Abstract
Background
Recent clinical trials have demonstrated the safety and efficacy of several non–vitamin K oral anticoagulants (NOACs) for the treatment of atrial fibrillation (AF). However, there are limited data on their use and outcomes in routine clinical practice, particularly among patients newly diagnosed as having AF and patients with AF recently transitioned to a NOAC.
Methods/Design
ORBIT-AF II is a multicenter, national registry of patients with AF that is enrolling up to 15,000 newly diagnosed patients with AF and/or those with AF recently transitioned to a NOAC from 300 US outpatient practices. These patients will be followed for up to 2 years, including clinical status, outcomes (major adverse cardiovascular events, bleeding), and management of anticoagulation surrounding bleeding events. In addition, detailed data regarding the use of these agents in and around cardiac procedures, their complications, and management of such complications will be collected.
Conclusions
The ORBIT-AF II registry will provide valuable insights into the safety and effectiveness of NOACs used in AF in community practice settings.
Atrial fibrillation (AF) represents the most common dysrhythmia worldwide and leads to significant morbidity, mortality, and cost.1 It is a major risk factor for stroke, and patients with AF who experience stroke experience worse survival and disability compared with stroke patients without AF.2 The use of warfarin for the prevention of stroke in patients with AF was a landmark public health advancement, decreasing all-cause mortality in clinical trials by 26%.3-5 Annual rates of stroke in this population can be reduced from5%-10% to less than 2%, depending on underlying risk.4 Although warfarin has been used for oral anticoagulation for more than 50 years, it has significant shortcomings including the need for routine monitoring and numerous drug and food interactions.
In October 2010, dabigatran etexilate (a direct thrombin inhibitor) became the first oral alternative to warfarin for the prevention of stroke or systemic embolism in patients with nonvalvular AF. Subsequently, several additional agents have been approved or are in late-stage development (eg, oral factor Xa inhibitors rivaroxaban, apixaban, and edoxaban) as alternatives for anticoagulation in these patients. Each of these agents has been proven to be equivalent to or better than warfarin with regard to prevention of stroke or systemic embolism and risk of bleeding.6-10
The ORBIT-AF I registry11 described the use, effectiveness, and outcomes of oral anticoagulation in more than 10,000 all-comer patients with AF treated at a diverse collection of electrophysiologists, cardiologists, and generalists from across the United States between June 2010 and August 2011. To date, this long-term follow-up registry has provided important insights into risk stratification, treatment, and outcomes of these patients.12-17 However, most patients and data in ORBIT-AF I involve anticoagulation with warfarin; that registry largely predated the development of non–vitamin K oral anticoagulants (<10% of patients in ORBIT-AF I were treated with such drugs).
Moving forward, the proliferation of alternatives to warfarin has generated significant interest in the utilization, management, and outcomes associated with non–vitamin K oral anticoagulants in clinical practice, outside clinical trials. Specifically, dosing, temporary interruptions, perioperative management, and management of bleeding are major considerations urgently requiring evidence-based approaches. Therefore, in an effort to address these knowledge gaps, phase II of ORBIT-AF was designed (ORBIT-AF II).
Registry objectives
The objectives of the ORBIT-AF II registry are as follows: (1) to evaluate the safety of non–vitamin K oral anticoagulants, including factor Xa inhibitors and direct thrombin inhibitors, in outpatients with AF; (2) to evaluate clinical outcomes in patients with AF treated with non–vitamin K oral anticoagulants; (3) to describe the management of patients with AF undergoing cardiac procedures and their outcomes; (4) to describe AF patient characteristics, with specific attention to the use of non–vitamin K oral anticoagulants and high-risk subgroups, such as those with chronic kidney disease, acute coronary syndromes, or risk factors for stroke or bleeding; and (5) to describe patterns of switching and discontinuation among anticoagulant strategies in patients with AF.
