoter of the gene for telomerase reverse transcriptase (TERT) in thyroid cancers,14 particularly the chr5:1,295,228C>T mutation (C228T), which represents the nucleotide change of −124 C>T from the ATG translation start site of the TERT gene. We also found that TERT C228T was particularly prevalent in aggressive types of thyroid cancer, such as anaplastic thyroid cancer and poorly differentiated thyroid cancer, as well as BRAF V600E mutation–positive PTC. These findings prompted us to propose and test in this study our hypothesis that BRAF V600E and TERT C228T mutations may cooperatively form a unique genetic background that identifies the most aggressive type of PTC and has important prognostic and therapeutic implications.
PATIENTS AND METHODS
Patients and Clinicopathologic Data
This study included 507 patients (365 women and 142 men) age 45.9 ± 14.0 years (mean ± SD) who were treated for PTC with total thyroidectomy and clinically observed between 1990 and 2012 at Johns Hopkins Hospital; the overall median follow-up time was 24 months (interquartile range, 8 to 78 months) after the initial treatments. Therapeutic neck dissection and radioiodine ablation were pursued following standard indications and criteria, as previously presented.11 The demographic data are listed in Table 1. After institutional review board approval and informed patient consenting, we obtained thyroid tumor specimens for genetic analysis and retrospectively collected clinicopathologic data. The pathologic diagnoses of PTC in our patients were formally established.11 Disease stages of PTC were defined on the basis of the American Joint Committee on Cancer staging system. Tumor recurrence was defined by the existence of histologically/cytologically/radioiodine radiographically confirmed recurrent/persistent PTC tumor. Follow-up time was defined as the time interval from the initial thyroidectomy to the discovery of disease recurrence or, in cases without disease recurrence, to the most recent clinical follow-up visit. All mutational analyses were performed after the surgical and radioiodine treatments of patients, and the genetic results had no influence on the treatment decision making.
Table 1.
Relationship of BRAF V600E and TERT C228T Mutations With Clinicopathologic Outcomes of PTC
| PTC Type and Clinicopathologic Outcomes |
BRAF Status |
TERT Status |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
BRAF V600E |
Wild-Type BRAF |
P |
TERT C228T |
Wild-Type TERT |
P | |||||||||
| No. | % | No. of Missing Cases | No. | % | No. of Missing Cases | No. | % | No. of Missing Cases | No. | % | No. of Missing Cases | |||
| All PTC | ||||||||||||||
| Total No. of cases | 194 | 313 | 61 | 446 | ||||||||||
| Age at diagnosis, years* | 47.1 ± 14.4 | 45.2 ± 13.8 | .138 | 51.7 ± 15.7 | 45.1 ± 13.6 | < .001 | ||||||||
| Sex, male | 69 | 35.6 | 73 | 23.3 | .003 | 32 | 52.5 | 110 | 24.7 | < .001 | ||||
| Tumor size, cm | 12 | 9 | .003 | 6 | 15 | .048 | ||||||||
| Median | 2.0 | 1.7 | 2.3 | 1.8 | ||||||||||
| Interquartile range | 1.3-3.0 | 1.0-2.8 | 1.2-3.5 | 1.1-3.0 | ||||||||||
| Multifocality | 68 | 36.4 | 7 | 122 | 39.1 | 1 | .542 | 20 | 33.9 | 2 | 170 | 38.6 | 6 | .482 |
| Extrathyroidal invasion | 58 | 31.3 | 9 | 35 | 11.2 | 1 | < .001 | 27 | 46.5 | 3 | 66 | 15.0 | 7 | < .001 |
| Vascular invasion | 36 | 20.0 | 14 | 41 | 13.2 | 2 | .045 | 14 | 25.9 | 7 | 63 | 14.4 | 9 | .028 |
| Lymph node metastasis | 85 | 46.2 | 10 | 68 | 21.7 | < .001 | 31 | 52.5 | 2 | 122 | 27.8 | 8 | < .001 | |
| Distant metastatic recurrence | 12 | 6.2 | 10 | 3.2 | .108 | 12 | 19.7 | 10 | 2.2 | < .001 | ||||
| Disease stage | 6 | 6 | ||||||||||||
| I | 114 | 60.6 | 237 | 75.7 | 26 | 42.6 | 325 | 73.9 | ||||||
| II | 13 | 6.9 | 31 | 9.9 | 6 | 9.8 | 38 | 8.6 | ||||||
| III | 39 | 20.7 | 35 | 11.2 | 12 | 19.7 | 62 | 14.1 | ||||||
| IV | 22 | 11.7 | 10 | 3.2 | < .001 | 17 | 27.9 | 15 | 3.4 | < .001 | ||||
| III + IV | 61 | 32.4 | 45 | 14.4 | < .001 | 29 | 47.5 | 77 | 17.5 | < .001 | ||||
| Tumor recurrence | 50 | 25.8 | 30 | 9.6 | < .001 | 29 | 47.5 | 51 | 11.4 | < .001 | ||||
| Total 131I dose, mCi | 16 | 7 | .004 | 7 | 16 | .001 | ||||||||
| Median | 87.7 | 74.9 | 100 | 75 | ||||||||||
| Interquartile range | 0-100 | 0-100 | 29.9-105 | 0-100 | ||||||||||
| Total follow-up, months | .027 | .056 | ||||||||||||
| Median | 18 | 31 | 30 | 24 | ||||||||||
| Interquartile range | 7-53 | 8-87 | 12-78 | 6-76 | ||||||||||
| CPTC | ||||||||||||||
| Total No. of cases | 164 | 219 | 47 | 336 | ||||||||||
| Age at diagnosis, years* | 46.7 ± 13.7 | 45.8 ± 13.9 | .511 | 51.6 ± 16.0 | 45.4 ± 13.4 | .004 | ||||||||
| Sex, male | 60 | 36.6 | 53 | 24.2 | .009 | 25 | 53.2 | 88 | 26.2 | < .001 | ||||
| Tumor size, cm | 12 | 9 | < .001 | 6 | 15 | .009 | ||||||||
| Median | 2 | 1.5 | 2.3 | 1.6 | ||||||||||
| Interquartile range | 1.3-3 | 0.8-2.3 | 1.2-3.5 | 1-2.5 | ||||||||||
| Multifocality | 54 | 34.4 | 7 | 87 | 39.9 | 1 | .277 | 16 | 35.6 | 2 | 125 | 37.9 | 6 | .763 |
| Extrathyroidal invasion | 48 | 31.0 | 9 | 29 | 13.3 | 1 | < .001 | 23 | 52.3 | 3 | 54 | 16.4 | 7 | < .001 |
| Vascular invasion | 27 | 18.0 | 14 | 27 | 14.4 | 2 | .140 | 10 | 25.0 | 7 | 44 | 13.5 | 9 | .052 |
| Lymph node metastasis | 76 | 49.0 | 9 | 58 | 26.5 | < .001 | 27 | 60.0 | 2 | 107 | 32.5 | 7 | < .001 | |
| Distant metastatic recurrence | 7 | 4.3 | 8 | 3.6 | .759 | 8 | 17.0 | 7 | 2.1 | < .001 | ||||
| Disease stage | 6 | 6 | ||||||||||||
| I | 98 | 62.0 | 172 | 78.5 | 19 | 40.3 | 251 | 76.1 | ||||||
| II | 10 | 6.3 | 15 | 6.9 | 3 | 6.4 | 22 | 6.7 | ||||||
