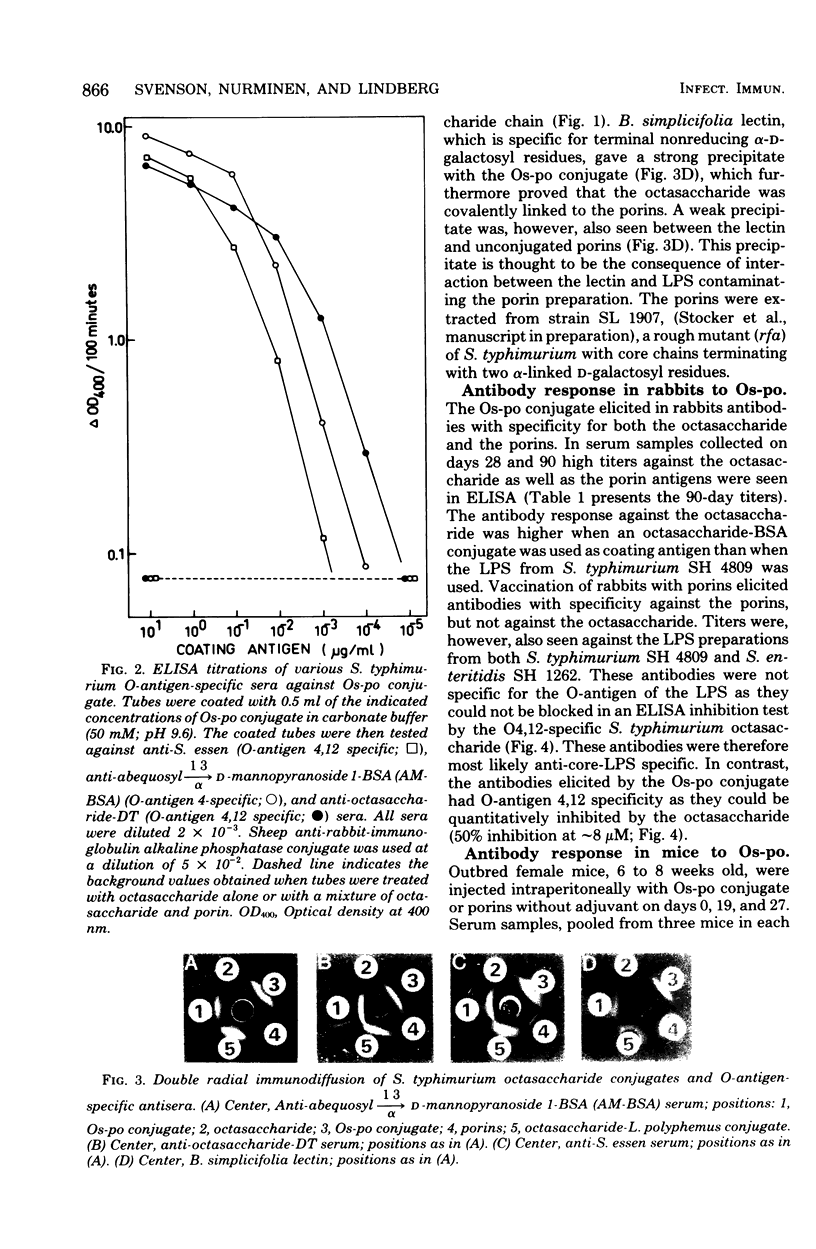
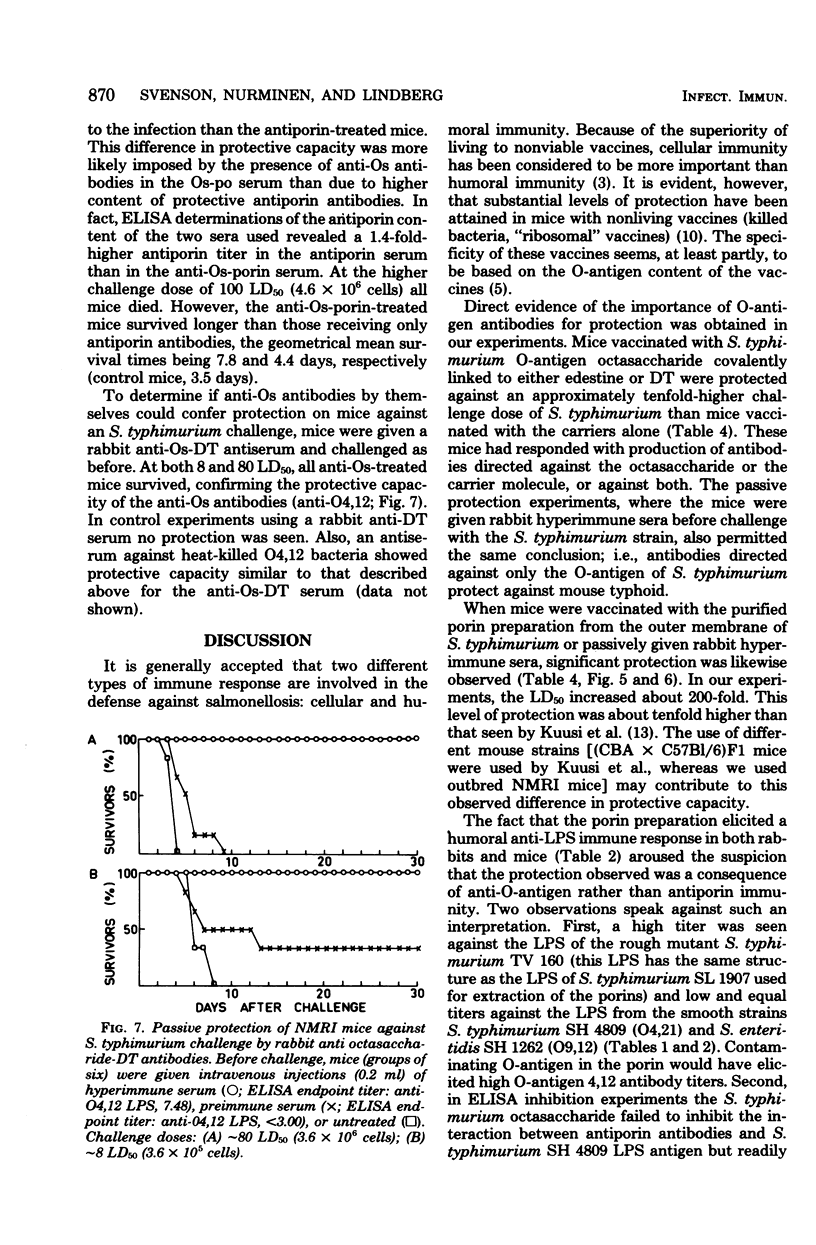
Abstract
Outbred mice were vaccinated with various artificial Salmonella vaccines and subsequently challenged intraperitoneally with graded doses of virulent Salmonella typhimurium. The Salmonella vaccines used were: (i) octasaccharide, obtained by hydrolysis of the O-antigenic polysaccharide chain of S. typhimurium strain SH 4809 with phage P22-associated endo-rhamnosidase and covalently linked to either diphtheria toxin or edestine; (ii) purified outer membrane proteins (porins) from S. typhimurium; and (iii) octasaccharide covalently linked to porins. All vaccines induced significant protection against experimental infection of mice with S. typhimurium. However, vaccination with the octasaccharide-porin conjugate resulted in better protection than that obtained by vaccination with octasaccharide or porin vaccines separately. Rabbit antibodies raised against the different vaccines were also passively administered intravenously to mice. Such mice were protected against challenge with virulent S. typhimurium by antibodies specific for the S. typhimurium O-antigen or for the porins. Thus, active immunization with more than one surface component of Salmonella bacteria improved the efficacy of the vaccine. The data from the passive immunization experiments also emphasized the role of humoral immunity for protection against S. typhimurium infection.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerman C. R., Eisenstein T. K. Comparative efficacy and toxicity of a ribosomal vaccine, acetone-killed cells, lipopolysaccharide, and a live cell vaccine prepared from Salmonella typhhimurium. Infect Immun. 1978 Feb;19(2):575–582. doi: 10.1128/iai.19.2.575-582.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson H. E., Hurvell B., Lindberg A. A. Enzyme-linked immunosorbent assay (ELISA) for titration of antibodies against Brucella abortus and Yersinia enterocolitica. Acta Pathol Microbiol Scand C. 1976 Jun;84(3):168–176. doi: 10.1111/j.1699-0463.1976.