Design
The ORBIT-AF II registry will be a prospective, observational study of outpatients with AF, followed up every 6 months to 2 years. By design, it will have a specific and unique focus on enrollment of patients with new-onset AF and those newly transitioned to non–vitamin K oral anticoagulants.
Site selection
Sites around the United States will be invited to participate, with particular attention to geographic and provider characteristics. Adaptive site enrollment will be used to ensure geographic heterogeneity, as well as diversity across practice type (eg, academic and private clinic) and provider type (primary care physician, neurologist, cardiologist, electrophysiologist). Approximately 300 sites will be activated to participate.
Patient enrollment criteria
The primary inclusion criteria will be age ≥21 years and with electrocardiographically confirmed AF (eg, by electrocardiogram, Holter monitoring, implanted device, etc) (Table I). In an effort to recruit patients on or eligible for non–vitamin K oral anticoagulants, the registry will include only patients who either (a) have a new diagnosis of AF within the previous 6 months or (b) have started taking a non–vitamin K oral anticoagulant for AF (dabigatran, rivaroxaban, apixaban, or any oral agent subsequently approved) within the previous 3 months (Figure). Patients can be enrolled fromboth inpatient and outpatient settings and are expected to be followed up clinically at least every 6 months.
Table I.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| • Age ≥21 y | • Anticipated life expectancy <6 mo |
| • Electrocardiographically confirmed AF | • Atrial flutter only without AF |
• ≥1 of the following:
|
• Transient AF secondary to a reversible condition (eg, hyperthyroidism, pulmonary embolism, and postcardiothoracic surgery) |
| • Anticipated, regular semiannual follow-up outpatient visits | • Participation in a randomized clinical study of any anticoagulation for AF |
| • Signed informed consent | • Any participation in the ORBIT-AF I registry |
Including dabigatran, rivaroxaban, and apixaban. If additional agents are approved during the study period, they will be added.
Figure.
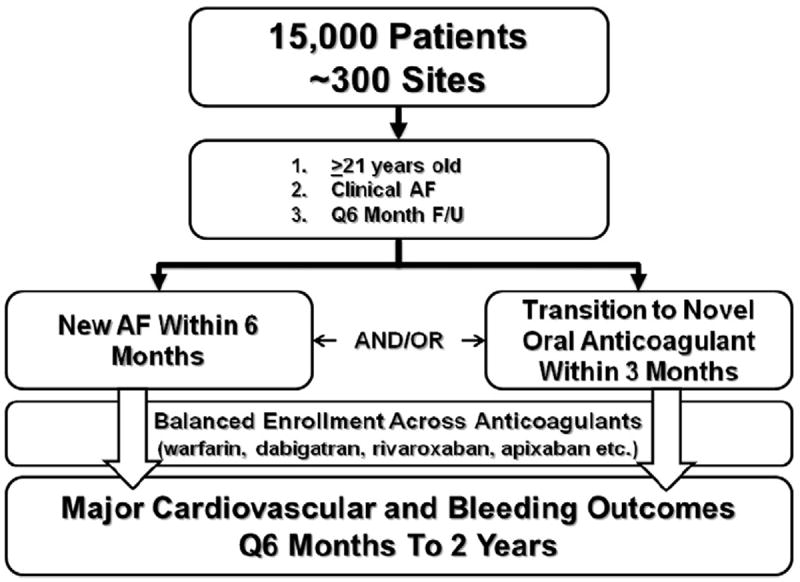
Design schematic of the ORBIT-AF II registry.
Principle exclusion criteria will be AF due to a reversible cause (eg, thyroid disease and pulmonary embolism), including postoperative AF, life expectancy less than 6 months, participation in ORBIT-AF I, or current enrollment in a clinical trial of an anticoagulant for AF. Patients with atrial flutter only will be excluded. Patients with both valvular and nonvalvular AF are eligible, and the use of warfarin is not an exclusion criterion.