| III | 32 | 20.2 | 23 | 10.5 | 10 | 21.3 | 45 | 13.6 | ||||||
| IV | 18 | 11.4 | 9 | 4.1 | .001 | 15 | 31.9 | 12 | 3.6 | < .001 | ||||
| III + IV | 50 | 31.6 | 32 | 14.6 | < .001 | 25 | 53.2 | 57 | 17.3 | < .001 | ||||
| Tumor recurrence | 42 | 25.6 | 22 | 10.0 | < .001 | 24 | 51.1 | 40 | 11.9 | < .001 | ||||
| Total 131I dose, mCi | 15 | 5 | .043 | 7 | < .001 | |||||||||
| Median | 75 | 51.7 | 100 | 75 | ||||||||||
| Interquartile range | 0-100 | 0-100 | 75-104 | 0-100 | ||||||||||
| Total follow-up, months | .026 | .026 | ||||||||||||
| Median | 19 | 32 | 48 | 24 | ||||||||||
| Interquartile range | 6.5-52 | 9-80 | 12-95 | 6-70 | ||||||||||
Abbreviations: CPTC, conventional papillary thyroid cancer; PTC, papillary thyroid cancer.
Data were summarized with means ± standard deviations.
Mutational Analyses
Genomic DNA was isolated from primary PTC tumors by standard phenol-chloroform extraction and ethanol precipitation procedures and subjected to classical Sanger sequencing for the detection of BRAF V600E and TERT C228T mutations. For BRAF V600E, the polymerase chain reaction (PCR) protocol and conditions described previously11 were used to amplify exon 15 of the BRAF gene containing the mutation hot spot, followed by a Big Dye (Applied Biosystems, Foster City, CA) reaction for Sanger sequencing. For TERT C228T, our recently described PCR conditions were used to amplify a fragment of the TERT promoter containing the C228T hot spot.14 BRAF V600E and TERT C228T were recognized on sequencing electropherograms.
Statistical Analyses
Categorical data were summarized with frequencies and percentages. Continuous data were summarized with means ± standard deviations (if normally distributed) or medians and interquartile ranges (if not normally distributed). Comparisons of categorical variables were performed using the χ2 test or, for small cell sizes, Fisher's exact test. The independent t and Wilcoxon-Mann-Whitney tests were used for normally and non-normally distributed continuous variables, respectively. Kaplan-Meier survival curves with log-rank tests and Cox proportional hazards regression analyses, censoring patients at the time of recurrence or, if no recurrence, at the time of last follow-up visit, were used to compare recurrence-free survival rates by mutation status. Independent associations of mutations with PTC recurrence were examined by Cox regression analyses. All P values were two sided, and a P value of <.05 was treated as statistically significant. The analyses were performed using Stata (Stata/SE version 10.1 for windows; Stata, College Station, TX) and GraphPad Prism (version 6 for Windows; GraphPad Software, San Diego, CA).
RESULTS
BRAF V600E and TERT C228T Mutations in PTC
We examined BRAF V600E and TERT C228T mutations in 507 cases of PTC that consisted of several variants (Appendix Table A1, online only). BRAF V600E was found in 164 of 383 (42.8%) CPTCs, 15 of 103 (14.6%) FVPTCs, 14 of 19 (73.7%) TCPTCs, and one of two (50%) columnar PTCs, with an overall prevalence of 38.3% (194 of 507). TERT C228T was found in 47 of 383 (12.3%) CPTCs, eight of 103 (7.8%) FVPTCs, five of 19 (26.3%) TCPTCs, and one of two (50.0%) columnar PTCs, with an overall prevalence of 12.0% (61 of 507). A significant association of TERT C228T with the BRAF mutation was observed (Appendix Table A2, online only). Specifically, on the overall analysis of all PTCs, TERT C228T was found in 26 of 313 (8.3%) BRAF mutation–negative cases versus 35 of 194 (18.0%) BRAF mutation–positive cases, and conversely, the BRAF mutation was found in 159 of 446 (35.7%) TERT mutation–negative cases versus 35 of 61 (57.4%) TERT mutation–positive cases (odds ratio [OR], 2.43; 95% CI, 1.40 to 4.21; P = .001). A significant association of the two mutations was similarly observed in CPTC (Appendix Table A2). Coexistence of BRAF and TERT mutations was found in 35 of 507 (6.9%) PTCs and 28 of 383 (7.3%) CPTCs (Appendix Table A1).
Relationship of BRAF V600E and TERT C228T Mutations With Clinicopathologic Outcomes of PTC
In the overall analysis of 507 PTCs (Table 1), the BRAF V600E mutation was found to be significantly associated with several high-risk clinicopathologic characteristics, including male sex of the patient, larger tumor size, extrathyroidal invasion, vascular invasion, lymph node metastasis, and stage III/IV. Tumor recurrence was 30 of 313 (9.6%; 22.88 recurrences per 1,000 person-years; 95% CI, 16.00 to 32.72) in BRAF mutation–negative patients versus 50 of 194 (25.8%; 77.60 recurrences per 1,000 person-years; 95% CI, 58.81 to 102.38) in BRAF mutation–positive patients (hazard ratio [HR], 3.22; 95% CI, 2.05 to 5.07; P < .001; Appendix Table A3, online only). Similarly, TERT C228T was significantly associated with these clinicopathologic characteristics in addition to older patient age and distant metastatic recurrence (Table 1). Tumor recurrence was 51 of 446 (11.4%; 30.21 recurrences per 1,000 person-years; 95% CI, 22.96 to 39.74) in TERT mutation–negative cases versus 29 of 61 (47.5%; 108.55 recurrences per 1,000 person-years; 95% CI, 75.43 to 156.20) in TERT mutation–positive cases (HR, 3.46; 95% CI, 2.19 to 5.45; P < .001; Appendix Table A3). The HRs of BRAF V600E and TERT C228T for tumor recurrence were all highly significant, which remained significant after adjustment for patient age and sex and, as may not be unexpected (see Discussion), they lost significance with the 95% CI marginally crossing 1.0 after additional adjustment for aggressive tumor behaviors (Appendix Table A3).