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974 Dec;38(4):371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOIS M., GILLES K., HAMILTON J. K., REBERS P. A., SMITH F. A colorimetric method for the determination of sugars. Nature. 1951 Jul 28;168(4265):167–167. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- Eisenstein T. K. Evidence for O antigens as the antigenic determinants in "ribosomal" vaccines prepared from Salmonella. Infect Immun. 1975 Aug;12(2):364–377. doi: 10.1128/iai.12.2.364-377.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekborg G., Eklind K., Garegg P. J., Gotthammar B., Carlsson H. E., Lindberg A. A., Svenungsson B. Artificial disaccharide-protein conjugates as immunogens for the preparation of specific anti-Salmonella O-antisera. Immunochemistry. 1977 Feb;14(2):153–157. doi: 10.1016/0019-2791(77)90295-6. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Eriksson U., Svenson S. B., Lönngren J., Lindberg A. A. Salmonella phage glycanases: substrate specificity of the phage P22 endo-rhamnosidase. J Gen Virol. 1979 Jun;43(3):503–511. doi: 10.1099/0022-1317-43-3-503. [DOI] [PubMed] [Google Scholar]
- Johnson W. Ribosomal vaccines. I. Immunogenicity of ribosomal fractions isolated from Salmonella typhimurium and Yersinia pestis. Infect Immun. 1972 Jun;5(6):947–952. doi: 10.1128/iai.5.6.947-952.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörbeck H., Carlsson H. E., Svenson S. B., Lindberg A. A., Alfredsson G., Garegg P. J., Svensson S., Wallin N. H. Immunochemistry of Salmonella O-antigens. Specificity and cross-reactivity of factor O9 serum and of antibodies against tyvelose (Formula: see text) mannose coupled to bovine serum albumin. Int Arch Allergy Appl Immunol. 1979;58(1):11–19. [PubMed] [Google Scholar]