Sample size and anticoagulation strategy
The target enrollment for ORBIT-AF II will be up to 15,000 patients from the 300 clinical sites. This will allow for balanced sampling across anticoagulant strategies, including each non–vitamin K agent, as well as warfarin or none (for patients with new-onset AF). In addition, we will use an adaptive design to allow for serial assessments of prevalent anticoagulation strategies. Thus, enrollment of patients receiving a specific agent may be capped to prevent any single drug or strategy from predominating in the registry.
Data collection
Primary data collection will be derived from the patient’s medical record, with input from the treating provider where needed. All data will be entered into a Web-based case report form, enabled with dynamic data checking for patient eligibility, validity, expected data ranges, and mandatory fields. Data entry will occur at 6-month intervals, regardless of patient clinic follow-up. All incident events and therapeutic changes will be entered at each collection interval (Table II).
Table II.
Timeline of data collection in ORBIT-AF II
| Baseline | 6 mo | 12 mo | 18 mo | 24 mo | |
|---|---|---|---|---|---|
| Demographics, medical history, AF diagnosis, provider/site information | X | ||||
| Contraindications to oral anticoagulant therapy | X | ||||
| Vital signs, laboratory data, ECG, Holter, and echo data | X | X | X | X | X |
| Current pharmacotherapies (including INR monitoring) and adverse events | X | X | X | X | X |
| Temporary and permanent discontinuation of antithrombotic therapies | X | X | X | X | |
| Details of cardiac procedures | X | X | X | X | |
| Outcomes | X | X | X | X |
Abbreviations: ECG, Electrocardiogram; INR, international normalized ratio.
Baseline data will include patient demographics, medical history, cardiovascular history, details of AF history and therapies, vital signs, laboratory measurements, electrocardiographic data, cardiac imaging parameters, details of medical management, and any contraindications to anticoagulation. At follow-up, major incident events and procedures (see “Outcomes,” below), as well as subsequent vital signs, laboratory studies, imaging parameters, and medication changes will be recorded. In-depth data regarding antithrombotic therapies, dosing, discontinuations, and reasons for discontinuations will be included in follow-up medication data. For patients on warfarin, international normalized ratio values will be recorded.
Outcomes
The primary safety outcome will be incidence of major bleeding during 2 years of follow-up, collected every 6 months, and defined by the International Society of Thrombosis and Haemostasis criteria; these include bleeding events meeting at least 1 of the following criteria18:
Fall in hemoglobin ≥2 g/dL
Transfusion of ≥2 units of packed red blood cells or whole blood
Any bleeding in a critical site (intracranial, intraspinal, intraocular, intra-articular, pericardial, retroperitoneal, or intramuscular with compartment syndrome)
Any fatal bleeding
Additional, unique, detailed data on management of bleeding events will be collected. This will include the use of any blood products or transfusions, potential reversal agents, and necessity for invasive management of bleeding events.
The primary effectiveness end point will be stroke or systemic embolism, which will be the only end point adjudicated by review of primary documentation. Additional outcomes will be captured, including transient ischemic attack, myocardial infarction, incident heart failure, cause-specific hospitalization, and cause-specific mortality. Specific design elements of the ORBIT-AF II case report form may also allow for longer-term follow-up and outcomes derived from administrative datasets, without the compromise of Health Insurance Portability and Accountability Act-protected (HIPAA-protected) information. These methods have been previously described and validated.19,20
Cardiac procedures
The management of non–vitamin K oral anticoagulants in the setting of trauma and/or invasive procedures (elective, urgent, or emergent) has become an area of great concern for patients and providers. These agents offer the benefits and the risks of being quick-acting, with half-lives that are relatively short compared with warfarin. Furthermore, there are few known reversible agents or antidotes, although several compounds including synthetics, biologics, and blood products have been tried or are in development.21-23 There is an unmet need for more information regarding outcomes following interruption of these agents, necessity of bridging therapies, and implications for bleeding management.