Similar results were obtained when analyses were performed only on CPTCs (Table 1; Appendix Table A3). For example, BRAF V600E and TERT C228T mutations were each associated with several high-risk clinicopathologic characteristics. Higher tumor recurrence rates and the number of recurrences per 1,000 person-years were associated with BRAF V600E or TERT C228T mutations. The HR of BRAF V600E for tumor recurrence was 3.10 (95% CI, 1.85 to 5.20; P < .001), and the HR of TERT C228T for PTC recurrence was 3.32 (95% CI, 2.00 to 5.52; P < .001).
Impacts of BRAF V600E or TERT C228T Alone or Their Coexistence on Clinicopathologic Outcomes of PTC
In the analysis of all PTCs (Table 2), in comparison with the group negative for either mutation, BRAF V600E alone was significantly associated with larger tumor size, extrathyroidal invasion, lymph node metastasis, disease stage III/IV, and tumor recurrences. TERT C228T alone was significantly associated with lymph node metastasis, and there was an insignificant association with other clinicopathologic characteristics. In contrast, the coexistence of BRAF V600E and TERT C228T was strongly associated with virtually all the classical high-risk characteristics as well as distant metastatic recurrence. Patients harboring both BRAF and TERT mutations had the highest recurrence rate as well, which was 24 of 35 (68.6%; 211.76 recurrences per 1,000 person-years; 95% CI, 141.94 to 315.94) versus only 25 of 287 (8.7%; 21.60 recurrences per 1,000 person-years; 95% CI, 14.59 to 31.97) in patients harboring neither mutation (HR, 8.51; 95% CI, 4.84 to 14.97; P < .001; Table 3).
Table 2.
Impact of BRAF V600E or TERT C228T or Their Coexistence on Clinicopathologic Outcomes of PTC
| PTC Type and Clinicopathologic Outcomes | No Mutation |
BRAF Mutation Only |
P |
TERT Mutation Only |
P |
BRAF + TERT Mutation |
P* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. of Missing Cases | No. | % | No. of Missing Cases | No. | % | No. of Missing Cases | No. | % | No. of Missing Cases | ||||
| All PTC | |||||||||||||||
| Total No. of cases | 287 | 159 | 26 | 35 | |||||||||||
| Age at diagnosis, years† | 45.3 ± 13.7 | 44.8 ± 13.5 | .724 | 44.0 ± 14.6 | .651 | 57.4 ± 14.1 | < .001 | ||||||||
| Sex, male | 65 | 22.6 | 45 | 28.3 | .185 | 8 | 30.8 | .348 | 24 | 68.6 | < .001 | ||||
| Tumor size, cm | 7 | 8 | .044 | 2 | .885 | 4 | .002 | ||||||||
| Median | 1.7 | 2.0 | 1.8 | 2.7 | |||||||||||
| Interquartile range | 1.0-3.0 | 1.3-3.0 | 1.1-2.5 | 1.3-4.0 | |||||||||||
| Multifocality | 114 | 39.7 | 56 | 36.6 | 6 | .522 | 8 | 32.0 | 1 | .448 | 12 | 35.3 | 1 | .617 | |
| Extrathyroidal invasion | 31 | 10.8 | 35 | 23.0 | 7 | .001 | 4 | 16.0 | 1 | .503 | 23 | 69.7 | 2 | < .001 | |
| Vascular invasion, n (%) | 35 | 12.2 | 1 | 28 | 18.5 | 8 | .074 | 6 | 24.0 | 1 | .096 | 8 | 27.6 | 6 | .022 |
| Lymph node metastasis | 58 | 20.2 | 64 | 42.4 | 8 | < .001 | 10 | 38.5 | .031 | 21 | 63.6 | 2 | < .001 | ||
| Distant metastatic recurrence | 8 | 2.8 | 2 | 1.3 | .506 | 2 | 7.7 | .198 | 10 | 28.6 | < .001 | ||||
| Disease stage | 6 | ||||||||||||||
| I | 219 | 76.3 | 106 | 69.3 | 18 | 69.2 | 8 | 22.9 | |||||||
| II | 27 | 9.4 | 11 | 7.2 | 4 | 15.4 | 2 | 5.7 | |||||||
| III | 33 | 11.5 | 29 | 18.9 | 2 | 7.7 | 10 | 28.6 | |||||||
| IV | 8 | 2.8 | 7 | 4.6 | .106 | 2 | 7.7 | .373 | 15 | 42.9 | < .001 | ||||
| III+IV | 41 | 14.3 | 36 | 23.5 | .015 | 4 | 15.4 | .776 | 25 | 71.4 | < .001 | ||||
| Tumor recurrence | 25 | 8.7 | 26 | 16.3 | .015 | 5 | 19.2 | .081 | 24 | 68.6 | < .001 | ||||
| Total 131I dose, mCi | 5 | 11 | .084 | 2 | .560 | 5 | < .001 | ||||||||
| Median | 74.6 | 75.4 | 77 | 100 | |||||||||||
| Interquartile range | 0-100 | 0-100 | 0-100 | 98-136 | |||||||||||
| Total follow-up, months | .048 | .030 | .864 | ||||||||||||
| Median | 28 | 17 | 66 | 24 | |||||||||||
| Interquartile range | 6-85 | 3-52 | 12-116 | 12-60 | |||||||||||
| CPTC | |||||||||||||||
| Total No. of cases | 200 | 136 | 19 | 28 | |||||||||||
| Age at diagnosis, years† | 46.0 ± 13.7 | 44.7 ± 12.8 | .398 | 44.2 ± 16.1 | .603 | 56.7 ± 14.2 | < .001 | ||||||||
| Sex, male | 47 | 23.5 | 41 | 30.1 | .174 | 6 | 31.6 | .432 | 19 | 67.9 | < .001 | ||||
| Tumor size, cm | 7 | 8 | < .001 | 2 | .349 | 4 | < .001 | ||||||||
| Median | 1.5 | 2 | 1.5 | 2.8 | |||||||||||
| Interquartile range | 0.8-2.3 | 1.3-2.5 | 1.0-2.3 | 1.7-3.5 | |||||||||||