- Kuusi N., Nurminen M., Saxen H., Valtonen M., Mäkelä P. H. Immunization with major outer membrane proteins in experimental salmonellosis of mice. Infect Immun. 1979 Sep;25(3):857–862. doi: 10.1128/iai.25.3.857-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindberg A. A., Holme T. Evaluation of some extraction methods for the preparation of bacterial lipopolysaccharides for structural analysis. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(5):751–759. doi: 10.1111/j.1699-0463.1972.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Lyman M. B., Steward J. P., Roantree R. J. Characterization of the virulence and antigenic structure of Salmonella typhimurium strains with lipopolysaccharide core defects. Infect Immun. 1976 Jun;13(6):1539–1542. doi: 10.1128/iai.13.6.1539-1542.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Song S. A., Shaltiel L., Nurminen M. Outer membrane of Salmonella XIV. Reduced transmembrane diffusion rates in porin-deficient mutants. Biochem Biophys Res Commun. 1976 May 23;76(2):324–330. doi: 10.1016/0006-291x(77)90728-8. [DOI] [PubMed] [Google Scholar]
- Paul W. E., Katz D. H., Benacerraf B. Augmented anti-S 3 antibody responses to an S 3 -protein conjugate. J Immunol. 1971 Sep;107(3):685–688. [PubMed] [Google Scholar]
- Plant J., Glynn A. A., Wilson B. M. Protective effects of a supernatant factor from Salmonella typhimurium on Salmonella typhimurium infection of inbred mice. Infect Immun. 1978 Oct;22(1):125–131. doi: 10.1128/iai.22.1.125-131.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson S. B., Larsen K. An enzyme-linked immunosorbent assay (ELISA) for the determination of diphtheria toxin antibodies. J Immunol Methods. 1977;17(3-4):249–256. doi: 10.1016/0022-1759(77)90107-7. [DOI] [PubMed] [Google Scholar]
- Svenson S. B., Lindberg A. A. Coupling of acid labile Salmonella specific oligosaccharides to macromolecular carriers. J Immunol Methods. 1979;25(4):323–335. doi: 10.1016/0022-1759(79)90025-5. [DOI] [PubMed] [Google Scholar]
- Svenson S. B., Lindberg A. A. Immunochemistry of Salmonella O-antigens: preparation of an octasaccharide-bovine serum albumin immunogen representative of Salmonella serogroup B O-antigen and characterization of the antibody response. J Immunol. 1978 May;120(5):1750–1757. [PubMed] [Google Scholar]