Therefore, an area of particular focus in the registry will be the management of these patients in and around cardiac procedures. Specific, additional data on (1) cardioversion, (2) electrophysiology procedures, (3) cardiac catheterization, and (4) cardiac surgery will be collected and will focus on risk of the procedure and provide granular detail on management of antithrombotic medications before, during, and after the procedure. In addition, data on complications and management of such events will be collected. This will include management and outcomes of bleeding complications, including blood product administration, reversal strategies, and the necessity of more aggressive management for bleeding. These data are unique to ORBIT-AF II, whereas ORBIT-AF I and other comparable registries have focused primarily on broader treatment strategies and outcomes.
Statistical analyses
Statistical considerations will vary according to specific analyses performed. However, there will be several important considerations common to all analyses from ORBIT-AF II. Principally, a variety of adjustment methodologies will be considered when attempting to compare patients; these may include simple multivariable adjustment, propensity scoring (matched or unmatched), inverse probability weighting, or other methods. Variables with <15% missing will routinely be imputed, using methods specific to individual analyses.24-26 Furthermore, within-site clustering of similar patients will need to be addressed in many analyses.
Of note, the registry is not designed or powered to address questions of direct comparative effectiveness between non–vitamin K anticoagulants. Although analyses may be stratified by anticoagulant strategy, such treatments are not randomized, and it would not be possible to adjust for all confounding.27 Thus, any conclusions of relative effectiveness between agents could be misleading.
Registry organization
Oversight and leadership
The protocol of the ORBIT-AF II registry has been approved by the Duke University Institutional Review Board. In addition, enrollment centers will obtain site-specific institutional review board approval pursuant to local regulations. All patients will be required to sign written, informed consent prior to collection of any study data. The ORBIT-AF II registry is led by independent executive and steering committees comprising electrophysiologists, cardiologists, anticoagulation specialists, and outcomes researchers. The Duke Clinical Research Institute will serve as the coordinating center, and the academic leadership will have full access to all of the primary data.
Study sponsorship
The ORBIT-AF II registry is sponsored by Janssen Scientific Affairs, LLC, Raritan, NJ. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
Timeline
Patient enrollment and data collection began in spring 2013. It is anticipated that enrollment will continue through the end of 2015. Follow-up will continue until all patients have 2-year data.
Reporting of potential severe adverse events
The ORBIT-AF II registry will provide postmarketing approval surveillance data regarding the safety of non–vitamin K oral anticoagulants. As such, all sites will be educated as to the importance and recognition of potential severe adverse events. Those events potentially related to rivaroxaban will be reported to the sponsor (which is responsible for marketing and approval of rivaroxaban in the United States); such events will be investigated, and the sponsor will be subsequently responsible for reporting of such events to regulatory authorities. Potential events related to all agents other than rivaroxaban (including nonanticoagulants) will be reported pursuant to local regulations and consistent with good clinical practice (ie, via the Food and Drug Administration’s MedWatch Safety Reporting Program: https://www.accessdata.fda.gov/scripts/medwatch/medwatch-online.htm).
Initial ORBIT-AF II patient recruitment
Characteristics of the initial 1,000 patients enrolled in ORBIT-AF II, compared with other contemporary AF registries, as well as controlled trials, are shown in Table III. This population is very similar to ORBIT-AF, phase I: 40% of the patients are women, and cardiovascular disease and risk factors are common. Consistently, patients studied are in their 70s and at significant risk for stroke or systemic embolism (as assessed by CHADS2 scores).
Table III.