| Multifocality | 81 | 40.5 | 44 | 33.8 | 6 | .223 | 6 | 33.3 | 1 | .552 | 10 | 37.0 | 1 | .730 | |
| Extrathyroidal invasion | 25 | 12.5 | 29 | 22.5 | 7 | .017 | 4 | 22.2 | 1 | .272 | 19 | 73.1 | 2 | < .001 | |
| Vascular invasion | 21 | 10.5 | 1 | 23 | 18.0 | 8 | .055 | 6 | 33.3 | 1 | .005 | 4 | 18.2 | 6 | .287 |
| Lymph node metastasis | 49 | 24.5 | 58 | 45.0 | 7 | < .001 | 9 | 47.4 | .031 | 18 | 69.2 | 2 | < .001 | ||
| Distant metastatic recurrence | 6 | 3.0 | 1 | 0.7 | .248 | 2 | 10.5 | .146 | 6 | 21.4 | < .001 | ||||
| Disease stage | 6 | ||||||||||||||
| I | 159 | 79.5 | 92 | 70.8 | 13 | 68.4 | 6 | 21.4 | |||||||
| II | 13 | 6.5 | 9 | 6.9 | 2 | 10.5 | 1 | 3.6 | |||||||
| III | 21 | 10.5 | 24 | 18.5 | 2 | 10.5 | 8 | 28.6 | |||||||
| IV | 7 | 3.5 | 5 | 3.8 | .212 | 2 | 10.5 | .429 | 13 | 46.4 | < .001 | ||||
| III+IV | 28 | 14.0 | 29 | 22.3 | .051 | 4 | 21.0 | .492 | 21 | 75.0 | < .001 | ||||
| Tumor recurrence | 18 | 9.0 | 22 | 16.2 | .046 | 4 | 21.0 | .107 | 20 | 71.4 | < .001 | ||||
| Total 131I dose, mCi | 3 | 10 | .193 | 2 | .110 | 5 | < .001 | ||||||||
| Median | 50.9 | 75 | 100 | 100 | |||||||||||
| Interquartile range | 0-100 | 0-100 | 0-103 | 75-131.5 | |||||||||||
| Total follow-up, months | .025 | .067 | .686 | ||||||||||||
| Median | 30.5 | 17 | 73 | 35.5 | |||||||||||
| Interquartile range | 8-79 | 2-48.5 | 12-108 | 12-61.5 | |||||||||||
Abbreviations: CPTC, conventional papillary thyroid cancer; PTC, papillary thyroid cancer.
P values are from the comparison of the indicated genetic group in the column immediately left of the P value column with the no mutation group.
Data were summarized with means ± standard deviations.
Table 3.
Hazard Ratios of BRAF V600E or TERT C228T or Their Coexistence for the Recurrence of PTC
| Type of PTC | Mutations | Recurrence | % | Recurrence per 1,000 Person-Years | 95% CI | Unadjusted |
Adjustment 1* |
Adjustment 2† |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratios | 95% CI | Hazard Ratios | 95% CI | Hazard Ratios | 95% CI | ||||||
| All PTC | No mutation | 25 of 287 | 8.7 | 21.60 | 14.59 to 31.97 | 1.00 | 1.00 | ||||
| BRAF mutation only | 26 of 159 | 16.3 | 48.96 | 33.34 to 71.91 | 2.24 | 1.29 to 3.88 | 2.16 | 1.24 to 3.75 | 1.17 | 0.62 to 2.20 | |
| TERT mutation only | 5 of 26 | 19.2 | 32.50 | 13.53 to 78.09 | 1.69 | 0.65 to 4.43 | 1.60 | 0.60 to 4.25 | 0.87 | 0.27 to 2.76 | |
| BRAF + TERT mutations | 24 of 35 | 68.6 | 211.76 | 141.94 to 315.94 | 8.51 | 4.84 to 14.97 | 8.41 | 4.44 to 15.94 | 3.10 | 1.24 to 7.75 | |
| CPTC | No mutation | 18 of 200 | 9.0 | 22.23 | 14.00 to 35.28 | 1.00 | 1.00 | ||||
| BRAF mutation only | 22 of 136 | 16.2 | 50.25 | 33.08 to 76.31 | 2.20 | 1.18 to 4.11 | 2.06 | 1.10 to 3.86 | 1.03 | 0.49 to 2.15 | |
| TERT mutation only | 4 of 19 | 21.0 | 35.22 | 13.22 to 93.83 | 1.82 | 0.61 to 5.38 | 1.71 | 0.56 to 5.22 | 0.50 | 0.12 to 2.00 | |
| BRAF + TERT mutations | 20 of 28 | 71.4 | 191.85 | 123.77 to 297.36 | 7.73 | 4.07 to 14.67 | 7.50 | 3.71 to 15.17 | 4.39 | 1.42 to 13.54 | |
NOTE. Hazard ratios and 95% CIs were calculated using Cox regression for the comparison of the indicated mutation group with the group harboring neither mutation.
Abbreviations: CPTC, conventional papillary thyroid cancer; PTC, papillary thyroid cancer.
Adjustment 1 was made for patient age at diagnosis and sex.
Adjustment 2 was made for patient age at diagnosis, sex, multifocality, tumor size, extrathyroidal invasion, vascular invasion, and lymph node metastasis.
Similar individual impacts of BRAF V600E and TERT C288T mutations on clinicopathologic outcomes were observed in CPTC (Table 2). In comparison with the group negative for either mutation, BRAF V600E was significantly associated with several high-risk clinicopathologic characteristics as well as tumor recurrences. The impacts of TERT C228T alone on clinicopathologic outcomes were significant for vascular invasion and lymph node metastasis and short of statistical significance for other parameters. In contrast, the coexistence of BRAF V600E and TERT C228T was highly associated with virtually all the high-risk clinicopathologic characteristics. Tumor recurrence was 20 of 28 (71.4%; 191.85 recurrences per 1,000 person-years; 95% CI, 123.77 to 297.36) in patients harboring both mutations versus 18 of 200 (9.0%; 22.23 recurrences per 1,000 person-years; 95% CI, 14.00 to 35.28) in patients harboring neither mutation (HR, 7.73; 95% CI, 4.07 to 14.67; P < .001; Table 3).