Characteristics of AF study populations
| Randomized controlled trials between non–vitamin K anticoagulants and warfarin
|
Contemporary observational registries
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| RE-LY6 (n = 18,113)
|
ROCKET-AF9 (n = 14,264)
|
ARISTOTLE8 (n = 18,201)
|
ENGAGE AF-TIMI 4810 (n = 21,105)
|
GLORIA-AF*34 (n = 56,000)
|
PINNACLE36 (n = 14,464)
|
GARFIELD (cohort 1, n = 10,164)35
|
ORBIT-AF I (n = 10,132)
|
ORBIT-AF II (preliminary, n = 1011)
|
|
| Anticoagulant(s) studied | Dabigatran | Rivaroxaban | Apixaban | Edoxaban | Warfarin, dabigatran | Warfarin | 58% warfarin, 4% non–vitamin K anticoagulant | 72% warfarin, 4% dabigatran | 22% warfarin, 9% dabigatran, 41% rivaroxaban, 14% apixaban |
| Locale | International, including the United States | International, including the United States | United States only | International, excluding the United States | United States only | United States only | |||
| Patient characteristics | |||||||||
| Age (y) | 72 | 73 | 70 | 72 | ≥18 | 67 | 70 | 73 | 73 |
| Female | 36% | 40% | 35% | 38% | N/A | 47% | 43% | 42% | 44% |
| Heart failure | 32% | 62% | 35% | 58% | N/A | 35% | 21% | 32% | 23% |
| Hypertension | 79% | 90% | 87% | 94% | N/A | 70% | 78% | 83% | 83% |
| Diabetes | 23% | 40% | 25% | 36% | N/A | 19% | 22% | 29% | 27% |
| Prior stroke/TIA | 20% | 55% | 20% | 28% | N/A | 3.1% | 14% | 15% | 11% |
| Prior MI | 17% | 17% | 14% | N/A | N/A | 12% | 10% | 16% | 34% |
| New-onset AF | N/A | 1.4% | N/A | N/A | 100% | N/A | 30% | 4.7% | 76% |
| Mean CHADS2 score | 2.1 | 3.5 | 3.5 | 2.8 | N/A | N/A | 1.9 | 2.3 | 2 |
Abbreviations: RELY, Randomized Evaluation of Long-Term Anticoagulation Therapy; ROCKET-AF, Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism; ARISTOTLE, Apixaban for Reduction in Stroke and Other Thrombo-embolic Events in Atrial Fibrillation; ENGAGE AFTIMI 48, Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48; GLORIA-AF, Global Registry on Long-Term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation; PINNACLE, Practice Innovation And Clinical Excellence; GARFIELD, Global Anticoagulant Registry in the FIELD; TIA, transient ischemic attack; MI, myocardial infarction; N/A, not applicable.
Data from GLORIA-AF are expected, based on its design.
Discussion
The ORBIT-AF II study will function as a postmarket surveillance study after the transition from clinical practice with a single available anticoagulant (ie, warfarin) to the era of widely available non–vitamin K, or target-specific, oral anticoagulants. This transition follows on the recent completion of several “mega-trials” of both direct thrombin and factor Xa inhibitors.6-10 These large, phase III trials were conducted to demonstrate safety and efficacy in specific populations, with a major objective being market approval by the Food and Drug Administration. Thus, they could not address myriad issues in the clinical implementation of the drugs, including transitions between therapies, perioperative management, and treatment of bleeding in patients receiving non–vitamin K oral anticoagulants. The ORBIT-AF II study will collect detailed data on patients experiencing these events and, importantly, their outcomes. Subsequent analyses of these data can help guide clinicians through many everyday management decisions where clinical trials cannot.
The ORBIT-AF I registry served as a primary observational cohort of more than 10,000 outpatients with AF, predominantly managed with vitamin K antagonism for stroke prevention. It has provided valuable data on the contemporary management of such patients, including rhythm strategies, symptom management, and anticoagulation, in the United States.12-15,17 However, the timing of enrollment in ORBIT-AF I precluded in-depth study of patients managed with each of the non–vitamin K oral anticoagulants. Furthermore, detailed data regarding periprocedural management and management of bleeding complications were not the focus of ORBIT-AF I. Whereas ORBIT-AF I provided novel data on contemporary, overall management, and outcomes in US patients with AF, ORBIT-AF II will provide complimentary, detailed insights regarding management of anticoagulants around specific clinical scenarios.
This will contrast with the methods previously used to assess postmarketing use of these drugs. Such studies have so far been limited to (1) post hoc analyses of the randomized trials,28,29 (2) experiences within a single or few centers,30,31 or (3) international and/or administrative claims data. 32,33 Although each method has strengths, there are inherent limitations that we hope to address in ORBIT-AF II. These include the following: the availability of data from community, “real-world” use of these drugs; broad enrollment across practice centers around the United States; and detailed data on medical diagnoses, medication use, dose, discontinuation, reasons for medication changes, periprocedural data, clinical outcomes, and management of adverse events.
A key feature of ORBIT-AF II is the careful attention to periprocedural management and transitions among stroke prevention strategies. Detailed data surrounding many of the most common cardiovascular procedures, including drug management, complications, and management of bleeding, will provide valuable insights for providers—implementation of these drugs can be relatively straightforward in the most stable patients. It is during these transitions and interventions where providers are challenged as to the best management for their patients, and up to now, data have been lacking.
However, ORBIT-AF II will also extend data from ORBITAF I—the culmination will be a cohort of approximately 25,000 outpatients with AF with long-term follow-up. It will be, by far, the largest detailed observational cohort of patients with AF in the United States, powered to explore a variety of outcomes, including ischemic, hemorrhagic, and mortality. This will have important implications for the assessment of rare events and outcomes, as well as continued refinement of risk stratification tools that are broadly applicable.
Additional observational studies of patients with AF are enrolling in the United States and around the world. Contemporary international cohorts have shown limited data on non–vitamin K oral anticoagulants, may focus on a single agent, and/or include minority representation of US patients.34,35 Others will have a specific focus on quality of care and implementation of guidelines, such as the American Heart Association’s expansion of “Get With The Guidelines” to patients with AF (GWTG-AFIB). Although GWTG-AFIB will generate feedback on short-term quality metrics among hospitalized patients to ensure that specific, previously identified best practices are followed, the ORBIT-AF program will identify broad patterns of treatment and outcomes longitudinally to help better inform what those best practices should be.
Limitations
The ORBIT-AF II registry is designed as a large, prospective, observational, clinical study in an effort to address specific questions regarding the care of patients with AF in community practice. However, there are specific limitations inherent in such methods. Primarily, there is no random assignment of patients to any treatments, and thus, comparisons among groups will be limited by residual and unmeasured bias. They could provide descriptive and hypotheses-generating observations. Second, data will be dependent on the quality of medical record abstraction. Lastly, the recruitment of sites prescribing non–vitamin K oral anticoagulants may represent unique providers (eg, “early-adopters”), difficult to extrapolate to a broader population of AF providers.
Conclusions
The ORBIT-AF II registry will fill a significant gap in the dynamic landscape of AF care and research. It will provide unique and necessary data on the management and outcomes of outpatients treated with emerging therapies and, combined with ORBIT-AF I, will yield the largest, contemporary longitudinal cohort of patients with AF in the United States.
Disclosures
The ORBIT-AF II registry is sponsored by Janssen Scientific Affairs, LLC, Raritan, NJ. Dr Steinberg was funded by National Institutes of Health T-32 Training Grant No. 5 T32 HL 7101-38.