There was an incremental impact of coexisting BRAF and TERT C228T mutations on PTC recurrence over either mutation alone (Table 4). Specifically, in the analysis of all PTCs, tumor recurrence was 24 of 35 (68.6%; 211.76 recurrences per 1,000 person-years; 95% CI, 141.94 to 315.94) in patients harboring both mutations versus 26 of 159 (16.3%; 48.96 recurrences per 1,000 person-years; 95% CI, 33.34 to 71.91) in patients harboring only the BRAF mutation (HR, 3.62; 95% CI, 2.07 to 6.33; P < .001) and five of 26 (19.2%; 32.5 recurrences per 1,000 person-years; 95% CI, 13.53 to 78.09) in patients harboring only TERT mutation (HR, 6.16; 95% CI, 2.29 to 16.61; P < .001). In fact, PTC recurrence associated with coexisting BRAF and TERT mutations was dramatically higher than the sum of those associated with the two mutations individually, demonstrating a synergistic effect of the two mutations on PTC recurrence. Similar results were also obtained in CPTC (Table 4).
Table 4.
Comparison of PTC Recurrence Between the BRAF V600E + TERT C228 Mutations Group and the BRAF V600E–Only or TERT C288T–Only Group
| PTC Type and Recurrence | BRAF V600E Only (A) | TERT C228T Only (B) | BRAF + TERT Mutations (C) | Comparison of C With A |
Comparison of C With B |
||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||||
| All PTC | |||||||||
| Recurrence | |||||||||
| No. | 26 of 159 | 5 of 26 | 24 of 35 | ||||||
| % | 16.3 | 19.2 | 68.6 | ||||||
| Recurrence per 1,000 person-years | 48.96 | 32.5 | 211.76 | 3.62 | 2.07 to 6.33 | < .001 | 6.16 | 2.29 to 16.61 | < .001 |
| 95% CI | 33.34 to 71.91 | 13.53 to 78.09 | 141.94 to 315.94 | ||||||
| CPTC | |||||||||
| Recurrence | |||||||||
| No. | 22 of 136 | 4 of 19 | 20 of 28 | ||||||
| % | 16.2 | 21.0 | 71.4 | ||||||
| Recurrence per 1,000 person-years | 50.25 | 35.22 | 191.85 | 3.30 | 1.79 to 6.06 | < .001 | 5.28 | 1.76 to 15.83 | .003 |
| 95% CI | 33.08 to 76.31 | 13.22 to 93.83 | 123.77 to 297.36 | ||||||
Abbreviations: CPTC, conventional variant papillary thyroid cancer; HR, hazard ratio; PTC, papillary thyroid cancer.
Impacts of BRAF V600E and TERT C228T Mutations on Disease-Free Survival of Patients With PTC
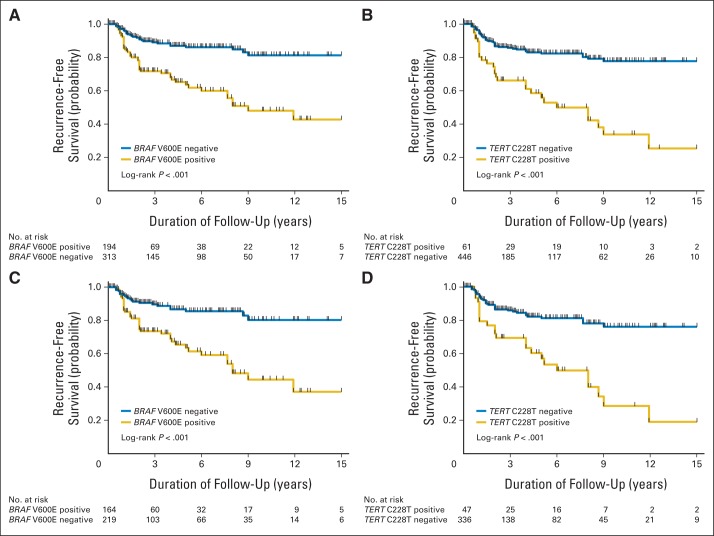
We performed Kaplan-Meier and log-rank analyses of disease-free survival rates of patients by genotype. In analyses of all PTCs (Fig 1A and 1B), tumor recurrence-free survival curves had a modest decline in patients negative for BRAF V600E (Fig 1A) or TERT C228T (Fig 1B). They declined further with either the BRAF mutation (Fig 1A) or the TERT mutation (Fig 1B). Similar results were obtained in the analyses of CPTCs (Figs 1C and 1D).
Fig 1.
Kaplan-Meier analyses of the impacts of BRAF V600E and TERT C228T mutations on disease-free survival of patients with papillary thyroid cancer (PTC). (A, B) Results of the analyses of patients with PTC of all types. (C, D) Results of the analyses of conventional variant PTC only. (A, C) Effects of the BRAF V600E mutation on tumor recurrence-free survival. (B, D) Effects of the TERT C228T mutation on tumor recurrence-free survival. Blue lines represent patients negative for the indicated mutation. Gold lines represent patients positive for the indicated mutation.
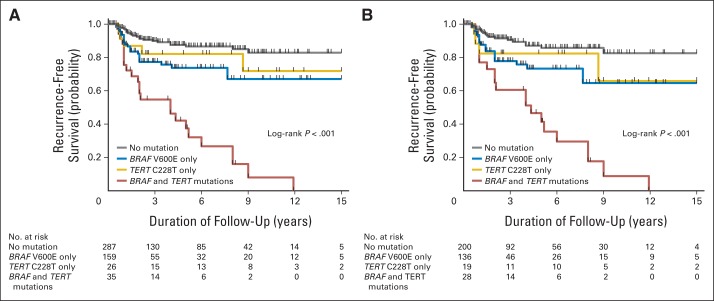
Figure 2A shows the impacts of individual BRAF V600E or TERT C228T mutations or their coexistence on tumor recurrence-free survival curves of all patients with PTC. There was an increasing decline in recurrence-free survival curves from patients with neither mutation to patients with the TERT mutation alone, those with the BRAF mutation alone, and those with both mutations. The curve decline with TERT mutation alone was modest, consistent with the modest effects of the TERT mutation alone on other clinicopathologic outcomes (Table 2). The curve decline with coexisting BRAF and TERT mutations was sharp and dramatic, and the curve decline with the BRAF mutation alone was intermediate. Virtually identical results were obtained in patients with CPTC (Fig 2B).
Fig 2.
Kaplan-Meier analyses of the impacts of BRAF V600E or TERT C288T alone or their coexistence on disease-free survival of patients with papillary thyroid cancer (PTC). (A) Results of the analyses of patients with PTC of all types. (B) Results of the analyses of conventional variant PTC only. Four groups of patients are indicated in A and B, including patients with neither mutation (gray lines), TERT C228T mutation only (gold lines), BRAF V600E mutation only (blue lines), and coexistence of the two mutations (red lines).
Table 3 summarizes the impacts of BRAF V600E, TERT C228T, and their coexistence on PTC recurrence after adjustment for classical clinicopathologic risk factors. The HR of BRAF mutation alone for tumor recurrence in all PTCs was 2.24 (95% CI, 1.29 to 3.88), and it remained significant at 2.16 (95% CI, 1.24 to 3.75) after the first adjustment for patient age at diagnosis and sex. This significance was lost after an additional adjustment for aggressive tumor behaviors, including tumor size, multifocality, extrathyroidal invasion, vascular invasion, and lymph node metastasis. The HR of TERT C228T alone for tumor recurrence was not significant, with the 95% CIs all crossing 1.0. In striking contrast, the HRs of coexisting BRAF and TERT mutations for tumor recurrence in all PTCs was 8.51 (95% CI, 4.84 to 14.97), and it remained significant at 8.41 (95% CI, 4.44 to 15.94) after the adjustment for patient age and sex and was still significant at 3.10 (95% CI, 1.24 to 7.75) after the additional adjustment for tumor behaviors.
We obtained similar HR results for tumor recurrence in CPTCs (Table 3). For example, the HRs of coexistence of the two mutations for tumor recurrence of CPTC—unadjusted, adjusted for the first level, and adjusted for the second level—were significant at 7.73 (95% CI, 4.07 to 14.67), 7.50 (95% CI, 3.71 to 15.17), and 4.39 (95% CI, 1.42 to 13.54), respectively.
DISCUSSION
We have identified a novel genetic background—coexistence of BRAF V600E and TERT C228T mutations—which defines the most aggressive subgroup of PTCs. The combined effects of the two mutations on recurrence compared with no mutation remained significant even on multivariable adjustments for the classical clinicopathologic risk factors. The PTC recurrence rate for patients with coexisting BRAF and TERT mutations was also significantly higher than that associated with either mutation alone or the sum of the recurrences associated with the two mutations individually, demonstrating an incremental and synergistic effect of the coexisting two mutations. These results were found both in the overall analyses of all PTCs and of the CPTC variant, establishing coexistence of the two mutations as an important novel genetic background for the worst aggressiveness of PTC.
This cooperative effect of BRAF and TERT promoter mutations can be explained at a molecular level. TERT maintains the length of chromosomes by adding telomeres to them, thus increasing the immortality of cells, and promotes cell proliferation and decreases apoptosis.15–17 Transgenic mouse models overexpressing TERT showed increased tumor development and malignant transformation.18,19 Consistent with this oncogenic role of TERT is its common overexpression in human cancers,15–17 including thyroid cancer.20,21 TERT C228T confers increased transcriptional activities of the TERT promoter by creating consensus binding motifs (GGA[A>T] or CCGGAA) for E-twenty-six (ETS)/ternary complex transcription factors.22,23 As activation of the mitogen-activated protein kinase pathway upregulates the ETS system,24–26 the coexistence of BRAF V600E and TERT C228T forms a unique mechanism upregulating the expression of TERT. Indeed, coexistence of the two mutations was associated with increased expression of the TERT mRNA in PTC.27 This oncogenic cooperation of TERT with BRAF mutation is interestingly similar to the finding in a transgenic mouse model in which p53 mutation and induced overexpression of TERT cooperatively promoted cancer development.18 Consistent with the role of TERT C228T in poor clinicopathologic outcomes of PTC were recent reports of the association of TERT promoter mutations with brain tumor-associated patient mortality,28 bladder cancer recurrence,29 and poor survival of patients with laryngeal cancer.30
Many studies have demonstrated a role of BRAF V600E in tumor aggressiveness8–10 and even patient mortality31 in PTC, but some studies failed to do so. In the present study, HRs for tumor recurrence remained significant on the multivariable adjustment for patient age and sex but fell short of significance when aggressive tumor pathologic behaviors were adjusted. This statistical result should not be interpreted as the lack of a role of BRAF mutation in the aggressiveness of PTC. Biologically, BRAF mutation uses various molecular mechanisms to promote the aggressive tumor behaviors.13 Because some of these tumor behaviors, particularly lymph node metastases, are the main source of PTC recurrence, it is not surprising that statistical adjustment for them could artificially (and misleadingly) diminish or even null the effect of BRAF mutation on recurrence. This study represents the largest uniform series of PTC to examine this role of the BRAF mutation, but perhaps an even larger study is needed to show an independent role of BRAF mutation. The persistently significant effects of coexisting BRAF and TERT mutations on PTC recurrence after multivariable adjustments for the classical clinicopathologic factors suggest that coexisting BRAF and TERT mutations have a more profound impact.
The effects of TERT C228T mutation fell short of significance when it was separated from the BRAF mutation and examined alone, suggesting that TERT mutation needs additional genetic alterations to cooperate to promote the aggressiveness of PTC. We previously reported a particularly high prevalence of TERT C228T in anaplastic thyroid cancer, poorly differentiated thyroid cancer, and thyroid cancer cell lines,14 which were confirmed in several subsequent publications.27,32,33 Thyroid cancer cell lines are usually undifferentiated34 and commonly harbor multiple genetic alterations, including the BRAF mutation.35–37 Thus, it is likely that their aggressiveness is cooperatively driven by coexisting TERT promoter mutations and other genetic alterations, similar to their cooperation found in this study. Our results in this American cohort of patients are consistent with our recent findings of the impact of coexisting BRAF V600E and TERT promoter mutations on aggressive behaviors of PTC in a Chinese cohort of patients.38
The follow-up time of patients in this study was relatively short. However, patients with PTC usually present recurrence within the first few years. Therefore, a median of 2 years should have captured the majority of recurrence events of PTC. The disease-free survival curves (Figs 1 and 2) show that, as time progresses, the separation of the mutation–positive and –negative curves becomes even more prominent, suggesting that in later follow-up years the impact of the mutations on PTC recurrence is even more profound. Therefore, if anything, a median follow-up time of 2 years likely caused an underestimate of the impacts of the BRAF and TERT mutations on PTC recurrence. The follow-up times were different among some groups. However, this variation was corrected by the Cox proportional and regression analyses, because these standard statistical methods take the time as a variable into the model. To further correct the time variations, we also additionally report recurrences per 1,000 person years.