The following relationships related to this manuscript exist: B.S., R.B., D.O., S.K., D.H., A.G., and L.T. report no disclosures. Dr Kowey reports modest consultant/advisory board support from Boehringer Ingelheim, Bristol-Myers Squibb, Johnson & Johnson, Portola, Merck, Sanofi, and Daiichi Sankyo. Dr Fonarow reports consultant to Janssen (modest) and Medtronic (modest). Dr Ansell reports modest consultant/advisory board from Bristol-Myers Squibb, Pfizer, Janssen, Daiichi, Boehringer Ingelheim, and Alere. Dr Gersh reports modest DSMB/advisory board support from Medtronic, Baxter Healthcare Corporation, InspireMD, Cardiovascular Research Foundation, PPD Development, LP, Boston Scientific, and St Jude. Dr Hylek reports modest speakers bureau support form Boehringer Ingelheim and Bayer and modest consultant/advisory board support from Johnson & Johnson, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Pfizer, and Ortho-McNeil-Janssen. Dr Mahaffey reports significant research grant support from Johnson & Johnson and significant consultant/advisory board support from Johnson & Johnson. Dr Chang reports significant employment with Johnson & Johnson. Dr Peterson reports significant research grant support from Eli Lilly & Company, Janssen Pharmaceuticals, Inc, and the American Heart Association and modest consultant/advisory board support from Boehringer Ingelheim, Bristol-Myers Squibb, Janssen Pharmaceuticals, Inc, Pfizer, and Genentech Inc. Dr Piccini reports receiving grants for clinical research from ARCA biopharma, Boston Scientific, GE Healthcare, Johnson & Johnson, and ResMed as well as consultancies to Janssen Scientific Affairs and Spectranetics.
References
- 1.Wolf PA, Mitchell JB, Baker CS, et al. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med. 1998;158(3):229–34. doi: 10.1001/archinte.158.3.229. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the FraminghamStudy. Stroke. 1991;22(8):983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 3.Ezekowitz MD, Levine JA. Preventing stroke in patients with atrial fibrillation. JAMA. 1999;281(19):1830–5. doi: 10.1001/jama.281.19.1830. [DOI] [PubMed] [Google Scholar]
- 4.Hart RG, Benavente O, McBride R, et al. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131(7):492–501. doi: 10.7326/0003-4819-131-7-199910050-00003. [DOI] [PubMed] [Google Scholar]
- 5.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–67. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 6.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versuswarfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 7.Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806–17. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 8.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versuswarfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 9.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 10.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 11.Piccini JP, Fraulo ES, Ansell JE, et al. Outcomes registry for better informed treatment of atrial fibrillation: rationale and design of ORBIT-AF. Am Heart J. 2011;162(4):606–612 e1. doi: 10.1016/j.ahj.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Cullen MW, Kim S, Piccini JP, Sr, et al. Risks and benefits of anticoagulation in atrial fibrillation: insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Circ Cardiovasc Qual Outcomes. 2013;6(4):461–9. doi: 10.1161/CIRCOUTCOMES.113.000127. [DOI] [PubMed] [Google Scholar]
- 13.Fosbol EL, Holmes DN, Piccini JP, et al. Provider specialty and atrial fibrillation treatment strategies in united states community practice: findings from the ORBIT-AF registry. J Am Heart Assoc. 2013;2(4):e000110. doi: 10.1161/JAHA.113.000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hess PL, Kim S, Piccini JP, et al. Use of evidence-based cardiac prevention therapy among outpatients with atrial fibrillation. Am J Med. 2013;126(7):625–632 e1. doi: 10.1016/j.amjmed.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinberg BA, Holmes DN, Ezekowitz MD, et al. Rate versus rhythm control for management of atrial fibrillation in clinical practice: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Am Heart J. 2013;165(4):622–9. doi: 10.1016/j.ahj.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinberg BA, Holmes DN, Piccini JP, et al. Early adoption of dabigatran and its dosing in US patients with atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation. J Am Heart Assoc. 