In summary, this study identified coexisting BRAF V600E and TERT C228T mutations as a novel genetic background for the most aggressive subgroup of PTC, whereas the two mutations alone have relatively less impact on the aggressiveness of PTC. These genetic patterns, by separating patients with PTC into different risk groups and particularly by defining the group with the most aggressive disease, have important prognostic and therapeutic implications.
Appendix
Table A1.
Prevalence of BRAF V600E and TERT C228T Mutations in Various Variants of PTC
| PTC Type |
BRAF V600E Mutation |
TERT C228T Mutation |
BRAF + TERT mutations |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| CPTC | 164 of 383 | 42.8 | 47 of 383 | 12.3 | 28 of 383 | 7.3 |
| FVPTC | 15 of 103 | 14.6 | 8 of 103 | 7.8 | 1 of 103 | 1.0 |
| TCPTC | 14 of 19 | 73.7 | 5 of 19 | 26.3 | 5 of 19 | 26.3 |
| Columnar PTC | 1 of 2 | 50.0 | 1 of 2 | 50.0 | 1 of 2 | 50.0 |
| All PTC | 194 of 507 | 38.3 | 61 of 507 | 12.0 | 35 of 507 | 6.9 |
Abbreviations: CPTC, conventional variant papillary thyroid cancer; FVPTC, follicular variant papillary thyroid cancer; PTC, papillary thyroid cancer; TCPTC, tall-cell variant papillary thyroid cancer.
Table A2.
Association of TERT Promoter C228T Mutation With BRAF V600E Mutation in PTC
| Tumor Type |
TERT C228T Mutation |
BRAF V600E Mutation |
OR | 95% CI | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
BRAF− |
BRAF+ |
TERT− |
TERT+ |
||||||||
| No. | % | No. | % | No. | % | No. | % | ||||
| All PTC | 26 of 313 | 8.3 | 35 of 194 | 18.0 | 159 of 446 | 35.7 | 35 of 61 | 57.4 | 2.43 | 1.40 to 4.21 | .001 |
| CPTC | 19 of 219 | 8.7 | 28 of 164 | 17.1 | 136 of 336 | 40.5 | 28 of 47 | 59.6 | 2.17 | 1.16 to 4.06 | .013 |
Abbreviations: CPTC, conventional papillary thyroid cancer; PTC, papillary thyroid cancer.
Table A3.
Association of BRAF or TERT C228T Promoter Mutation With PTC Recurrence
| Tumor Type and Mutation Status | Recurrence |
Recurrence per 1,000 Person-Years | 95% CI | Unadjusted |
Adjusted* |
Adjusted† |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | No. | % | Hazard Ratios | 95% CI | Hazard Ratios | 95% CI | Hazard Ratios | 95% CI | |||
| All PTC | |||||||||||
| BRAF V600E | |||||||||||
| Negative | 30 | 313 | 9.6 | 22.88 | 16.00 to 32.72 | 1.00 | 1.00 | 1.00 | |||
| Positive | 50 | 194 | 25.8 | 77.60 | 58.81 to 102.38 | 3.22 | 2.05 to 5.07 | 3.02 | 1.91 to 4.77 | 1.51 | 0.87 to 2.60 |
| TERT C228T | |||||||||||
| Negative | 51 | 446 | 11.4 | 30.21 | 22.96 to 39.74 | 1.00 | 1.00 | 1.00 | |||
| Positive | 29 | 61 | 47.5 | 108.55 | 75.43 to 156.20 | 3.46 | 2.19 to 5.45 | 3.21 | 2.02 to 5.09 | 1.78 | 0.97 to 3.25 |
| CPTC | |||||||||||
| BRAF V600E | |||||||||||
| Negative | 22 | 219 | 10.0 | 23.82 | 15.69 to 36.18 | 1.00 | 1.00 | 1.00 | |||
| Positive | 42 | 164 | 25.6 | 77.48 | 57.26 to 104.84 | 3.10 | 1.85 to 5.20 | 2.88 | 1.71 to 4.86 | 1.46 | 0.77 to 2.75 |
| TERT C228T | |||||||||||
| Negative | 40 | 336 | 11.9 | 32.06 | 23.52 to 43.71 | 1.00 | 1.00 | 1.00 | |||
| Positive | 24 | 47 | 51.1 | 110.18 | 73.85 to 164.38 | 3.32 | 2.00 to 5.52 | 3.15 | 1.89 to 5.24 | 1.54 | 0.76 to 3.12 |
NOTE. Hazard ratios and 95% CIs were calculated with Cox regression.
Abbreviations: CPTC, conventional papillary thyroid cancer; PTC, papillary thyroid cancer.
Adjustment was made for patient age at diagnosis and sex.
Adjustment was made for patient age at diagnosis, sex, multifocality, tumor size, extrathyroidal invasion, vascular invasion, and lymph node metastasis.
Footnotes
See accompanying editorial on page 2683
Supported by US National Institutes of Health Grants No. RO1CA113507 and R01CA134225 (M.X.).