2013;2(6):e000535. doi: 10.1161/JAHA.113.000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinberg BA, Kim S, Piccini JP, et al. Use and associated risks of concomitant aspirin therapy with oral anticoagulation in patients with atrial fibrillation: insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Circulation. 2013;128(7):721–8. doi: 10.1161/CIRCULATIONAHA.113.002927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692–4. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 19.Hammill BG, Hernandez AF, Peterson ED, et al. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157(6):995–1000. doi: 10.1016/j.ahj.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernandez AF, Hammill BG, Peterson ED, et al. Relationships between emerging measures of heart failure processes of care and clinical outcomes. Am Heart J. 2010;159(3):406–13. doi: 10.1016/j.ahj.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siegal DM, Cuker A. Reversal of novel oral anticoagulants in patients with major bleeding. J Thromb Thrombolysis. 2013;35(3):391–8. doi: 10.1007/s11239-013-0885-0. [DOI] [PubMed] [Google Scholar]
- 22.Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013;19(4):446–51. doi: 10.1038/nm.3102. [DOI] [PubMed] [Google Scholar]
- 23.Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573–9. doi: 10.1161/CIRCULATIONAHA.111.029017. [DOI] [PubMed] [Google Scholar]
- 24.Enders CK. Applied missing data analysis. Guilford Press; 2010. [Google Scholar]
- 25.Little RJA, Rubin DB. Statistical analysis with missing data. 2. New York: John Wiley & Sones; 2002. [Google Scholar]
- 26.Rubin DB. Inference and missing data. Biometrika. 1976;63:581–92. [Google Scholar]
- 27.Barbash GI, Reiner J, White HD, et al. Evaluation of paradoxic beneficial effects of smoking in patients receiving thrombolytic therapy for acute myocardial infarction: mechanism of the “smoker’s paradox” from the GUSTO-I trial, with angiographic insights. Global Utilization of Streptokinase and Tissue-Plasminogen Activator for Occluded Coronary Arteries. J Am Coll Cardiol. 1995;26(5):1222–9. doi: 10.1016/0735-1097(95)00299-5. [DOI] [PubMed] [Google Scholar]
- 28.Lip GY, Larsen TB, Skjoth F, et al. Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2012;60(8):738–46. doi: 10.1016/j.jacc.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 29.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacyand safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2013 doi: 10.1016/S0140-6736(13)62343-0. http://dx.doi.org/10.1016/S0140-6736(13)62343-0. [DOI] [PubMed]
- 30.Lakkireddy D, Reddy YM, Di Biase L, et al. Feasibility and safety of dabigatran versus warfarin for periprocedural anticoagulation in patients undergoing radiofrequency ablation for atrial fibrillation: results from a multicenter prospective registry. J Am Coll Cardiol. 2012;59(13):1168–74. doi: 10.1016/j.jacc.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Lakkireddy D, Reddy YM, Di Biase L, et al. Feasibility & safety of uninterrupted rivaroxaban for periprocedural anticoagulation in patients undergoing radiofrequency ablation for atrial fibrillation: results from a multicenter prospective registry. J Am Coll Cardiol. 2014;63(10):982–8. doi: 10.1016/j.jacc.2013.11.039. [DOI] [PubMed] [Google Scholar]
- 32.Southworth MR, Reichman ME, Unger EF. Dabigatran and postmarketing reports of bleeding. N Engl J Med. 2013;368(14):1272–4. doi: 10.1056/NEJMp1302834. [DOI] [PubMed] [Google Scholar]
- 33.Larsen TB, Rasmussen LH, Skjoth F, et al. Efficacy and safety of dabigatran etexilate and warfarin in “real-world” patients with atrial fibrillation: a prospective nationwide cohort study. J Am Coll Cardiol. 2013;61(22):2264–73. doi: 10.1016/j.jacc.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Huisman MV, Lip GY, Diener HC, et al. Design and rationale of Global Registry on Long-Term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation: a global registry program on longterm oral antithrombotic treatment in patients with atrial fibrillation. Am Heart J. 2014;167(3):329–34. doi: 10.1016/j.ahj.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Kakkar AK, Mueller I, Bassand JP, et al. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD registry. PLoS One. 2013;8(5):e63479. doi: 10.1371/journal.pone.0063479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan PS, Oetgen WJ, Buchanan D, et al. Cardiac performance measure compliance in outpatients: the American College of Cardiology and National Cardiovascular Data Registry’s PINNACLE (Practice Innovation And Clinical Excellence) program. J Am Coll Cardiol. 2010;56(1):8–14. doi: 10.1016/j.jacc.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]