Disclaimer: The content of this article is solely the responsibility of the authors and does not necessarily reflect the official views of the funding agency (US National Institutes of Health). The funding organizations had no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Patents, Royalties, and Licenses: Mingzhao Xing, BRAF mutation in thyroid carcinoma, USA patent 7,378,233 Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Mingzhao Xing
Financial support: Mingzhao Xing
Administrative support: Mingzhao Xing
Provision of study materials or patients: Mingzhao Xing, Martha A. Zeiger, Sara Pai, Justin Bishop
Collection and assembly of data: Mingzhao Xing, Rengyun Liu, Xiaoli Liu, Avaniyapuram Kannan Murugan, Guangwu Zhu, Justin Bishop
Data analysis and interpretation: Mingzhao Xing, Rengyun Liu, Xiaoli Liu, Avaniyapuram Kannan Murugan, Martha A. Zeiger, Sara Pai, Justin Bishop
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2010. Bethesda, MD: National Cancer Institute; http://seer.cancer.gov/csr/1975_2010/ [Google Scholar]
- 3.Brown RL, de Souza JA, Cohen EE. Thyroid cancer: Burden of illness and management of disease. J Cancer. 2011;2:193–199. doi: 10.7150/jca.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 5.Tuttle RM, Ball DW, Byrd D, et al. Thyroid carcinoma. J Natl Compr Canc Netw. 2010;8:1228–1274. doi: 10.6004/jnccn.2010.0093. [DOI] [PubMed] [Google Scholar]
- 6.Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet. 2013;381:1058–1069. doi: 10.1016/S0140-6736(13)60109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xing M. Prognostic utility of BRAF mutation in papillary thyroid cancer. Mol Cell Endocrinol. 2010;321:86–93. doi: 10.1016/j.mce.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim TH, Park YJ, Lim JA, et al. The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: A meta-analysis. Cancer. 2012;118:1764–1773. doi: 10.1002/cncr.26500. [DOI] [PubMed] [Google Scholar]
- 9.Tufano RP, Teixeira GV, Bishop J, et al. BRAF mutation in papillary thyroid cancer and its value in tailoring initial treatment: A systematic review and meta-analysis. Medicine (Baltimore) 2012;91:274–286. doi: 10.1097/MD.0b013e31826a9c71. [DOI] [PubMed] [Google Scholar]
- 10.Xing M. BRAF mutation in papillary thyroid cancer: Pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 11.Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–6379. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 12.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 13.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–199. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Bishop J, Shan Y, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer. 2013;20:603–610. doi: 10.1530/ERC-13-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blasco MA. Telomeres and human disease: Ageing, cancer and beyond. Nat Rev Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 16.Mocellin S, Pooley KA, Nitti D. Telomerase and the search for the end of cancer. Trends Mol Med. 2013;19:125–133. doi: 10.1016/j.molmed.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Smekalova EM, Shubernetskaya OS, Zvereva MI, et al. Telomerase RNA biosynthesis and processing. Biochemistry (Mosc) 2012;77:1120–1128. doi: 10.1134/S0006297912100045. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Suarez E, Flores JM, Blasco MA. Cooperation between p53 mutation and high telomerase transgenic expression in spontaneous cancer development. Mol Cell Biol. 2002;22:7291–7301. doi: 10.1128/MCB.22.20.7291-7301.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Suarez E, Samper E, Ramirez A, et al. Increased epidermal tumors and increased skin wound healing in transgenic mice overexpressing the catalytic subunit of telomerase, mTERT, in basal keratinocytes. EMBO J. 2001;20:2619–2630. doi: 10.1093/emboj/20.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capezzone M, Marchisotta S, Cantara S, et al. Telomeres and thyroid cancer. Curr Genomics. 2009;10:526–533. doi: 10.2174/138920209789503897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saji M, Xydas S, Westra WH, et al. Human telomerase reverse transcriptase (hTERT) gene expression in thyroid neoplasms. Clin Cancer Res. 1999;5:1483–1489. [PubMed] [Google Scholar]
- 22.Horn S, Figl A, Rachakonda PS, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 23.Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janknecht R, Ernst WH, Nordheim A. SAP1a is a nuclear target of signaling cascades involving ERKs. Oncogene. 1995;10:1209–1216. [PubMed] [Google Scholar]
- 25.Strahl T, Gille H, Shaw PE. Selective response of ternary complex factor Sap1a to different mitogen-activated protein kinase subgroups. Proc Natl Acad Sci U S A. 1996;93:11563–11568. doi: 10.1073/pnas.93.21.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitmarsh AJ, Shore P, Sharrocks AD, et al. Integration of MAP kinase signal transduction pathways at the serum response element. Science. 1995;269:403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- 27.Vinagre J, Almeida A, Populo H, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185. doi: 10.1038/ncomms3185. [DOI] [PubMed] [Google Scholar]
- 28.Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110:6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinde I, Munari E, Faraj SF, et al. TERT promoter mutations occur early in urothelial neoplasia and are biomarkers of early disease and disease recurrence in urine. Cancer Res. 2013;73:7162–7167. doi: 10.1158/0008-5472.CAN-13-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qu Y, Dang S, Wu K, et al. TERT promoter mutations predict worse survival in laryngeal cancer patients. Int J Cancer. doi: 10.1002/ijc.28728. [epub ahead of print on January 16, 2014] [DOI] [PubMed] [Google Scholar]
- 31.Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309:1493–1501. doi: 10.1001/jama.2013.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landa I, Ganly I, Chan TA, et al. Frequent somatic TERT promoter mutations in thyroid cancer: Higher prevalence in advanced forms of the disease. J Clin Endocrinol Metab. 2013;98:E1562–E1566. doi: 10.1210/jc.2013-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu T, Wang N, Cao J, et al. The age- and shorter telomere-dependent TERT promoter mutation in follicular thyroid cell-derived carcinomas. Oncogene. doi: 10.1038/onc.2013.446. [epub ahead of print on October 21, 2013] [DOI] [PubMed] [Google Scholar]
- 34.van Staveren WC, Solis DW, Delys L, et al. Human thyroid tumor cell lines derived from different tumor types present a common dedifferentiated phenotype. Cancer Res. 2007;67:8113–8120. doi: 10.1158/0008-5472.CAN-06-4026. [DOI] [PubMed] [Google Scholar]
- 35.Liu D, Xing J, Trink B, et al. BRAF mutation-selective inhibition of thyroid cancer cells by the novel MEK inhibitor RDEA119 and genetic-potentiated synergism with the mTOR inhibitor temsirolimus. Int J Cancer. 2010;127:2965–2973. doi: 10.1002/ijc.25304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu R, Liu D, Trink E, et al. The Akt-specific inhibitor MK2206 selectively inhibits thyroid cancer cells harboring mutations that can activate the PI3K/Akt pathway. J Clin Endocrinol Metab. 2011;96:E577–E585. doi: 10.1210/jc.2010-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Z, Hou P, Ji M, et al. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab. 2008;93:3106–3116. doi: 10.1210/jc.2008-0273. [DOI] [PubMed] [Google Scholar]
- 38.Liu X, Qu S, Liu R, et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathologic characteristics of thyroid cancer. J Clin Endocrinol Metab. 2014;99:E1130–E1136. doi: 10.1210/jc.2013-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]